Abstract
The use of biotechnology for the genetic improvement of wheat (Triticum aestivum L.) has been hampered by its recalcitrance to standard transformation and regeneration protocols. Male gametes present a potentially useful option for introducing gene edits via clustered regularly interspaced short palindromic repeats (CRISPR). However, the utility of male gametes for introducing genetic improvements would be dependent on the retention of viability after treatment to introduce the CRISPR components. We have studied wheat pollen morphology and its viability in a range of germination media to identify conditions that optimize the viability of in vitro hydrated pollen. The size, shape, and aperture from seven different wheat genotypes were compared using scanning electron microscope (SEM). The SEM results revealed that the pollen of all of the wheat genotypes examined in this study were monoporate; however, a significant variation in the size of the mature pollen grains was observed. The hydrated pollen of the wheat genotypes remained viable for up to five hours at 20 ± 2 °C. Of all of the germination media tested, the medium containing 5% sucrose, 10% PEG4000, 100 mg/L boric acid, 200 mg/L calcium nitrate, 100 mg/L potassium nitrate, and 100 mg/L magnesium sulphate at pH 6.5 achieved the highest percentage of pollen germination after 5 h of hydration. Impedance Flow Cytometry (IFC) provided similar results to the in vitro germination study. This work elucidates important factors that can form the basis for a pollen-based non-genetically modified system for gene editing in wheat.
1. Introduction
Pollen has given plants the ability to transfer male gametes to create new genetic variations through cross-fertilization. The fate of fertilization, embryogenesis, and seed development depends on the ability of pollen to maintain its viability for delivering the enclosed gametes to an awaiting ovule. Pollen’s easy accessibility and its clear role in delivering the genetic material to the ovule has seen it being used as a carrier of foreign DNA since the 19th century [1]. The pollen-mediated genetic transformation can be achieved with either immature, developing pollen, known as microspores [2], or with mature pollen grains [3]. The advantage of using mature pollen grains is that the transfected pollen grains have the potential to be used directly to fertilize ovules to produce genetically modified progeny. In contrast, the genetic transformation of microspores requires tissue culture regeneration techniques to produce doubled haploid transgenic plants [4].
The disadvantages of the tissue culture-based techniques [3] and the innate advantages of mature pollen have led to the development of several interesting mature pollen-based methods. Maize genetic stocks were genetically modified via the irradiation of pollen [5], and the complexing of DNA and pollen prior to the use of the treated pollen for fertilization [6,7]. In the DNA–pollen complexing method, the DNA from a donor parent is mixed with freshly collected pollen from a recipient parent in a solution of 0.3 M sucrose [6,7]. After a brief incubation, the complex can be applied to the silk of the recipient parent for fertilization. This simple technique can lead to a transformation efficiency of up to 9.29% [6,7]. Several attempts were made to use mature pollen-mediated genetic transformations in wheat, including a direct transformation with Agrobacterium [8] and using pollen as a DNA delivery system [9,10]. Unfortunately, there has been no satisfactory evidence for transformation using mature pollen grains in any study [3,4]. Due to this failure, the focus shifted to methods based on other tissues. Although the tissues including microspores, leaf explants, and embryos have the drawback of requiring the tissue culture regeneration of transformed cells, they have been used successfully for the genetic modification of several species of crop plants [11].
Despite enormous efforts by the scientific community, the use of advanced genetic engineering techniques is still challenging in wheat compared to other species. This is due to the complex genomic constitution of wheat and its highly genotype-specific response to anther/tissue culture techniques. Agrobacterium tumifaciens has been shown to have only 5% efficiency in wheat [12]. Most of the studies in wheat were conducted using the genotypes “Fielder” and “Bobwhite”, the two cultivars widely recognized as being amenable to Agrobacterium transformation [11,13,14]. The consumer resistance to genetically modified wheat has, however, hampered their widespread use for economic and nutritional trait improvement. The development of clustered regularly interspaced short palindromic repeats (CRISPR)-based gene editing, and its potential for modifying the genomes in a manner that can avoid the classification of the modified plants as genetically modified organisms (GMOs), makes it a method of choice [15]. One possibility for the efficient incorporation of gene edits in plants is to develop technologies based on the use of mature pollen. A significant difficulty with this approach in wheat is that the pollen readily loses viability. The maintenance of pollen viability in germination medium is fundamental for the development of mature pollen-based genetic transformation techniques. Cheng and Mcomb [16,17] reported an in vitro pollen germination medium for wheat, this medium was unsuitable for a broad range of the genotypes used in this study, presumably due to genotypic specificity. This study reports the results on different aspects of the pollen biology of wheat with the aim of developing a robust protocol for its use in the delivery of genetic material for gene-editing in this important crop.
2. Materials and Methods
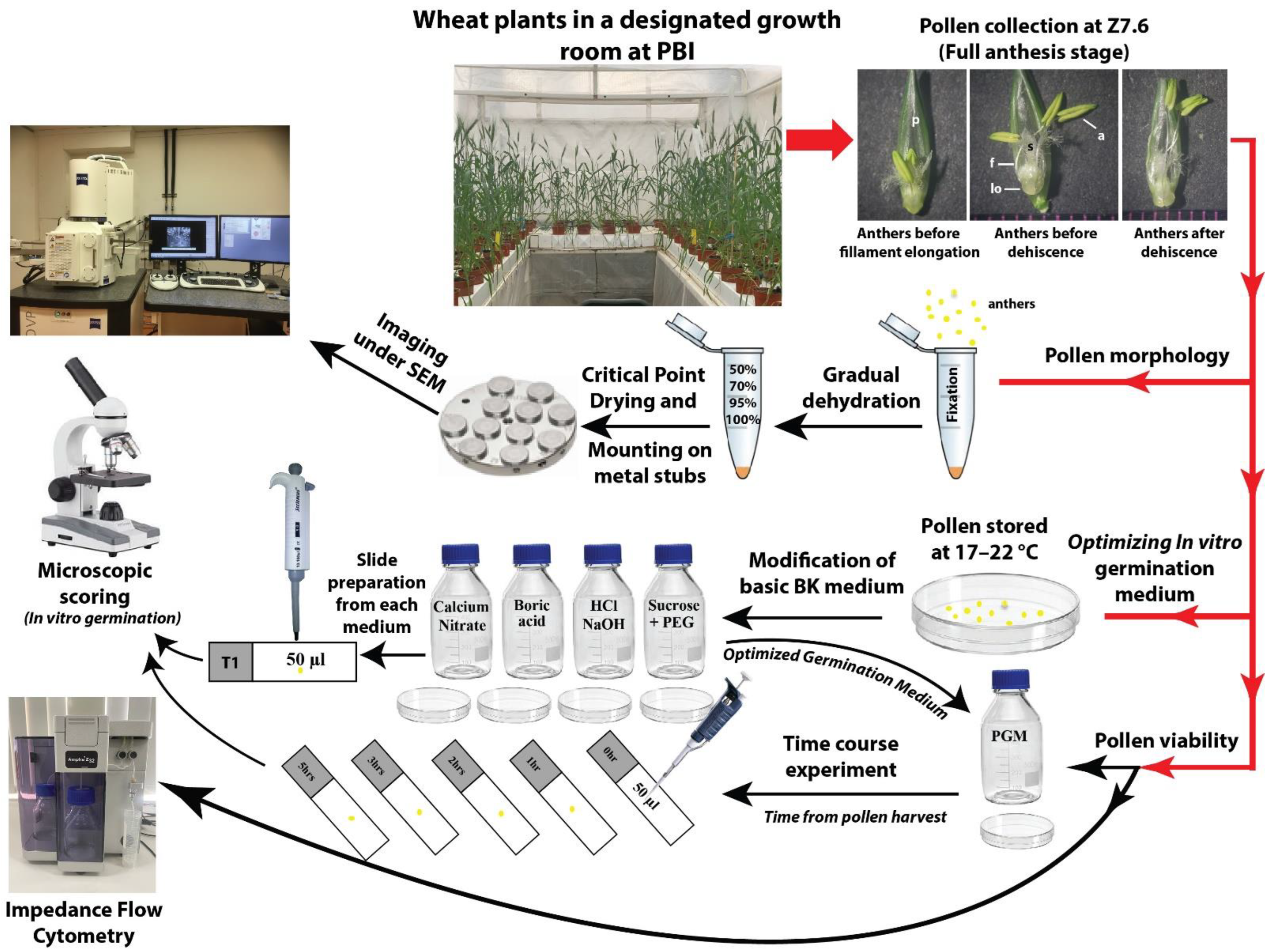
Figure 1 shows a schematic representation of the methodology used in this study. The mature pollen grains were collected at the anther dehiscence stage from plants grown in a microclimate room under controlled temperature and humidity conditions. These pollen grains were used for the pollen morphological study, using scanning electron microscopy (SEM) analysis, and for optimizing the organic and inorganic composition of an in vitro pollen germination medium. The optimized medium was used to study the longevity of pollen grains at room temperature, as well as to test the efficacy of Impedance Flow Cytometry (IFC) as a quick viability test.
