Cereal Husks: Versatile Roles in Grain Quality and Seedling Performance
Abstract
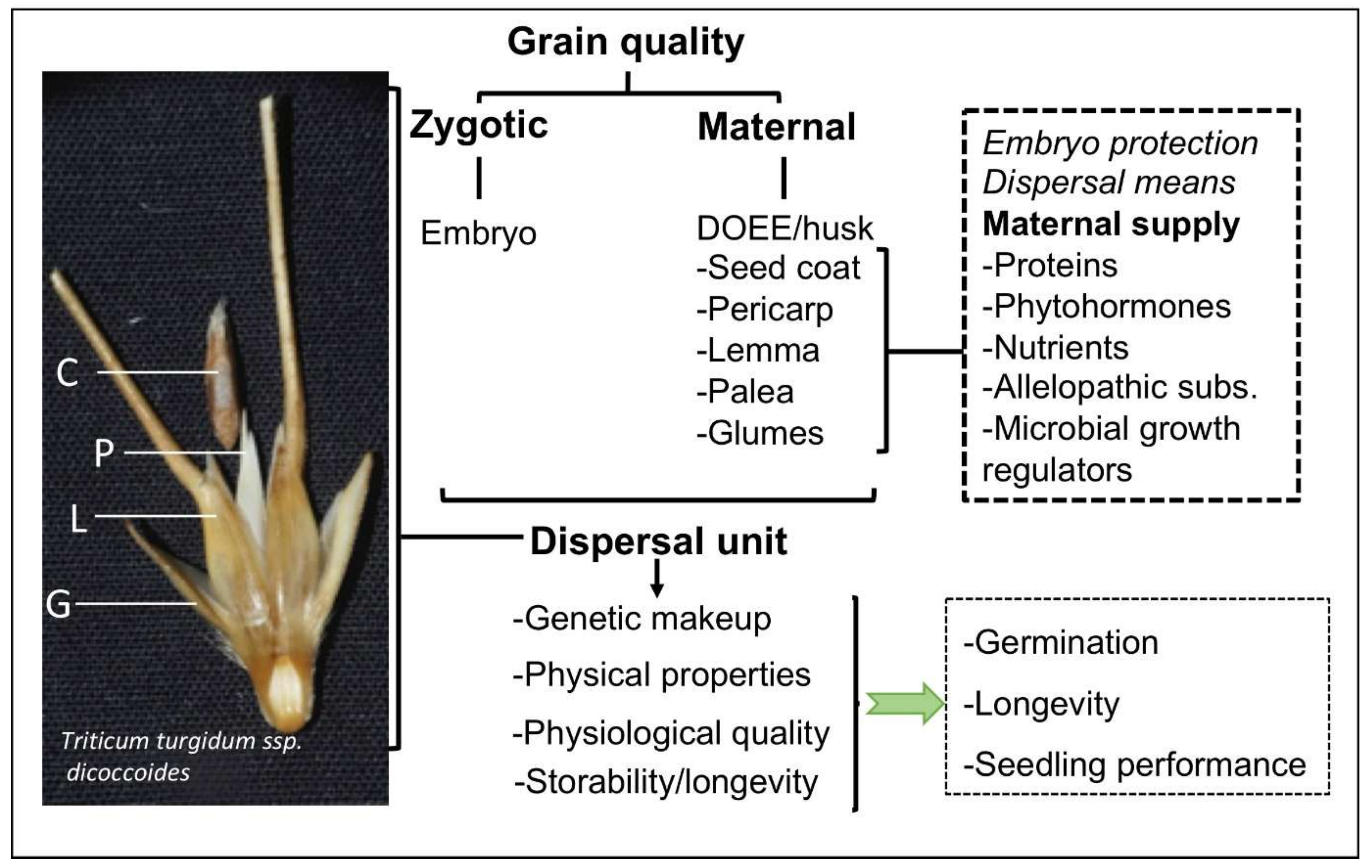
:1. Introduction: Dispersal Units of Cereals
2. The Role of Husk and Other Dead Coverings in Seed Biology and Ecology
3. Husks Function as Long-Term Storage for Beneficial Substances
4. The Husk and Storability of Seeds in Seed Banks
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, M. Seed quality assessment. Seed Sci. Res. 1998, 8, 265–276. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Tan, D.; Baskin, C.; Baskin, J. Seed dormancy in six cold desert Brassicaceae species with indehiscent fruits. Seed Sci. Res. 2015, 25, 276–285. [Google Scholar] [CrossRef]
- Godwin, J.; Raviv, B.; Grafi, G. Dead pericarps of dry fruits function as long-term storage for active hydrolytic enzymes and other substances that affect germination and microbial growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huarte, R.; Staltari, S.; Chorzempa, S.E.; García, M.D. Tripsacum dactyloides (L.) L. caryopses water uptake dynamics and germination responses to gibberellic acid, fluctuating temperatures and pericarp scarification. Seed Sci. Technol. 2007, 35, 255–265. [Google Scholar] [CrossRef]
- Peart, M.H. Experiments on the biological significance of the morphology of seed-dispersal units in grasses. J. Ecol. 1979, 67, 843–863. [Google Scholar] [CrossRef]
- Peart, M.H. Further experiments on the biological significance of the morphology of seed-dispersal units in grasses. J. Ecol. 1981, 69, 425–436. [Google Scholar] [CrossRef]
- Cavanagh, A.M.; Morgan, J.W.; Godfree, R.C. Awn Morphology Influences Dispersal, Microsite Selection and Burial of Australian Native Grass Diaspores. Front. Ecol. Evol. 2020, 8, 581967. [Google Scholar] [CrossRef]
- Elbaum, R.; Zaltzman, L.; Burgert, I.; Fratzl, P. The role of wheat awns in the seed dispersal unit. Science 2007, 316, 884–886. [Google Scholar] [CrossRef]
- Booth, D.T. Plant diaspore functions. J. Seed Technol. 1990, 14, 61–73. [Google Scholar]
- Wurzburger, J.; Leshem, Y. Physiological action of the germination inhibitor in the husk of Aegilops kotschyi Boiss. New Phytol. 1969, 68, 337–341. [Google Scholar] [CrossRef]
- Fandrich, L.; Mallory-Smith, C.A. Factors affecting germination of jointed goatgrass (Aegilops cylindrica) seed. Weed Sci. 2006, 54, 677–684. [Google Scholar] [CrossRef]
- Webb, J.; Miao, S.; Zhang, X.-H. Factors and mechanisms influencing seed germination in a wetland plant sawgrass. Plant Growth Regul. 2009, 57, 243–250. [Google Scholar] [CrossRef]
- Takahashi, N. Inhibitory effect of oxygen on seed germination in rice. Ann. Bot. 1985, 55, 597–600. [Google Scholar] [CrossRef]
- Ma, H.Y.; Liang, Z.W.; Wang, Z.C.; Chen, Y.; Huang, L.H.; Yang, F. Lemmas and endosperms significantly inhibited germination of Leymus chinensis (Trin.) Tzvel. (Poaceae). J. Arid Environ. 2008, 72, 573–578. [Google Scholar] [CrossRef]
