Biofertilizer with Bacillus pumilus TUAT1 Spores Improves Growth, Productivity, and Lodging Resistance in Forage Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Biofertilizer Preparation
2.2. Seed Preparation
2.3. Seed Inoculation with Bacterial Culture
2.4. Nursery Preparation and Rice Transplantation
2.5. Plant Growth Analysis
2.6. Yield Analysis
2.7. Evaluation of Plant Lodging Resistance
- Equation (1). The formula for Bending moment
- Equation (2). The formula for Lodging index
2.8. Statistical Analysis
3. Results
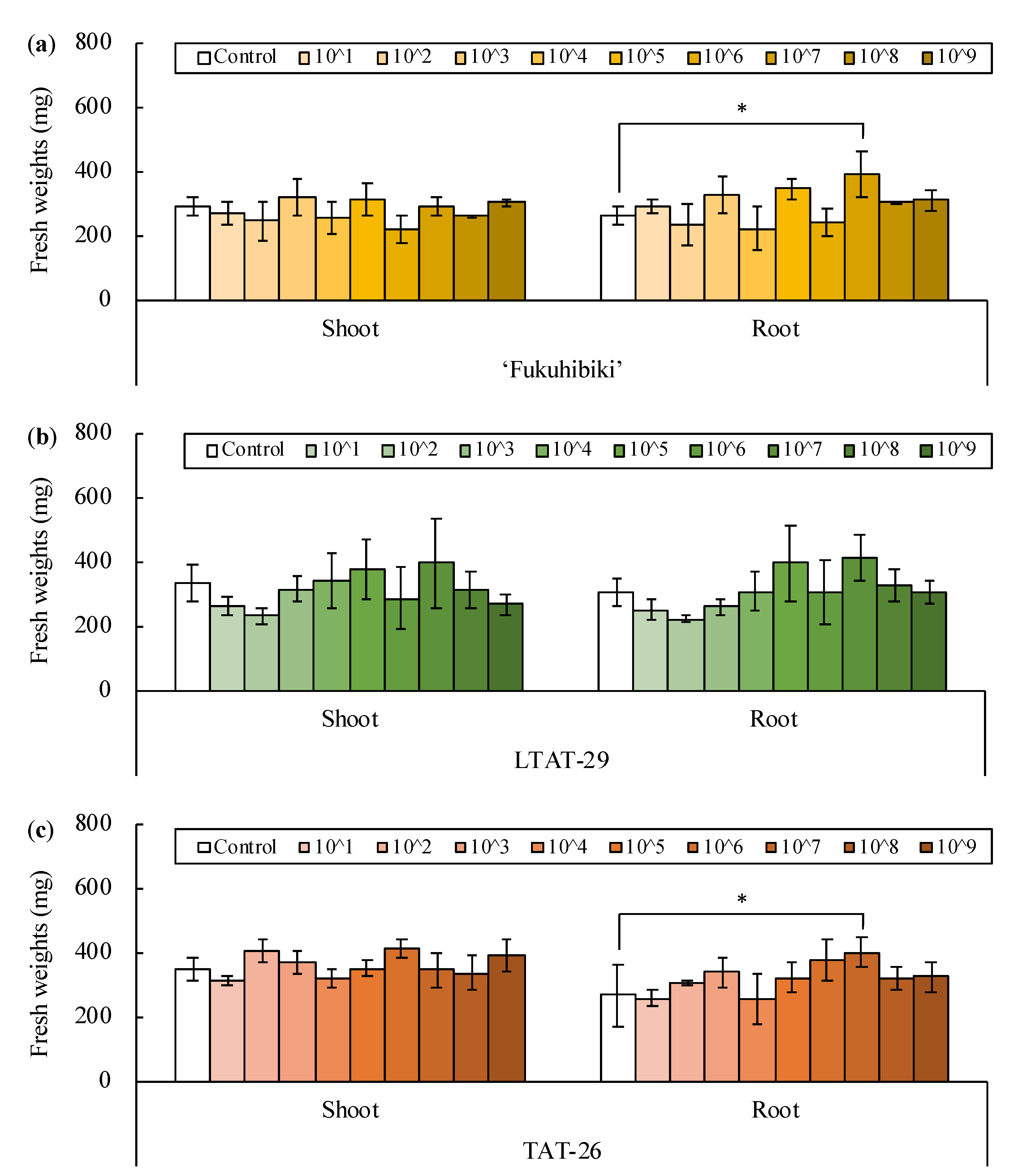
3.1. Inoculation of Bacillus pumilus TUAT1 Spores Suspension to the Forage Rice in the Plant Box
3.2. The Effects of the Biofertilizer on the Nursery Seedlings of Forage Rice
3.3. The Effects of the Biofertilizer on Tillers Number, Plant Height, and Nitrogen Concentration in the Vegetative Growth Stage of Forage Rice
3.4. The Effects of the Biofertilizer on the Feed Rice Yields
3.5. The Effects of the Biofertilizer on the Fodder Rice Yields
3.6. The Effects of the Biofertilizer on the Lodging Resistance of Forage Rice
4. Discussion
4.1. The Short-Term and Long-Term Effects of the Biofertilizer on the Forage Rice Growths and Yields
4.2. The Contribution of the Biofertilizer to the Quality of Fodder Rice as WCS and Lodging Resistance Improvements of Forage Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phung, L.D.; Ichikawa, M.; Pham, D.V.; Sasaki, A.; Watanabe, T. High yield of protein-rich forage rice achieved by soil amendment with composted sewage sludge and topdressing with treated wastewater. Sci. Rep. 2020, 10, 10155. [Google Scholar] [CrossRef] [PubMed]
- Senda, M.; Ishikawa, T.; Kusa, K. Practical Analyses of High-Yield Technology for Forage Rice. Jpn. J. Farm Manag. 2010, 48, 1–10. (In Japanese) [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]
- Böhlke, J.K. Groundwater recharge and agricultural contamination. Hydrogeol. J. 2002, 10, 153–179. [Google Scholar] [CrossRef]
- Glibert, P.M.; Maranger, R.; Sobota, D.J.; Bouwman, L. The Haber Bosch-harmful algal bloom (HB-HAB) link. Environ. Res. Lett. 2014, 9, 105001. [Google Scholar] [CrossRef]
- Showers, W.J.; Genna, B.; Mcdade, T.; Bolich, R.; Fountain, J.C. Nitrate contamination in groundwater on an urbanized dairy farm. Environ. Sci. Technol. 2008, 42, 4683–4688. [Google Scholar] [CrossRef]
- Wick, K.; Heumesser, C.; Schmid, E. Groundwater nitrate contamination: Factors and indicators. J. Environ. Manag. 2012, 111, 178–186. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Okazaki, S.; Sano, N.; Yamada, T.; Ishii, K.; Kojima, K.; Djedidi, S.; Artigas Ramírez, M.D.; Yuan, K.; Kanekatsu, M.; Ohkama-Ohtsu, N.; et al. Complete Genome Sequence of Plant Growth-Promoting Bacillus pumilus TUAT1. Microbiol. Resour. Announc. 