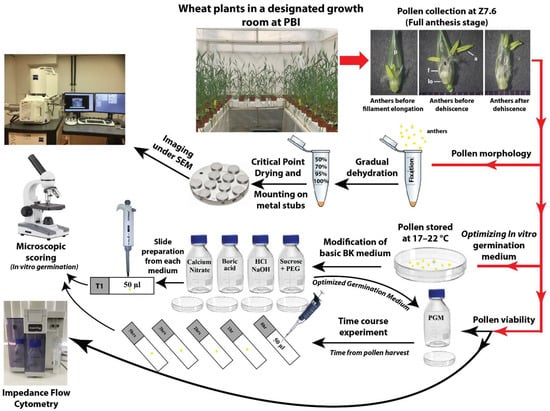
Figure 1.
Schematic presentation of overall methodology used in this study. Abbreviations: a, anther; f, filament; lo, lodicule; p, palea; PGM, pollen germination medium; s, stigma.
2.1. Plant Material
The 12 wheat genotypes used in the study were: Thatcher+Lr22a; CO1568+Sr33; Lorikeet (Sr33); Kulin+Sr45; Thornbill (Sr45); Gladius+Sr50; P1 638536 (Yr36); P1 638537 (Yr36); Lilian (Yr36); Chinese Spring; Fielder; and Mace. This set covered stocks carrying the cloned rust resistance genes Lr22a, Sr33, Sr45, Sr50, and Yr36. Chinese Spring was chosen because of the availability of a well-curated whole genome sequence for this genotype, Fielder was included for its transformation amenability, and Mace was included because it has been a leading Australian cultivar. These genotypes were grown in a specialized microclimate room at the University of Sydney Plant Breeding Institute, Cobbitty. Small pots (9-cm diameter) were filled with a potting mixture comprising of composted pine bark and sand in 2:1 ratio, mixed with a fungicide (Sapphire) treated fertilizer (10 gm per pot). Two seeds of each wheat genotype were sown in a pot to obtain 10–12 tillers. The pots were then transferred to the microclimate room that was set at 18–20 °C and 72–73% humidity, with a 16/8 h of day/night duration (natural light and light from Osram Metal halide lamps, 250 Watt). The fertilizer was applied weekly (0.0025 w/v Urea). The plants were grown to the Z6.5–Z7.6 stage [18] and the anthers were collected at this stage and used in all of the experiments.
2.2. Pollen Collection
The awns, glumes, and lemmas were carefully removed from stage Z7.6 wheat flowers to allow for filament elongation. The ideal stage for pollen collection from the wheat flowers is once the filament protrudes, the lodicules swell, and the anthers enlarge, turning from a greenish to a yellowish color. The intact anthers for the scanning electron microscope (SEM)-based morphological analysis and pollen for the media incubation experiments were harvested at this stage of the flower development. Figure S1 (Supplementary Materials) shows the developmental changes in the reproductive organs within the wheat floret from pre-anthesis to post-anthesis stages. Supplementary Figure S1M,N represents the ideal stage for pollen collection.
2.3. Pollen Morphology under Scanning Electron Microscope (SEM)
The morphology of the pollen from the seven wheat genotypes: Kulin+Sr45; Gladius+Sr50; Lilian (Yr36); CO1568+Sr33; Thatcher+Lr22a; Chinese Spring; and Fielder was examined with SEM (Zeiss Sigma VP HD, Carl Zeiss Microscopy, WP, United States) at the Australian Centre for Microscopy and Microanalysis (ACMM), the University of Sydney, Australia. Following the biological specimen preparation methods described by Ruzin [19], the whole wheat anthers were fixed just before dehiscence in formalin acetic acid (FAA; five parts formalin: five parts glacial acetic acid: 90 parts 50% ethanol (v/v/v)) for four hours. This was followed by gradual dehydration with a graded ethanol (EtOH) series: 50% EtOH (2 × 5 min), 70% EtOH (2 × 5 min), 95% EtOH (3 × 5 min) and 100% EtOH (4 × 10 min). The dehydrated anthers were then further dried, using critical point drying in carbon dioxide with a Leica EM CPD300 critical point dryer (Leica Microsystems Pty Ltd., Wetzlar, Germany). The dried pollen grains were removed from the anthers and mounted on carbon tape affixed to aluminum stubs. The mounted specimens were coated with 20 nm of gold palladium in a sputter coater (Emitech K550 Gold Sputter Coater, 20 mA for 3 min) and then examined with a Zeiss Sigma VP HD SEM, operating at an accelerating voltage of 5 KV and photographed with the integral digital camera. The polar axis (P), equatorial axis (E), and the P/E ratio were measured on at least 30 fully developed pollen grains. The morphology results are expressed as mean ± standard error.
2.4. Development of a Standardized In Vitro Pollen Germination Medium
An initial objective of the work was to develop an optimized germination medium that enables the highest percentage of the viable wheat pollen grains to germinate in vitro with negligible pollen bursting. We used the pollen of wheat genotype Gladius+Sr50 for the standardization of the medium. This was achieved by modifying the composition of the basic Brewbaker and Kwack (BK) germination medium, which includes 10% sucrose, 100 mg/L boric acid (H3BO3), 300 mg/L calcium nitrate (CaNO3)2.4H2O), 200 mg/L magnesium sulphate (MgSO4.7H2O), and 100 mg/L potassium nitrate (KNO3) in deionized water [20]. An additional component, polyethylene glycol (PEG), was used as an osmo-regulator. For the formulation of an optimized pollen germination medium, stepwise modifications were made to the basic BK germination medium by adding different amounts of the components: sucrose; PEG4000; boric acid; and calcium nitrate.