- Li, M.; Han, J.; Wang, Y.; Sun, J.; Haferkamp, M. Different seed dormancy levels imposed by tissues covering the Caryopsis in zoysiagrass (Zoysia japonica Steud.). Seed Sci. Technol. 2010, 38, 320–331. [Google Scholar] [CrossRef]
- Ahring, R.M.; Todd, G. The bur enclosure of the caryopses of buffalograss as a factor affecting germination. Agron. J. 1977, 69, 15–17. [Google Scholar] [CrossRef]
- Ogawa, K.; Iwabuchi, M. A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 2001, 42, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Mamut, J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Role of trichomes and pericarp in the seed biology of the desert annual Lachnoloma lehmannii (Brassicaceae). Ecol. Res. 2014, 29, 33–44. [Google Scholar] [CrossRef]
- Fulbright, T.E.; Flenniken, K.S. Causes of dormancy in Paspalum plicatulum (Poaceae) seeds. Southwest Nat. 1988, 33, 35–39. [Google Scholar] [CrossRef]
- Gallart, M.; Verdu, A.M.C.; Mas, M.T. Dormancy breaking in Digitaria sanguinalis seeds: The role of the caryopsis covering structures. Seed Sci. Technol. 2008, 36, 259–270. [Google Scholar] [CrossRef]
- Booth, D.T.; Schuman, G.E. Seedbed ecology of winterfat: Fruits versus threshed seeds. J. Range Manag. 1983, 38, 387–390. [Google Scholar] [CrossRef]
- Hu, X.W.; Wang, Y.R.; Wu, Y.P. Effects of the pericarp on imbibition, seed germination, and seedling establishment in seeds of Hedysarum scoparium Fisch. et Mey. Ecol. Res. 2009, 24, 559–564. [Google Scholar] [CrossRef]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raviv, B.; Khadka, J.; Swetha, B.; Singiri, J.R.; Grandhi, R.; Shapira, E.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Sternberg, M.; et al. Extreme drought alters progeny dispersal unit properties of winter wild oat (Avena sterilis L.). Planta 2020, 252, 77. [Google Scholar] [CrossRef]
- Ueno, K.; Miyoshi, K. Difference of optimum germination temperature of seeds of intact and dehusked japonica rice during seed development. Euphytica 2005, 143, 271–275. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Elgabra, M.; Mosa, K.A.; Fakhry, A.; Soliman, S. Roles of hardened husks and membranes surrounding Brachypodium hybridum grains on germination and seedling growth. Plants 2019, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Pedrini, S.; Lewandrowski, W.; Stevens, J.C.; Dixon, K.W. Optimising seed processing techniques to improve germination and sowability of native grasses for ecological restoration. Plant Biol. 2019, 21, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Meredith, W.O.S. Note on the malting quality of peeled barley. J. Inst. Brew. 1959, 65, 31–33. [Google Scholar] [CrossRef]
- Grant, K.R.; Brennan, M.; Hoad, S.P. The structure of the barley husk influences its resistance to mechanical stress. Front. Plant Sci. 2021, 11, 614334. [Google Scholar] [CrossRef]