2019, 8, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Agtuca, B.J.; Stopka, S.A.; Tuleski, T.R.; Do Amaral, F.P.; Evans, S.; Liu, Y.; Xu, D.; Monteiro, R.A.; Koppenaal, D.W.; Paša-Tolić, L.; et al. In-situ metabolomic analysis of Setaria viridis roots colonized by beneficial endophytic bacteria. Mol. Plant Microbe Interact. 2020, 33, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Cherif-Silini, H.; Silini, A.; Yahiaoui, B.; Ouzari, I.; Boudabous, A. Phylogenetic and plant-growth-promoting characteristics of Bacillus isolated from the wheat rhizosphere. Ann. Microbiol. 2016, 66, 1087–1097. [Google Scholar] [CrossRef]
- Dertz, E.A.; Xu, J.; Stintzi, A.; Raymond, K.N. Bacillibactin-mediated iron transport in Bacillus subtilis. J. Am. Chem. Soc. 2006, 128, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Dilfuza, E.; Adesemoye, A.O. Improvement of Crop Protection and Yield in Hostile Agroecological Conditions with PGPR-Based Biofertilizer Formulations. In Bioformulations: For Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 199–211. [Google Scholar] [CrossRef]
- Ferreira, C.M.H.; Soares, H.M.V.M.; Soares, E.V. Promising bacterial genera for agricultural practices: An insight on plant growth-promoting properties and microbial safety aspects. Sci. Total Environ. 2019, 682, 779–799. [Google Scholar] [CrossRef]
- García-Fraile, P.; Menéndez, E.; Rivas, R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Bioeng. 2015, 2, 183–205. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; He, P.; Ho, H.; Wu, Y.; He, Y. Whole-genome sequencing of Bacillus subtilis XF-1 reveals mechanisms for biological control and multiple beneficial properties in plants. J. Ind. Microbiol. Biotechnol. 2015, 42, 925–937. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Ohkama-Ohtsu, N.; Sarhadi, W.A.; Kojima, K.; Rallos, R.V.; Ramirez, M.D.A.; Yamaya, H.; Sekimoto, H.; Yokoyama, T. Isolation and Screening of Indigenous Plant Growth-promoting Rhizobacteria from Different Rice Cultivars in Afghanistan Soils. Microbes Environ. 2019, 34, 347–355. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Spaepen, S.; du Jardin, P.; Delaplace, P. Biostimulant effects of rhizobacteria on wheat growth and nutrient uptake depend on nitrogen application and plant development. Arch. Agron. Soil Sci. 2019, 65, 58–73. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Pare, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [Green Version]
- Umamaheswari, T.; Anbukkarasi, K.; Hemalatha, T.; Chendrayan, K. Original Research Article Studies on phytohormone producing ability of indigenous endophytic bacteria isolated from tropical legume crops. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 127–136. [Google Scholar] [CrossRef]