In the first phase of the work, the osmoticum of the germination medium was optimized by modifying the concentrations of sucrose and PEG4000. The percent pollen germination was tested in 12 different concentration combinations. After optimizing the sucrose and PEG4000 concentrations, the pH of the best osmo-regulated media composition was adjusted with 1M NaOH or 1mM HCL at pH levels 4.5, 5.5, 6.5, and 7.5 and tested for enhanced germination response. Further modification of the BK medium was tested by the addition of various concentrations of mineral salts, which could greatly or marginally augment the germination response. The concentrations of boric acid (100, 200, 300, and 400 mg/L) and calcium nitrate (50, 100, 200, and 300 mg/L) at the optimized osmoticum and pH levels were tested for enhancing the germination percentage. All of the chemicals used in this experiment were of Analytical Reagent (AR) grade and the pH of each medium was adjusted after adding all of the components and having the final volume adjusted with deionized water. The germination media were pre-sterilized by autoclaving for 15 min at 121 °C.
2.5. Assaying for Percentage of Pollen Germination
Fifty µL of each germination medium were added onto a glass microscope slide. The pollen grains were pooled from three freshly dehisced and randomly selected anthers and uniformly spread onto the medium with the help of fine forceps. A coverslip was placed over the pollen grains and the samples were then kept on moist tissue paper in a covered Petri dish for 30 min at 37 °C to germinate. Three separate slides were prepared per treatment.
The pollen grain germination count was performed under a Leica DMIL light microscope (Leica Microsystems Pty Ltd., Wetzlar, Germany) at a magnification of ×100 and photographs were taken, using a mounted Nikon Coolpix camera. Six fields were selected randomly across the samples and the pollen grains were counted as germinated when the length of a pollen tube was equal to or greater than the diameter of the pollen grain from which it emerged. The germination percentages from each microscopic field were calculated, using the following formula:
2.6. Assessment of Pollen Viability
The standardized germination medium from the above experiments with the highest germination percentage and the lowest percentage of pollen bursting was used to test the pollen viability of the wheat genotypes used in this study. A time–course experiment was planned with 0, 1, 2, 3, and 5 h after storage to study the pollen viability. The anthers were collected before dehiscence randomly from a spike and were stored at temperature (20 ± 2 °C) in glass petri dishes. The microscopic slides for each time–course experiment were prepared by using these stored pollen grains. The pollen viability (%) was estimated based on the number of germinated pollen grains at each timepoint.
The pollen grain viability was also tested with the Impedance Flow Cytometer (IFC). The mature pollen grains were collected at the anthesis stage [21] and tested using an Ampha Z32 cytometer (Amphasys, Technopark Luzern, Switzerland). The biological relevance of the IFC data was confirmed by comparing it to the results of the in vitro pollen germination assays. In vitro pollen germination was determined by using pollen grains from the same samples collected for the IFC analysis. The IFC—in vitro germination analyses were performed on five replicates, with the correlations determined between the Ampha Z32 and the germination assays.
2.7. Statistical Analysis
The data from all of the experiments were statistically analyzed by using the package R (version 3.6.1) for the analysis of variance (ANOVA) and for the post-hoc analysis for significant pairwise comparison [22]. The correlation co-efficient between the IFC assay and the in vitro germination value was determined, and the linear regression analysis conducted, using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Pollen Morphology under Scanning Electron Microscope (SEM)
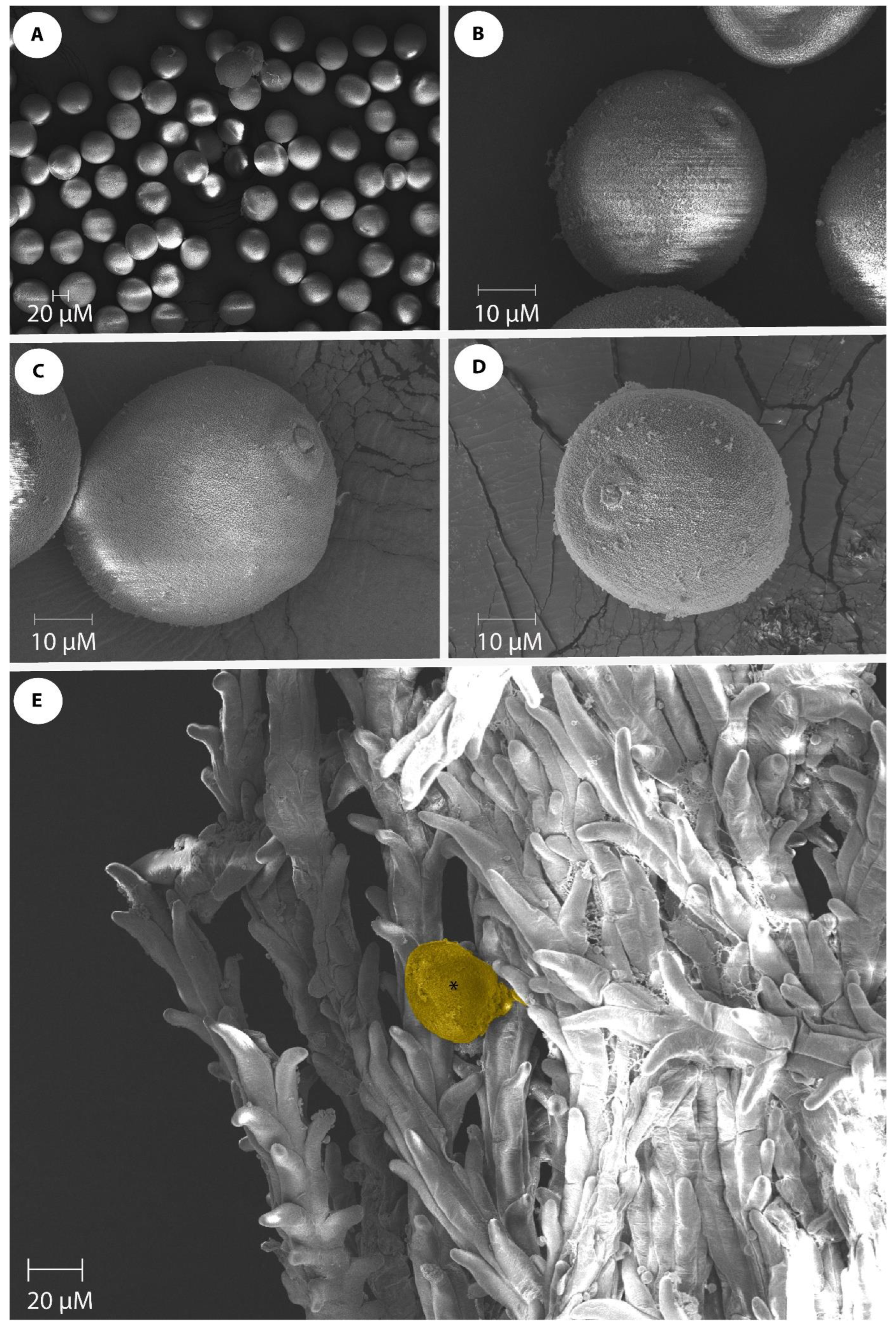
The pollen morphology of seven genotypes (Kulin+Sr45, Gladius+Sr50, Lilian, CO1568+Sr33, Thatcher+Lr22a, Chinese Spring, and Fielder) was examined via SEM. Three palynological traits were observed in the dehydrated pollen grains (Table 1; Figure 2A). The pollen size was determined by measuring the polar axis length and equatorial axis length. A highly significant (p = 0.00) variation was observed for both the pollen polar axis length and the pollen equatorial axis length among the genotypes used in this study (Table 1). The mean values for the polar axis length ranged from 40.1 µm (Fielder) to 47.8 µm (Thatcher+Lr22a). The pollen equatorial axis length ranged from 37.8 µm (Fielder) to 46.07 µm (Kulin+Sr45) (Table 1). The pollen from all of the genotypes had a single aperture and the genotypes used in this study can all be characterized as “monoporate”. Figure 2B–D show the different views of the pollen of Gladius+Sr50, Thatcher, and Lilian, respectively. The aperture size varied among the genotypes, with the smallest aperture (3.0 µm) being observed in Lilian, and the largest aperture (5.6 µm) in the CO1568+Sr33 genotype (Table 1). The comparison of the mean polar axis lengths and the pollen equatorial axis lengths revealed that the wheat genotype Fielder was statistically different from the other wheat genotypes in this parameter. Fielder also differed from all of the genotypes, except Chinese Spring, in terms of the pollen equatorial axis length (Table 1).