- Windsor, J.B.; Symonds, V.V.; Mendenhall, J.; Lloyd, A.M. Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 2000, 22, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Radchuk, V.; Tran, V.; Radchuk, R.; Diaz-Mendoza, M.; Weier, D.; Fuchs, J.; Riewe, D.; Hensel, G.; Kumlehn, J.; Munz, E.; et al. Vacuolar processing enzyme 4 contributes to maternal control of grain size in barley by executing programmed cell death in the pericarp. New Phytol. 2018, 218, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Haughn, G.; Chaudhury, A. Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci. 2005, 10, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence–A genomics approach. Plant Biotechnol. J. 2003, 1, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, F.; Cejudo, F.J. Programmed cell death (PCD): An essential process of cereal seed development and germination. Front. Plant Sci. 2014, 5, 366. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [Green Version]
- Raviv, B.; Godwin, J.; Granot, G.; Grafi, G. The dead can nurture: Novel insights into the function of dead organs enclosing embryos. Int. J. Mol. Sci. 2018, 19, 2455. [Google Scholar] [CrossRef] [Green Version]
- Grafi, G. Dead but not dead end: Multifunctional role of dead organs enclosing embryos in seed biology. Int. J. Mol. Sci. 2020, 21, 8024. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Scheler, C.; Weitbrecht, K.; Pearce, S.P.; Hampstead, A.; Büttner-Mainik, A.; Lee, K.J.; Voegele, A.; Oracz, K.; Dekkers, B.J.; Wang, X.; et al. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015, 167, 200–215. [Google Scholar] [CrossRef] [Green Version]
- Müller, K.; Levesque-Tremblay, G.; Bartels, S.; Weitbrecht, K.; Wormit, A.; Usadel, B.; Haughn, G.; Kermode, A.R. Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 2013, 161, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Galiana, E.; Bonnet, P.; Conrod, S.; Keller, H.; Panabières, F.; Ponchet, M.; Poupet, A.; Ricci, P. RNase activity prevents the growth of a fungal pathogen in tobacco leaves and increases upon induction of systemic acquired resistance with elicitin. Plant Physiol. 1997, 115, 1557–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hugot, K.; Ponchet, M.; Marais, A.; Ricci, P.; Galiana, E. A tobacco S-like RNase inhibits hyphal elongation of plant pathogens. Mol. Plant Microbe Interact. 2002, 15, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, N.; Sharma, K.P.; Gaur, R.K.; Gupta, V.K. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Chandrashekar, N.; Ali, S.; Grover, A. Exploring expression patterns of PR-1, PR-2, PR-3, and PR-12 like genes in Arabidopsis thaliana upon Alternaria brassicae inoculation. Biotechnology 2018, 8, 230. [Google Scholar] [CrossRef]