- Ngo, N.P.; Yamada, T.; Higuma, S.; Ueno, N.; Saito, K.; Kojima, K.; Maeda, M.; Yamaya-Ito, H.; Ohkama-Ohtsu, N.; Kanekatsu, M.; et al. Spore inoculation of Bacillus pumilus TUAT1 strain, a biofertilizer microorganism, enhances seedling growth by promoting root system development in rice. Soil Sci. Plant Nutr. 2019, 65, 598–604. [Google Scholar] [CrossRef]
- Seerat, A.Y.; Ookawa, T.; Kojima, K.; Ohkama-Ohtsu, N.; Maeda, M.; Djedidi, S.; Habibi, S.; Sekimoto, H.; Abe, A.; Yokoyama, T. Evaluation of the effects of spores and their heat-treated residues from different Bacillus strains on the initial growth of rice plants. Soil Sci. Plant Nutr. 2019, 65, 122–136. [Google Scholar] [CrossRef]
- Yamada, T.; Yokoyama, T. Tokyo University of Agriculture and Technology: Tokyo, Japan, 2022; Manuscript in preparation; to be submitted.
- Owada, M. Adaption and Extension of Rice Variety “Fukuhibiki” in Fukushima Prefecture. Tohoku J. Crop Sci. 1993, 36, 111–115. [Google Scholar] [CrossRef]
- Wang, Y.; Kuroda, E.; Hirono, M.; Murata, T. Analysis of High Yielding Mechanism of Rice Varieties Belonging to Different Plant Types: I. Comparison of growth and yield characteristics and dry matter production. Jpn. J. Crop Sci. 1997, 66, 293–299. [Google Scholar] [CrossRef]
- Abe, A. Assessment of the Quality of Forage from Its Chemical Composition and Application to Feeding Program. Japan Int. Res. Cent. Agric. Sci. 1984, 18, 133–150. [Google Scholar]
- Kato, H. Development of rice varieties for Whole Crop Silage (WCS) in Japan. Japan Agric. Res. Q. 2008, 42, 231–236. [Google Scholar] [CrossRef]
- Sakai, M.; Iida, S.; Maeda, H.; Sunohara, Y.; Nemoto, H.; Imbe, T. New rice varieties for whole crop silage use in Japan. Breed. Sci. 2003, 53, 271–275. [Google Scholar] [CrossRef]
- Guo, J.R.; Fan, H.; Wang, B.S. Lodging markedly reduced the biomass of sweet sorghum via decreasing photosynthesis in saline-alkali field. E3S Web Conf. 2018, 38, 02016. [Google Scholar] [CrossRef]
- Lang, Y.Z.; Yang, X.D.; Wang, M.E.; Zhu, Q.S. Effects of Lodging at Different Filling Stages on Rice Yield and Grain Quality. Rice Sci. 2012, 19, 315–319. [Google Scholar] [CrossRef]
- Shah, L.; Yahya, M.; Shah, S.M.A.; Nadeem, M.; Ali, A.; Ali, A.; Wang, J.; Riaz, M.W.; Rehman, S.; Wu, W.; et al. Improving lodging resistance: Using wheat and rice as classical examples. Int. J. Mol. Sci. 2019, 20, 4211. [Google Scholar] [CrossRef] [Green Version]
- Endo, S.; Yokoyama, T.; Motobayashi, T.; Katsura, K.; Ookawa, T. Characterization of biomass production, nitrogen accumulation and physiological nitrogen use efficiency in the super long-culm rice line ‘TAT-26’. In Abstracts of Meeting of the CSSJ; The Crop Science Society of Japan: Tokyo, Japan, 2018; p. 14. [Google Scholar] [CrossRef]