Table 1.
Pollen morphology of seven wheat genotypes based on Scanning Electron Microscopy (SEM).
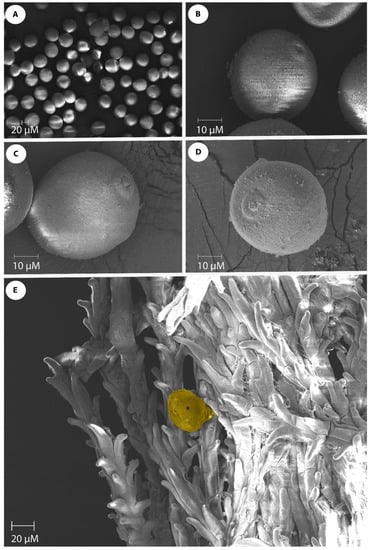
Figure 2.
Wheat pollen grains of wheat genotypes. (A) Monoporate wheat pollen grains; (B) Equatorial view of Gladius+Sr50 pollen showing 5 µm surface aperture; (C) Equatorial view of Thatcher pollen showing 4 µm aperture size; (D) Slightly oblique polar view of Lilian pollen with 3 µm aperture size; (E) Pollen germination (indicated by an asterisk) on the feathery branched stigmatic surface. Scale bars are shown on each SEM image.
3.2. Optimization of In Vitro Pollen Germination Media
The in vitro pollen germination for the wheat was standardized by a stepwise modification of the BK germination medium to enable the highest germination and the lowest pollen-bursting percentages.
3.2.1. Optimization of Sucrose and Polyethylene Glycol (PEG)
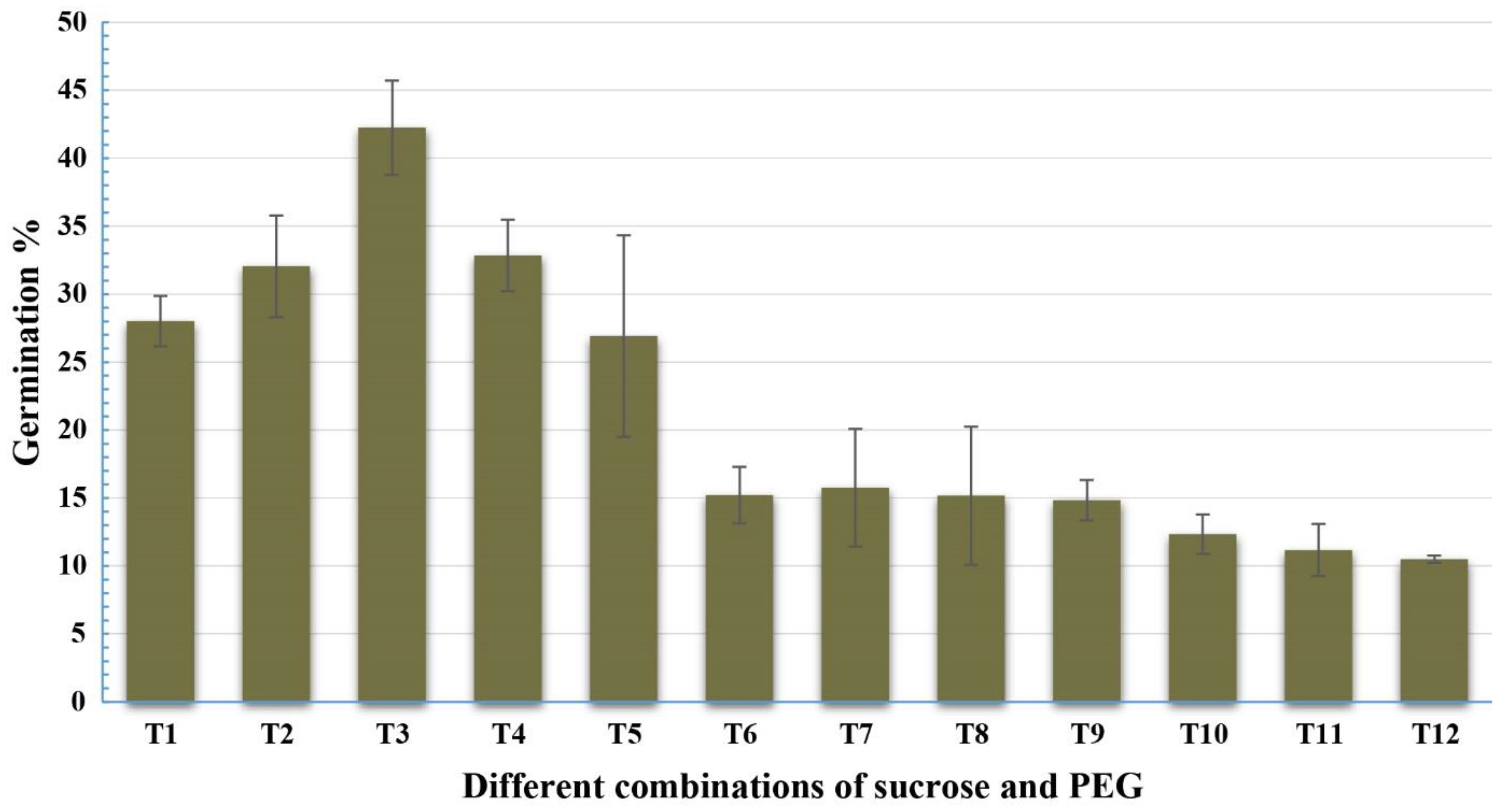
Twelve concentrations of sucrose and PEG4000 were tested with the pollen grains of Gladius+Sr50 (Table 2), and the ANOVA indicated highly significant differences among the various treatments (Table 3). The treatment: 5% sucrose and 10% PEG in combination with boric acid 100 mg/L; calcium nitrate 300 mg/L; potassium nitrate 100 mg/L; and magnesium sulphate 200 mg/L at pH 6.0 showed the highest pollen germination percentage. Increasing the concentration of sucrose in the media resulted in a significant reduction in the pollen germination (Figure 3).

Table 2.
The combinations of sucrose and PEG used to modify the composition of the Brewbaker and Kwack (BK) basal medium.

Table 3.
ANOVA of pollen viability of the wheat genotype Gladius+Sr50 in different treatments of PEG, pH, boric acid and calcium nitrate.