- Lamkemeyer, P.; Laxa, M.; Collin, V.; Li, W.; Finkemeier, I.; Schöttler, M.A.; Holtkamp, V.; Tognetti, V.B.; Issakidis-Bourguet, E.; Kandlbinder, A.; et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J. 2006, 45, 968–981. [Google Scholar] [CrossRef]
- Sun, W.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins-evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Worrall, D.; Holroyd, G.H.; Moore, J.P.; Glowacz, M.; Croft, P.; Taylor, J.E.; Paul, N.D.; Roberts, M.R. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol. 2012, 193, 770–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.Y.; Liang, Z.W.; Liu, M.; Wang, M.M.; Wang, S.H. Mechanism of the glumes in inhibiting seed germination of Leymus chinensis (Trin.) Tzvel. (Poaceae). Seed Sci. Technol. 2010, 38, 655–664. [Google Scholar] [CrossRef]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Fan, J.; Hill, L.; Crooks, C.; Doerner, P.; Lamb, C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009, 150, 1750–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.S.; MacTaggart, J.M.; Elofson, R.M. Chemical constituents in wild oat (Avena fatua) hulls and their effects on seed germination. Can. J. Plant Sci. 1982, 62, 155–161. [Google Scholar] [CrossRef]
- Waisel, Y.; Adler, Y. Germination behavior of Aegilops kotschyi Boiss. Can J. Bot. 1959, 37, 741–742. [Google Scholar] [CrossRef]
- Ben-Hammouda, M.; Kremer, R.J.; Minor, H.C. Phytotoxicity of extracts from sorghum plant components on wheat seedlings. Crop Sci. 1995, 35, 1652–1656. [Google Scholar] [CrossRef]
- Kushima, M.; Kakuta, H.; Kosemura, S.; Yamamura, S.; Yamada, K.; Yokotani-Tomita, K.; Hasegawa, K. An allelopathic substance exuded from germinating watermelon seeds. Plant Growth Regul. 1998, 25, 1–4. [Google Scholar] [CrossRef]
- Ohno, S.; Tomita-Yokotani, K.; Kosemura, S.; Node, M.; Suzuki, T.; Amano, M.; Yasui, K.; Goto, T.; Yamamura, S.; Hasegawa, K. A species-selective allelopathic substance from germinating sunflower (Helianthus annuus L.) seeds. Phytochemistry 2001, 56, 577–581. [Google Scholar] [CrossRef]
- Evenari, M. Germination inhibitors. Bot. Rev. 1949, 15, 153–194. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Ku, J.J.; Cho, W.; Kang, H. Effects of rice hull cover for seed germination, types of tray and soil, shading conditions for seedling growth of Codonopsis pilosuala. J. Korean For. Soc. 2013, 102, 66–73. [Google Scholar] [CrossRef]
- Yokota, T.; Handa, H.; Yamada, Y.; Yoneyama, K.; Takeuchi, Y. Mechanism of the rice hull-induced germination of Monochoria vaginalis seeds in darkness. Weed Biol. Manag. 2014, 14, 138–144. [Google Scholar] [CrossRef]
- Swetha, B.; Singiri, J.R.; Novoplansky, N.; Grandhi, R.; Srinivasan, J.; Khadka, J.; Galis, I.; Grafi, G. Single and Combined Salinity and Heat Stresses Impact Yield and Dead Pericarp Priming Activity. Plants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [Green Version]