- Nomura, T.; Arakawa, N.; Yamamoto, T.; Ueda, T.; Adachi, S.; Yonemaru, J.; Abe, A.; Takagi, H.; Yokoyama, T.; Ookawa, T. Next generation long-culm rice with superior lodging resistance and high grain yield, Monster Rice 1. PLoS ONE 2019, 1, e0221424. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Yamamoto, T.; Ueda, T.; Yonemaru, J.; Abe, A.; Adachi, S.; Hirasawa, T.; Ookawa, T. Novel quantitative trait loci for the strong-culm and high-yield related traits in rice detected from the F2 population between the super thick-culm and super grain-bearing line ‘LTAT-29’ and the high-yielding variety ‘Takanari’. In Proceedings of the Korean Society of Crop Science Conference, ICC, Jeju, Korea, 4–7 June 2017; p. 95. [Google Scholar]
- Tahara, K.; Yamamoto, T.; Ueda, T.; Adachi, S.; Hirasawa, T.; Ookawa, T. Analysis on the quantitative trait loci for the super strong-culm traits associated with lodging resistance in rice, using F2 populations derived from a cross between the high biomass line TAT-26 and Takanari. Kanto J. Crop Sci. 2016, 31, 32–33. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, J.; Liu, Y.; Huang, N.; Tian, K.; Shah, F.; Liang, K.; Zhong, X.; Liu, B. Optimized nitrogen management enhances lodging resistance of rice and its morpho-anatomical, mechanical, and molecular mechanisms. Sci. Rep. 2019, 9, 20274. [Google Scholar] [CrossRef] [PubMed]
- Duy, P.Q.; Tanaka, D.; Abe, A.; Sagawa, S.; Kuroda, E. Analysis of the number of spikelets per panicle on the main stems, primary and secondary tillers of different rice genotypes grown under the conventional and nitrogen-free basal dressing accompanied with sparse planting density practices. Plant Prod. Sci. 2004, 7, 456–462. [Google Scholar] [CrossRef]
- Wang, D.; Chen, S.; Wang, Z.; Ji, C.; Xu, C.; Zhang, X.; Chauhan, B.S. Optimizing hill seeding density for high-yielding hybrid rice in a single rice cropping system in South China. PLoS ONE 2014, 9, e109417. [Google Scholar] [CrossRef]
- Win, K.T.; Oo, A.Z.; Ohkama-Ohtsu, N.; Yokoyama, T. Bacillus pumilus Strain TUAT-1 and Nitrogen Application in Nursery Phase Promote Growth of Rice Plants under Field Conditions. Agronomy 2018, 8, 216. [Google Scholar] [CrossRef]
- Win, K.T.; Okazaki, K.; Ookawa, T.; Yokoyama, T.; Ohwaki, Y. Influence of rice-husk biochar and Bacillus pumilus strain TUAT-1 on yield, biomass production, and nutrient uptake in two forage rice genotypes. PLoS ONE 2019, 14, e0220236. [Google Scholar] [CrossRef]
- Win, K.T.; Okazaki, K.; Ookawa, T.; Yokoyama, T.; Ohwaki, Y. Effects of biochar and TUAT-1 bio-inoculant on grain yield and nitrogen efficiency of forage rice ‘Monster rice 1’ under different N application modes. Arch. Agron. Soil Sci. 2021, 1–15. [Google Scholar] [CrossRef]
- Win, K.T.; Okazaki, K.; Ohkama-Ohtsu, N.; Yokoyama, T.; Ohwaki, Y. Short-term effects of biochar and Bacillus pumilus TUAT-1 on the growth of forage rice and its associated soil microbial community and soil properties. Biol. Fertil. Soils 2020, 56, 481–497. [Google Scholar] [CrossRef]