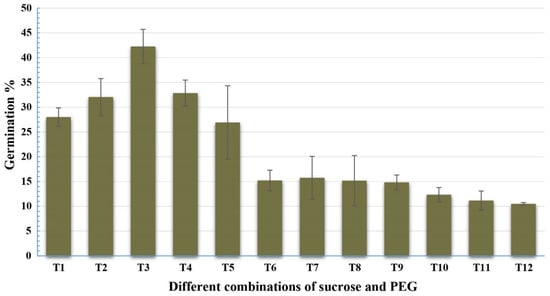
Figure 3.
Effect of sucrose and PEG on pollen germination (%) of Gladius+Sr50. The value for each treatment is the mean percentage counts from three microscopic fields of three slides. Least significant difference (LSD; p = 0.05) for treatments was 4.97%. Error bars represent SE of the means.
3.2.2. Optimization of pH, Boric Acid, and Calcium Nitrate
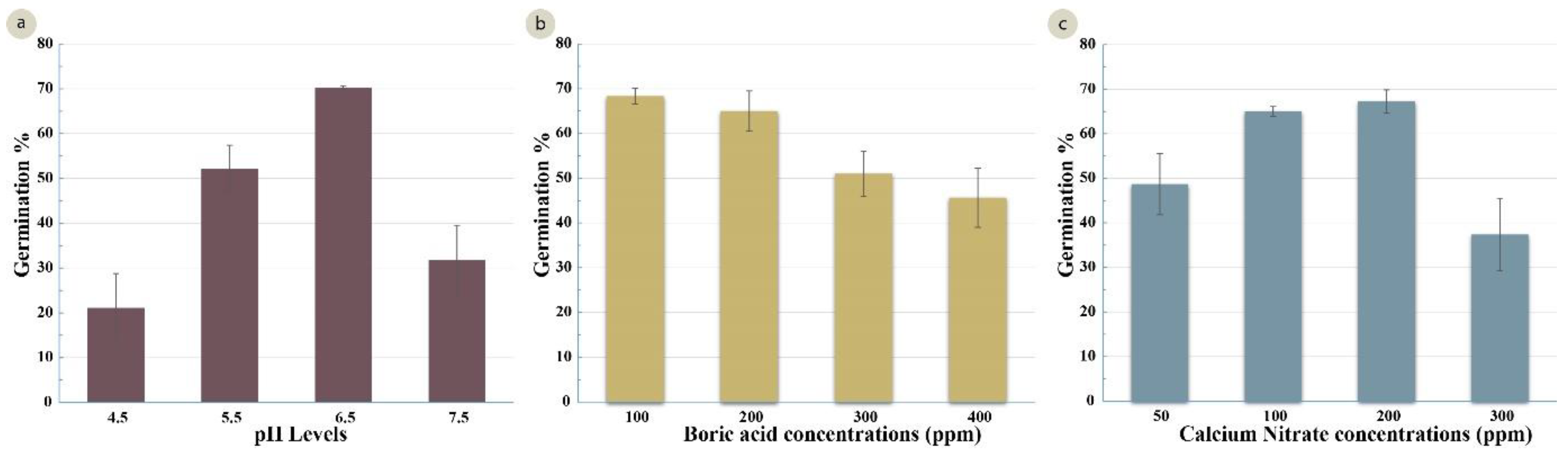
Following identification of the best osmoticum concentration (sucrose and PEG4000), the pH of the medium was optimized. Four treatments at pH levels 4.5, 5.5, 6.5 and 7.5 were examined. Highly significant differences were observed between the treatments (Table 3). The highest germination percentage (65%) was recorded in a media with a pH 6.5 (Figure 4a). The comparative assays were also conducted to identify the optimum levels of boric acid and calcium nitrate. A significant variation (Table 3) was observed in the four boric acid treatments (B1 = 100 mg/L, B2 =200 mg/L, B3 =300 mg/L, and B4 = 400 mg/L). Treatment B1 had the highest germination percentage (68%). Increasing the concentration of boric acid resulted in lower germination (46% with 400 mg/L) (Figure 4b). The germination percentage also differed with the calcium nitrate concentration (C1 = 50 mg/L, C2 = 100 mg/L, C3 = 200 mg/L, and C4 = 300 mg/L) (Table 3). The highest germination percentages (65% and 67%) were achieved with 100 mg/L and 200 mg/L of calcium nitrate, and a significant decline in germination (37%) was observed at the higher calcium nitrate concentration (300 mg/L) (Figure 4c).
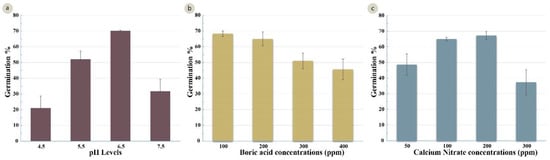
Figure 4.
Effect of several factors on germination (%) of the wheat cultivar Gladius+Sr50. (a) different levels of pH (LSD 0.05 = 8.49%); (b) different concentrations of boric acid in mg/L (LSD 0.05 = 6.81%); (c) different concentrations of calcium nitrate in mg/L (LSD 0.05 = 7.76%). Error bars represent SE of the means.
3.3. Assessment of Pollen Viability
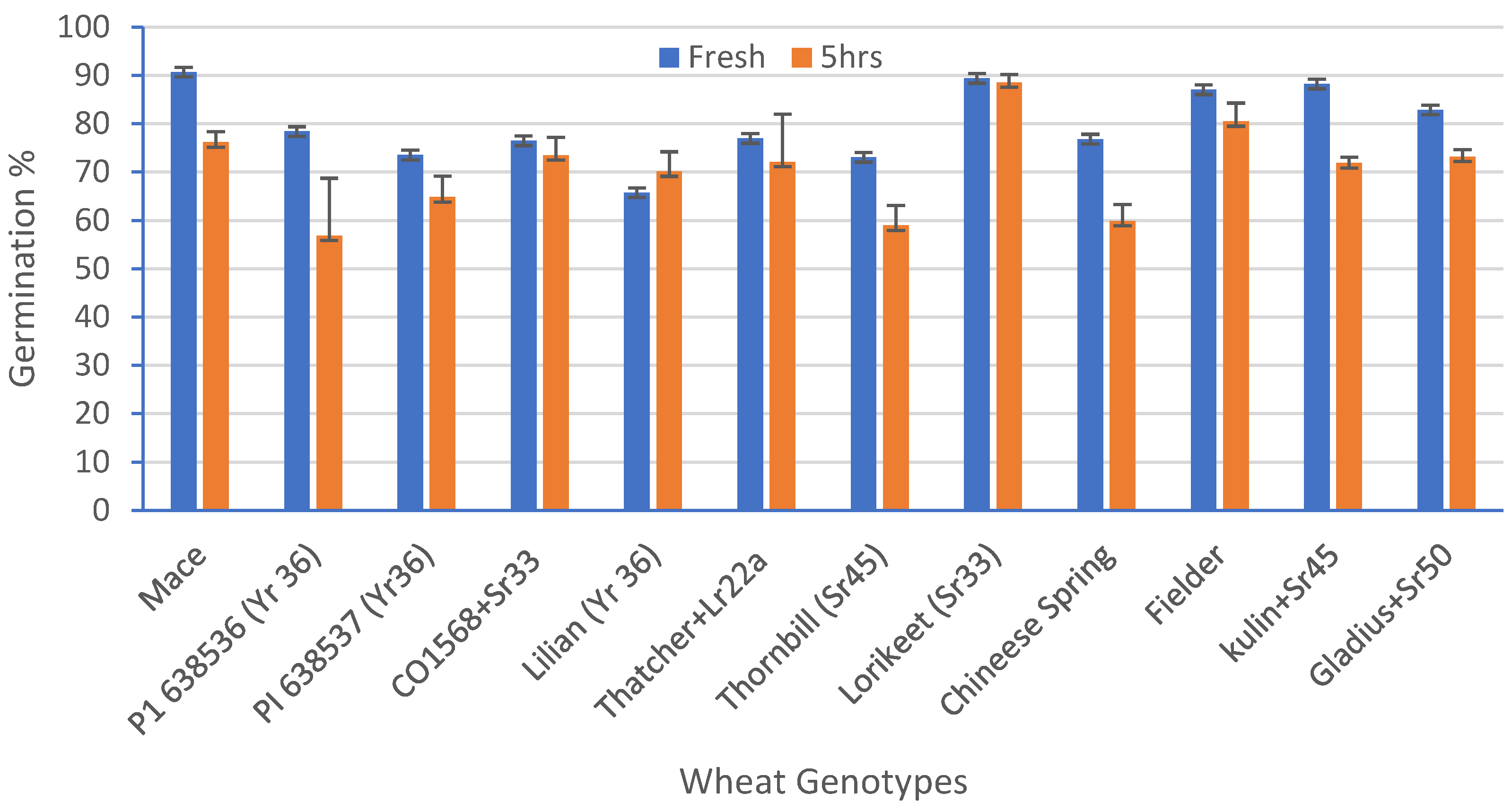
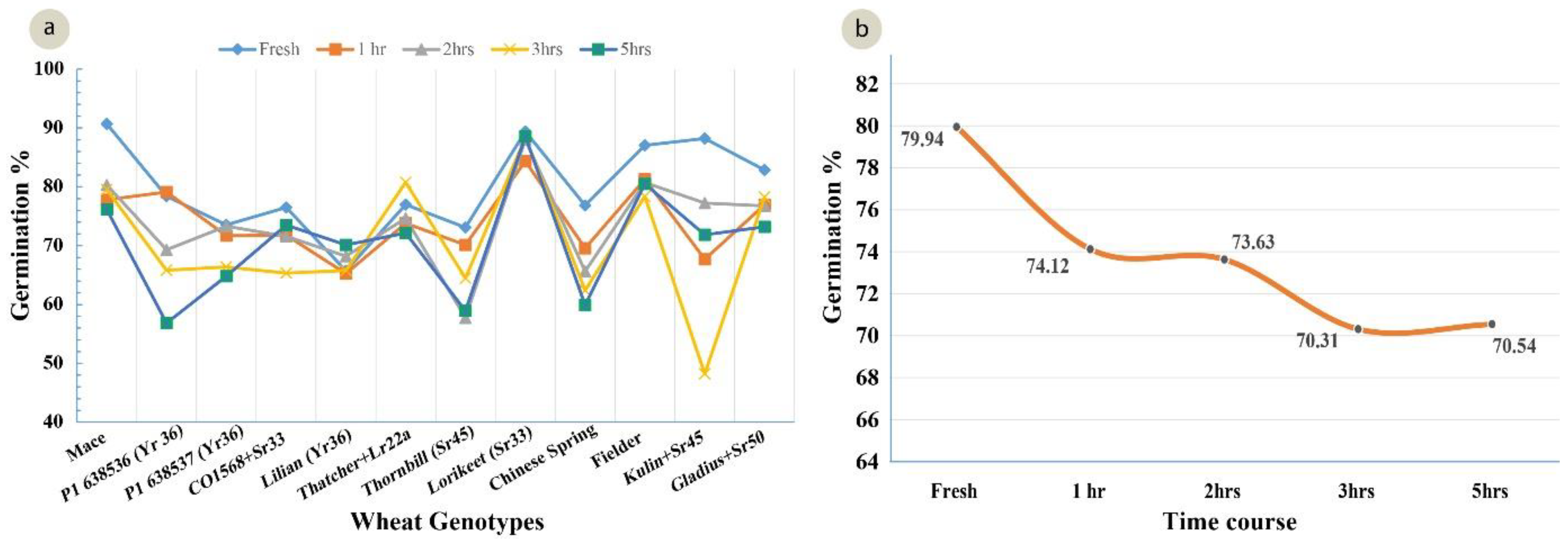