- Khadka, J.; Raviv, B.; Swetha, B.; Grandhi, R.; Singiri, J.R.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Huang, Z.; Grafi, G. Maternal environment alters dead pericarp biochemical properties of the desert annual plant Anastatica hierochuntica L. PLoS ONE 2020, 15, e0237045. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Frankel, O.H. Genetic conservation: Our evolutionary responsibility. Genetics 1974, 78, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peres, S. Saving the gene pool for the future: Seed banks as archives. Stud. His. Philosop. Biol. Biomed. Sci. 2016, 55, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Walters, C. Optimising seed banking procedures. In Seed Conservation: Turning Science into Practice; Smith, R.D., Dickie, J.B., Linington, S.H., Pritchard, H.W., Probert, R.J., Eds.; Royal Botanic Gardens, Kew: London, UK, 2003; pp. 723–743. [Google Scholar]
- Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manual of Seed Handling in Genebanks; Handbooks for Genebanks No. 8; Bioversity International: Rome, Italy, 2006; ISBN1 978-92-9043-740-6. ISBN2 92-9043-740-5. [Google Scholar]
- Rao, N.K.; Dulloo, M.E.; Engels, J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar]
- Justice, O.L.; Bass, L.N. Principles and Practices of Seed Storage; Agriculture Handbook No. 506; US Government Printing Office: Washington, DC, USA, 1978.
- Haferkamp, M.E.; Smith, L.; Nilan, R.A. Studies on aged seeds. I. relation of age of seed to germination and longevity. Agron. J. 1953, 45, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Goff, E.S. Comparative vitality of hulled and unhulled seeds. Wis. Agron. Expt. Sta. Ann. Rpt. 1890, 7, 202–204. [Google Scholar]
- Stevens, O.A. Germination studies on aged and injured seeds. J. Agron. Res. 1935, 51, 1093–1106. [Google Scholar]
- Ricciardi, P.; Cillari, G.; Carnevale Miino, M.; Collivignarelli, M.C. Valorization of agro-industry residues in the building and environmental sector: A review. Waste Manag. Res. 2020, 38, 487–513. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-T.; Lin, Y.-Q.; Huang, H.-J. Valorization of Rice Husk for the Production of Porous Biochar Materials. Fermentation 2021, 7, 70. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafi, G.; Singiri, J.R. Cereal Husks: Versatile Roles in Grain Quality and Seedling Performance. Agronomy 2022, 12, 172. https://doi.org/10.3390/agronomy12010172
Grafi G, Singiri JR. Cereal Husks: Versatile Roles in Grain Quality and Seedling Performance. Agronomy. 2022; 12(1):172. https://doi.org/10.3390/agronomy12010172
Chicago/Turabian StyleGrafi, Gideon, and Jeevan R. Singiri. 2022. "Cereal Husks: Versatile Roles in Grain Quality and Seedling Performance" Agronomy 12, no. 1: 172. https://doi.org/10.3390/agronomy12010172
APA StyleGrafi, G., & Singiri, J. R. (2022). Cereal Husks: Versatile Roles in Grain Quality and Seedling Performance. Agronomy, 12(1), 172. https://doi.org/10.3390/agronomy12010172