- Hindersah, R.; Rahmadina, I.; Harryanto, R.; Suryatmana, P.; Arifin, M. Bacillus and Azotobacter counts in solid biofertilizer with different concentration of zeolite and liquid inoculant. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 3–8. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, M.; Hou, R.; Li, L. Changes in the soil microbial community are associated with the occurrence of Panax quinquefolius L. root rot diseases. Plant Soil 2019, 438, 143–156. [Google Scholar] [CrossRef]
- Dunn, B.L.; Singh, H.; Payton, M.; Kincheloe, S. Effects of nitrogen, phosphorus, and potassium on SPAD-502 and atLEAF sensor readings of Salvia. J. Plant Nutr. 2018, 41, 1674–1683. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Mamaev, A.V.; Sherudilo, E.G. Evaluation of a SPAD-502 Plus Chlorophyll Meter to Estimate Chlorophyll Content in Leaves with Interveinal Chlorosis. Russ. J. Plant Physiol. 2020, 67, 690–696. [Google Scholar] [CrossRef]
- Süb, A.; Danner, M.; Obster, C.; Locherer, M.; Hank, T.; Richter, K. Measuring Leaf Chlorophyll Content with the Konica Minolta SPAD-502Plus—Theory, Measurement, Problems, Interpretation. In EnMAP Field Guides Technical Report; GFZ Data Service: Potsdam, Germany, 2015; pp. 1–13. [Google Scholar] [CrossRef]
- Fukushima, A.; Ohta, H.; Yokogami, N.; Tsuda, N. Dry Matter Trait and Feed Composition of Rice Varieties for WCS in the Tohoku Region of Japan. Jpn. J. Crop Sci. 2017, 86, 1–6. [Google Scholar] [CrossRef]
- Ogawa, M.; Yahara, N.; Masubuchi, T.; Oshibe, A.; Kamo, M.; Nakagawasai, H. Estimation of TDN and DCP Contents in Ammoniated Hay. J. Jpn. Soc. Grassl. Sci. 1987, 32, 408–413. [Google Scholar] [CrossRef]
- Furuhata, M.; Chosa, T.; Shioya, Y.; Tsukamoto, T.; Seki, M.; Hosokawa, H. Developing Direct Seeding Cultivation Using an Air-Assisted Strip Seeder. Japan Agric. Res. Q. 2015, 49, 227–233. [Google Scholar] [CrossRef]
- Taguchi, Y.; Maruyama, S. Effect of Planting Pattern on Growth, Yield and Lodging Resistance in Rice Direct-Sown in Flooded Paddy Field. Bull. Agr. For. Res. U. Tsukuba 2012, 25, 19–30. [Google Scholar]
- Nguyen, T.H.; Phan, T.C.; Choudhury, A.T.M.A.; Rose, M.T.; Deaker, R.J.; Kennedy, I.R. Biogro: A plant growth-promoting biofertilizer validated by 15 years’ research from laboratory selection to rice farmer’s fields of the mekong delta. In Agro-Environmental Sustainability: Managing Crop Health; Singh, J.S., Seneviratne, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1, pp. 237–254. [Google Scholar] [CrossRef]
- Omer, A.M. Bioformulations of Bacillus spores for using as biofertilizer. Life Sci. J. 2010, 7, 124–131. [Google Scholar]
- Tanimoto, E. Regulation of root growth by plant hormones—Roles for auxin and gibberellin. CRC. Crit. Rev. Plant Sci. 2005, 24, 249–265. [Google Scholar] [CrossRef]