A standardized germination medium with 5% of sucrose, 10% PEG, 100 mg/L boric acid, 200 mg/L calcium nitrate, 100 mg/L potassium nitrate, and 100 mg/L magnesium sulphate, with an optimum pH 6.5, was used to study the pollen viability of 12 genotypes over a time course analysis. The ANOVA for the set of 12 wheat genotypes showed a highly significant (p = 0.00) variation for the pollen viability across the genotypes (Table 4). The highest viability (91%) was observed in Mace and the lowest viability was observed in Lilian (66%), at 0 h in solution (Figure 5). After 5 h, the highest viability (87%) was observed in Lorikeet and the lowest (57%) in the wheat line P1 638536 (Figure 5). Although a highly significant variation was observed among the treatments (0, 1, 2, 3, and 5 h), only non-significant interactions among the genotypes and the time-course treatments were observed. There was a decline in the pollen viability over time in all of the genotypes (Figure 5 and Figure 6a,b).

Table 4.
ANOVA of pollen viability in 12 wheat genotypes using the optimum in vitro germination medium in a time course experiment.
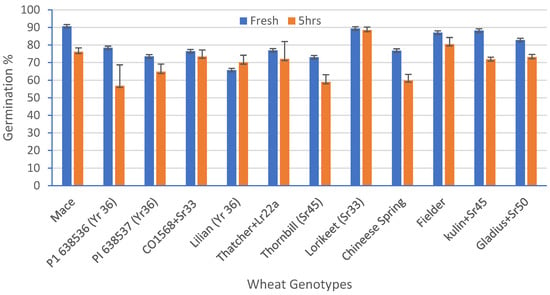
Figure 5.
Percentage reduction in pollen viability (%) for 12 wheat genotypes when freshly harvested pollen was compared with pollen five hours after harvest. Least significant difference (LSD; p = 0.05) for genotypes = 9.77%, for time = 3.99%, and for the interaction between genotype and time = 13.81%. Error bars represent SE of the means.
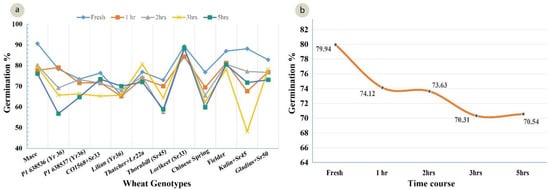
Figure 6.
(a) Mean pollen viability of 12 wheat genotypes in their response to time course experiment (LSD 0.05 = 4.11); (b) Decline in pollen viability averaged over all 12 genotypes during the time period from 0 (fresh pollen) to 5 h after pollen harvest (LSD 0.05 = 6.36).
The in vitro pollen germination data were compared with the IFC data for the genotype PI 638537. The pollen viability values with IFC (74.44 ± 2.80) and in vitro (73.11 ± 8.62) were comparable and showed non-significant differences (Figure S2, Supplementary Materials). A strong correlation (r2 = 0.947) was observed between the two assays.
4. Discussion
This study examined the pollen morphology and viability in seven wheat genotypes to assess the feasibility of the use of pollen as a vehicle for delivering genetic modifications to progeny. A single aperture was observed in all of the genotypes examined. The aperture size ranged from a maximum of ~5.6 µm in the wheat genotype CO1568+Sr33 to a minimum of ~3.0 µm in the Lilian genotype. The aperture is the site from which the pollen tube emerges during germination [23]. This site facilitates the various physiological processes, including the diffusion and endocytosis that contribute to the uptake of exogenous materials [24,25]. It is likely that the uptake of gene editing materials will occur via this aperture. Zhao et al. [26] reported that the pollen morphology, including size, shape, aperture number, and aperture size, are important features for use in genetic transformation studies. The wheat genotype Fielder, known to be amenable to transformation [11,13,27,28,29,30,31], had the smallest overall pollen size (p = 40.1 µm; E = 37.8 µm) and an aperture size in the middle range (4.5 µm) of the genotypes examined. These results indicated that there is no fixed ratio between pollen and aperture sizes for transformation amenability. It is unclear whether Fielder will be more amenable than other genotypes for the pollen-based genetic modification.
Significant structural differences were observed in the morphologies of wheat, triticale, and rye pollen [32]. Ressayre et al. [33] suggested that a structural variation might exist in the pollen grains within a species. Our results agree with this observation, as the overall pollen and aperture sizes differed considerably among the seven wheat genotypes studied. The pollen grains of the genotypes examined in this study can be classified as medium in terms of their size (ranging from 37 µm to 47 µm). This contrasts with the suggested size range of 51 µm to 100 µm indicated in the palynological database (https://www.paldat.org/pub/Triticum_aestivum/301222, accessed on 25 August 2022). However, this difference in the size range may be due to differences in the pollen fixation protocols. We examined dehydrated pollen grains with SEM, the best method for the morphological studies of any given species [23].
Wheat pollen at the time of anther dehiscence has a 30–35% moisture content and is prone to a decrease in viability when exposed to desiccation or drying [17,34]. It is therefore classified as a ‘recalcitrant pollen’, being very difficult to germinate in vitro. Only a few reports exist for the establishment of a successful in vitro germination medium for wheat pollen [16,17]. These previously published media did not support successful pollen germination in our conditions. This may be the result of different plant growth conditions, or that these media were specifically suited to genotypes used in those studies [35,36]. The basic BK [20] germination medium resulted in pollen bursting, which suggested an insufficient osmoticum (sucrose and PEG) in the medium. By modifying the basic BK germination medium through adding PEG4000, adjusting the pH and calibrating the sucrose, boric acid, and calcium nitrate concentrations, we were able to obtain high percentages of germination with negligible pollen bursting. Impe et al. [36] reported that sucrose is the major sugar in fresh wheat pollen, with a soluble sucrose concentration of 84.5%, 88.7%, and 91.0% in three wheat genotypes, Ferrum, Hermann, and TRI 9102, respectively. Given these high levels of endogenous sucrose, an optimum sucrose level in the germination medium would potentially avoid the stress that can hinder pollen tube growth. After testing various osmotic combinations, we observed that a sucrose concentration of 5% along with 10% PEG4000 in the medium best supported the wheat pollen germination. We also determined that a pH level of 6.5 was optimal. The previous studies on different plant species showed that a range of pH levels from 6 to 7.5 is suitable for in vitro pollen germination [17,37,38,39]. We also empirically determined the optimal concentrations of minerals, including boron and calcium that are known to be regulatory [17,40], and involved in the signaling that initiates the in vitro pollen germination [17,19,41,42,43].
There are various protocols for the assessment of pollen viability, including in vitro germination, staining-based quick viability tests [44,45,46,47], and IFC [21,48]. The elementary principle in the staining-based assays is the staining of the cytoplasm. This can unfortunately lead to an overestimation of the pollen viability due to the presence of staining, even in dead cells. The IFC technique is based on the dielectric characteristic of the pollen grain. The main drawback of IFC is that smaller pollen may not be detected as viable, which underestimates the ability of pollen to germinate and most of the environmentally stressed non-viable deformed pollen can be discarded as debris, resulting in an overestimation of viability [21,36]. In our study, the IFC results correlated strongly with the germination percentages obtained from the in vitro germination assay. This means that the IFC assay can be used efficiently to assess the actual pollen viability of the genotypes examined in this study. The efficiency of the IFC assay can be improved if the mature anthers are collected at the right stage from the plants growing under optimum conditions. The strong correlation (r = 0.973) between IFC and in vitro germination in our study compared favorably with the IKI staining assay [21], which overestimated the viability.
Various published studies have shown the effect of temperature on pollen viability as a crucial factor [49,50,51]. Baninasab et al. [51] showed that wheat pollen remained viable after 24 h when it was stored at 5 °C. The wheat pollen remained viable for up to 5 h when stored at 20 ± 2 °C in this study. Interestingly, the highest pollen viability (87%) in our study was observed for the long-season wheat cultivar, Lorikeet. This indicates that the long-season genotypes might have vigorous pollen to allow the maintenance of viability for a long time under controlled conditions.
Attempts have been made to use pollen as a carrier of foreign material for genetic transformation protocols in different crop species, including tobacco [52], maize [53], sorghum [54], and cotton [26]. The attempt to stably integrate the nptII gene into the transformed wheat plants resulted in only 1% transformation frequency in the first and second generations [8]. Although this attempt was unsuccessful, it highlighted the possibility of pollen-mediated genetic transformation. The pollen viability and the availability of a suitable in vitro germination medium is an essential first step for the establishment of a pollen-based transformation in wheat.
5. Conclusions
This study examined wheat genotypes to determine the optimal conditions for maintaining viability in hydrated pollen grains. The maintenance of pollen viability over the treatment period, such as transfecting pollen with plasmids or CRISPR/Cas9 ribonucleoprotein (RNP) complexes, is critical for pollen-based approaches in plant genetic modifications. The study of pollen morphology revealed no significant structural variation between the genotypes, but there was a substantial variation in the pollen size and aperture size, that may affect the ability of exogenous materials (e.g., plasmid DNA, CRISPR RNPs) to be internalized into hydrated pollen. Although wheat pollen is known to be labile/short lived compared to the pollen from other species [55,56], our results showed that it can remain viable for up to five hours, if stored under controlled conditions and can successfully germinate in the optimized medium. This study provides important information for moving forward with pollen-mediated approaches to genetic transformation and gene editing protocols in wheat.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12092009/s1, Figure S1: Developmental changes of wheat floret at pre-anthesis, anthesis and post-anthesis stages. (A–I) Pre-anthesis stage showing the reproductive organs (anthers and pistils), (J–M) anthesis stage showing yellowish anthers and fully receptive stigma, (N–T) post anthesis stage showing dehisced anthers. Abbreviations: a, anther; aw, awn; f, filament; le, lemma; lo, lodicule; o, ovary; p, palea; pi, pistil; r, rachilla; s, stigma; st, stamen. Ruler divisions = 1.0 mm; Figure S2: Ampha cytometer chart at 12.00 MHz for categories of cell count using mature pollen grains of the genotype P1 638537 (Yr36).
Author Contributions
U.B., N.A., B.J. and H.B. conceived the idea and supervised the project. M.K., N.A. (SEM) and N.G. conducted experiments and statistical analysis. M.K. prepared the initial draft and N.A., U.B., B.J. and H.B. edited it. All authors have read and agreed to the published version of the manuscript.
Funding
First author acknowledges the University of Sydney for providing funding through the International Scholarship and Postgraduate Research Supplementary Scholarship in Excellence in Genome Editing.
Data Availability Statement
All data associated with this manuscript are included in the main manuscript and in the Supplementary Materials.