- Agake, S.; Artigas Ramirez, M.D.; Kojima, K.; Ookawa, T.; Ohkama-Ohtsu, N.; Yokoyama, T. Seed coating by biofertilizer containing spores of Bacillus pumilus TUAT1 strain enhanced initial growth of Oryza sativa L. Agron. J. 2021, 113, 3708–3717. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Y.; Chen, Z.F.; Xing, Z.P.; Lei, Z.; Liu, Q.Y.; Zhang, Z.Z.; Jiang, Y.; Hu, Y.J.; Zhu, J.Y.; Cui, P.Y.; et al. Effects of slow or controlled release fertilizer types and fertilization modes on yield and quality of rice. J. Integr. Agric. 2018, 17, 2222–2234. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.H.; Ding, Y.F.; Tao, W.K.; Gao, S.; Li, Q.X.; Li, W.W.; Liu, Z.H.; Li, G.H. Effects of different types of slow- and controlled-release fertilizers on rice yield. J. Integr. Agric. 2021, 20, 1503–1514. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, S.; Li, G.; Liu, Z.; Ding, C.; Chen, L.; Wang, S.; Ding, Y. Application of nitrogen fertilizer at heading stage improves rice quality under elevated temperature during grain-filling stage. Crop Sci. 2017, 57, 2183–2192. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; He, X.; Li, C.; Chang, T.; Chang, S.; Zhang, H.; Zhang, Y. Scheduling of nitrogen fertilizer topdressing during panicle differentiation to improve grain yield of rice with a long growth duration. Sci. Rep. 2020, 10, 15197. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Rais, A.; Hassan, M.N.; Hafeez, F.Y. Root associated Bacillus sp. improves growth, yield and zinc translocation for basmati rice (Oryza sativa) varieties. Front. Microbiol. 2015, 6, 1286. [Google Scholar] [CrossRef] [PubMed]
- Kusa, K.; Ishikawa, T.; Moriya, N. High nitrogen application increases N concentration in the foliage of the rice (Oryza sativa L.) cultivar ‘Leaf Star’ but decreases the quality of whole-crop silage. Jpn. J. Soil Sci. Plant Nutr. 2016, 87, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Wu, X.; Ding, Y.; Li, G.; Li, J.; Weng, F.; Liu, Z.; Tang, S.; Ding, C.; et al. Lodging resistance of japonica rice (Oryza sativa L.): Morphological and anatomical traits due to top-dressing nitrogen application rates. Rice 2016, 9, 31. [Google Scholar] [CrossRef] [Green Version]




| Distance (Length by Width, cm) | Chemical N Fertilization | Biofertilizer |
|---|---|---|
| 15 × 30 | N2 | Control |
| 15 × 30 | N2 | BF |
| 15 × 30 | N4 | Control |
| 15 × 30 | N4 | BF |
| 30 × 30 | N2 | Control |
| 30 × 30 | N2 | BF |
| 30 × 30 | N4 | Control |
| 30 × 30 | N4 | BF |
| DAT (DAS) | Trans- Planting | 1st Survey (8 Weeks) | 2nd Survey (13 Weeks) | Heading Stage | Lodging Measurement | Harvest |
|---|---|---|---|---|---|---|
| ‘Fukuhibiki’ | 0 (21) | 60 (81) | 92 (113) | 86 (107) | 119 (140) | 137 (158) |
| LTAT-29 | 0 (14) | 61 (75) | 91 (105) | 103 (117) | 137 (151) | 147 (161) |
| TAT-26 | 0 (14) | 61 (75) | 92 (106) | 110 (124) | 147 (161) | 137 (151) |
| Variety | Distance | Fertilization | Biofertilizer | NSP | PRR | WBR | TPN | GBRY |
|---|---|---|---|---|---|---|---|---|
| Panicle−1 | % | g | m−2 | kg | ||||
| ‘Fukuhibiki’ | 15 × 30 | N2 | Control | 109 | 87 | 22.8 | 235 | 483 |
| ‘Fukuhibiki’ | 15 × 30 | N2 | BF | 115 | 90 | 23.1 | 282 ** | 650 ** |
| (BF/Control) | (1.06) | (1.03) | (1.01) | (1.20) | (1.34) | |||
| ‘Fukuhibiki’ | 15 × 30 | N4 | Control | 114 | 89 | 23.2 | 247 | 581 |
| ‘Fukuhibiki’ | 15 × 30 | N4 | BF | 102 | 91 | 23.2 | 313 ** | 651 |
| (BF/Control) | (0.89) | (1.03) | (1.00) | (1.27) | (1.12) | |||
| ‘Fukuhibiki’ | 30 × 30 | N2 | Control | 129 | 85 | 23.0 | 179 | 429 |
| ‘Fukuhibiki’ | 30 × 30 | N2 | BF | 108 * | 79 | 23.5 | 210 ** | 471 |
| (BF/Control) | (0.84) | (0.94) | (1.02) | (1.17) | (1.10) | |||
| ‘Fukuhibiki’ | 30 × 30 | N4 | Control | 131 | 79 | 22.7 | 200 | 417 |
| ‘Fukuhibiki’ | 30 × 30 | N4 | BF | 117 | 89 | 24.0 * | 212 * | 485 |
| (BF/Control) | (0.89) | (1.13) | (1.06) | (1.06) | (1.16) | |||
| LTAT-29 | 15 × 30 | N2 | Control | 215 | 71 | 18.8 | 158 | 438 |
| LTAT-29 | 15 × 30 | N2 | BF | 212 | 72 | 19.2 | 181 ** | 507 * |
| (BF/Control) | (0.99) | (1.01) | (1.02) | (1.15) | (1.16) | |||
| LTAT-29 | 15 × 30 | N4 | Control | 220 | 64 | 18.6 | 180 | 454 |
| LTAT-29 | 15 × 30 | N4 | BF | 222 | 63 | 18.8 | 189 | 453 |
| (BF/Control) | (1.01) | (0.98) | (1.01) | (1.05) | (1.00) | |||
| LTAT-29 | 30 × 30 | N2 | Control | 248 | 67 | 17.9 | 128 | 369 |
| LTAT-29 | 30 × 30 | N2 | BF | 268 | 67 | 18.1 | 146 * | 390 |
| (BF/Control) | (1.08) | (0.99) | (1.01) | (1.14) | (1.06) | |||
| LTAT-29 | 30 × 30 | N4 | Control | 272 | 63 | 17.9 * | 146 | 360 ** |
| LTAT-29 | 30 × 30 | N4 | BF | 274 | 59 | 16.0 | 149 | 279 |
| (BF/Control) | (1.01) | (0.93) | (0.89) | (1.02) | (0.70) | |||
| ANOVA (p value) | ||||||||
| Biofertilizer | n.s. | n.s. | n.s. | <0.000 | <0.000 | |||
| Variety | <0.000 | <0.000 | < 0.000 | <0.000 | <0.000 | |||
| Distance | <0.000 | 0.001 | 0.01 | <0.000 | <0.000 | |||
| Fertilization | n.s. | n.s. | n.s. | <0.000 | n.s. | |||
| Biofertilizer × Variety | n.s. | n.s. | n.s. | <0.000 | <0.000 | |||
| Biofertilizer × Distance | n.s. | n.s. | n.s. | 0.002 | 0.005 | |||
| Biofertilizer × Fertilization | n.s. | n.s. | n.s. | n.s. | 0.007 | |||
| Distance | Fertilization | Biofertilizer | TPN | Straw DW | Panicle DW | Total DW | Panicle/Total | N Conc. | N Uptake | TDN | YTDN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| m−2 | g m−2 | g m−2 | g m−2 | mg g−1 | g m−2 | mg g−1 | g m−2 | ||||
| 15 × 30 | N2 | Control | 113 | 613 | 500 | 1112 | 0.45 | 7.48 | 8.27 | 630 | 700.4 |
| 15 × 30 | N2 | BF | 120 | 653 | 503 | 1156 | 0.43 | 7.36 | 8.52 | 631 | 729.1 |
| (BF/Control) | (1.06) | (1.07) | (1.01) | (1.04) | (0.97) | (0.98) | (1.03) | (1.00) | (1.04) | ||
| 15 × 30 | N4 | Control | 128 | 653 | 458 | 1111 | 0.41 | 7.68 | 8.55 | 628 | 697.6 |
| 15 × 30 | N4 | BF | 123 | 714 | 446 | 1160 | 0.38 | 8.15 | 9.42 | 628 | 729.2 |
| (BF/Control) | (0.96) | (1.09) | (0.97) | (1.04) | (0.93) | (1.06) | (1.10) | (1.00) | (1.05) | ||
| 30 × 30 | N2 | Control | 96 | 610 | 394 | 1003 | 0.39 | 7.36 | 7.37 | 628 | 630.3 |
| 30 × 30 | N2 | BF | 97 | 641 | 394 | 1035 | 0.38 | 7.27 | 7.54 | 628 | 649.6 |
| (BF/Control) | (1.01) | (1.05) | (1.00) | (1.03) | (0.97) | (0.99) | (1.02) | (1.00) | (1.03) | ||
| 30 × 30 | N4 | Control | 97 | 664 | 322 | 985 | 0.33 | 8.05 | 7.92 | 628 | 618.7 |
| 30 × 30 | N4 | BF | 103 | 742 ** | 298 | 1040 | 0.29 | 8.37 | 8.69 * | 627 | 652.8 |
| (BF/Control) | (1.06) | (1.12) | (0.93) | (1.06) | (0.87) | (1.04) | (1.10) | (1.00) | (1.06) | ||
| ANOVA (p value) | |||||||||||
| Biofertilizer | n.s. | 0.037 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Distance | <0.000 | n.s. | <0.000 | 0.007 | <0.000 | 0.006 | 0.045 | n.s. | 0.007 | ||
| Fertilization | n.s. | 0.015 | 0.014 | n.s. | <0.000 | n.s. | 0.026 | n.s. | n.s. | ||
| Biofertilizer × Distance | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Biofertilizer × Fertilization | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agake, S.-i.; Ohwaki, Y.; Kojima, K.; Yoshikawa, E.; Artigas Ramirez, M.D.; Bellingrath-Kimura, S.D.; Yamada, T.; Ookawa, T.; Ohkama-Ohtsu, N.; Yokoyama, T. Biofertilizer with Bacillus pumilus TUAT1 Spores Improves Growth, Productivity, and Lodging Resistance in Forage Rice. Agronomy 2022, 12, 2325. https://doi.org/10.3390/agronomy12102325
Agake S-i, Ohwaki Y, Kojima K, Yoshikawa E, Artigas Ramirez MD, Bellingrath-Kimura SD, Yamada T, Ookawa T, Ohkama-Ohtsu N, Yokoyama T. Biofertilizer with Bacillus pumilus TUAT1 Spores Improves Growth, Productivity, and Lodging Resistance in Forage Rice. Agronomy. 2022; 12(10):2325. https://doi.org/10.3390/agronomy12102325
Chicago/Turabian StyleAgake, Shin-ichiro, Yoshinari Ohwaki, Katsuhiro Kojima, Emon Yoshikawa, Maria Daniela Artigas Ramirez, Sonoko Dorothea Bellingrath-Kimura, Tetsuya Yamada, Taiichiro Ookawa, Naoko Ohkama-Ohtsu, and Tadashi Yokoyama. 2022. "Biofertilizer with Bacillus pumilus TUAT1 Spores Improves Growth, Productivity, and Lodging Resistance in Forage Rice" Agronomy 12, no. 10: 2325. https://doi.org/10.3390/agronomy12102325
APA StyleAgake, S. -i., Ohwaki, Y., Kojima, K., Yoshikawa, E., Artigas Ramirez, M. D., Bellingrath-Kimura, S. D., Yamada, T., Ookawa, T., Ohkama-Ohtsu, N., & Yokoyama, T. (2022). Biofertilizer with Bacillus pumilus TUAT1 Spores Improves Growth, Productivity, and Lodging Resistance in Forage Rice. Agronomy, 12(10), 2325. https://doi.org/10.3390/agronomy12102325