Acknowledgments
Our thanks are due to Anowarul Bokshi from the Plant Breeding Institute, University of Sydney for his assistance in using the impedance flow cytometer. We also express our thanks to all the staff of the Australian Centre for Microscopy and Microanalysis (ACMM), University of Sydney for their help in using the scanning electron microscope.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alwen, A.; Eller, N.; Kastler, M.; Moreno, R.M.B.; Heberle-Bors, E. Potential of in vitro pollen maturation for gene transfer. J. Physiol. Plant. 1990, 79, 194–196. [Google Scholar] [CrossRef]
- Touraev, A.; Stöger, E.; Voronin, V.; Heberle-Bors, E. Plant male germ line transformation. Plant J. 1997, 12, 949–956. [Google Scholar] [CrossRef]
- Eapen, S. Pollen grains as a target for introduction of foreign genes into plants: An assessment. Physiol. Mol. Biol. Plants 2011, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hess, D. Genetic transformation of wheat via pollen 25 years of plant transformation attempts II. In In Vitro Haploid Production in Higher Plants; Springer: Dordrecht, The Netherlands, 1996; pp. 393–409. [Google Scholar]
- Pandey, K.K. Sexual transfer of specific genes without gametic fusion. Nature 1975, 256, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Hess, D. Uptake of DNA and bacteriophage into pollen and genetic manipulation. In Genetic Manipulations with Plant Material; Springer: Berlin/Heidelberg, Germany, 1975; pp. 519–537. [Google Scholar]
- Ohta, Y. High-efficiency genetic transformation of maize by a mixture of pollen and exogenous DNA. Proc. Natl. Acad. Sci. USA 1986, 83, 715–719. [Google Scholar] [CrossRef]
- Hess, D.; Dressler, K.; Nimmrichter, R. Transformation experiments by pipetting Agrobacterium into the spikelets of wheat (Triticum aestivum L.). Plant Sci. 1990, 72, 233–244. [Google Scholar] [CrossRef]
- Martin, N.; Forgeois, P.; Picard, E. Investigations on transforming Triticum aestivum via the pollen tube pathway. Agron. EDP Sci. 1992, 12, 537–544. [Google Scholar] [CrossRef]
- Picard, E.; Jacquemin, J.; Granier, F.; Bobin, M.; Forgeois, P. Genetic transformation of wheat (Triticum aestivum) by plasmid DNA uptake during pollen tube germination. In Proceedings of the Seventh International Wheat Genetics Symposium, Cambridge, UK, 13–19 July 1988; pp. 779–781. [Google Scholar]
- Borisjuk, N.; Kishchenko, O.; Eliby, S.; Schramm, C.; Anderson, P.; Jatayev, S.; Shavrukov, Y. Genetic modification for wheat improvement: From transgenesis to genome editing. BioMed Res. Int. 2019, 2019, 6216304. [Google Scholar] [CrossRef]
- Risacher, T.; Craze, M.; Bowden, S.; Paul, W.; Barsby, T. Highly efficient Agrobacterium-mediated transformation of wheat via in planta inoculation. In Transgenic Wheat, Barley and Oats; Humana Press: Totowa, NJ, USA, 2009; pp. 115–124. [Google Scholar]
- Ishida, Y.; Tsunashima, M.; Hiei, Y.; Komari, T. Wheat (Triticum aestivum L.) transformation using immature embryos. In Agrobacterium Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 189–198. [Google Scholar]
- Pellegrineschi, A.; Noguera, L.M.; Skovmand, B.; Brito, R.M.; Velazquez, L.; Salgado, M.M.; Hoisington, D. Identification of highly transformable wheat genotypes for mass production of fertile transgenic plants. Genome 2002, 45, 421–430. [Google Scholar] [CrossRef]
- Jouanin, A.; Gilisen, L.J.W.J.; Schaart, J.G.; Leigh, F.J.; Cockram, J.; Wallington, E.J.; Boyd, L.A.; van den Broeck, H.C.; van der Meer, I.M.; America, A.H.P.; et al. CRISPR/CAS9 gene editing of gluten in wheat to reduce gluten content and exposure-reviewing methods to screen for coeliac safety. Front. Nutr. 2020, 7, 51. [Google Scholar] [CrossRef]
- Cheng, C.; Mcomb, J. In-vitro germination of wheat pollen on raffinose medium. New Phytol. 1992, 120, 459–462. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Annapoorani, S.; Vikas, V.K.; Sivasamy, M.; Kumar, J.; Saravannan, K.; Sheeba, D. An improved in-vitro germination medium for recalcitrant bread wheat (Triticum aestivum L.) pollen. Indian J. Genet. Plant Breed. 2015, 75, 446–452. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. The growth stage code for cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999; p. 322. [Google Scholar]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Bokshi, A.I.; Tan, D.K.; Trethowan, R.M. A robust and rapid pollen viability test using impedance flow cytometry for high throughput screening of heat tolerant wheat (Triticum aestivum) germplasm. In Proceedings of the 2019 Agronomy Australia Conference, Wagga Wagga, NSW, Australia, 25–29 August 2019; Pratley, J., Ed.; Australian Society of Agronomy: Wagga Wagga, NSW, Australia, 2019. Available online: www.agronomyaustralia.org/ (accessed on 19 July 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. DJU hcr-powpnih (2011) the R Development Core Team (2011) nlme: Linear and Nonlinear Mixed Effects Models, R package version 3 1–117.
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Frosch-Radivo, A. Illustrated Pollen Terminology; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. J. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, Z.; Wang, Y.; Chen, W.; Sun, C.; Cui, B.; Luo, D. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants 2017, 3, 956–964. [Google Scholar] [CrossRef]
- Vasil, V.; Castillo, A.M.; Fromm, M.E.; Vasil, I.K. Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. J. Biotechnol. 1992, 10, 667–674. [Google Scholar] [CrossRef]
- Maës, O.C.; Chibbar, R.N.; Caswell, K.; Leung, N.; Kartha, K.K. Somatic embryogenesis from isolated scutella of wheat: Effects of physical, physiological and genetic factors. Plant Sci. 1996, 121, 75–84. [Google Scholar] [CrossRef]
- Richardson, T.; Thistleton, J.; Higgins, T.J.; Howitt, C.; Ayliffe, M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell Tissue Organ Culture 2014, 119, 647–659. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Du, L.; Ye, X. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol. J. 2017, 15, 614–623. [Google Scholar] [CrossRef]
- Ishida, Y.; Hiei, Y.; Komari, T. High-efficiency transformation techniques. In Applications of Genetic and Genomic Research in Cereals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–120. [Google Scholar]
- Liu, Z.; Zhang, Z.; Shen, Y. Scanning electron microscope observation on pollen morphology and leaf epidermis in wheat, triticale and rye. Acta Agric. Boreali-Sin. 1992, 7, 23–28. [Google Scholar]
- Ressayre, A.; Godelle, B.; Mignot, A.; Gouyon, P.H. A morphogenetic model accounting for pollen aperture pattern in flowering plants. J. Theor. Biol. 1998, 193, 321–334. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Media for Isolation, Cultivation, Identification and Maintenance of Medical Bacteria; Williams, Wilkims: Baltimore, MD, USA, 1985. [Google Scholar]
- Liu, J.X.; Liao, D.Q.; Oane, R.; Estenor, L.; Yang, X.E.; Li, Z.C.; Bennett, J. Genetic variation in the sensitivity of anther dehiscence to drought stress in rice. Field Crops Res. 2006, 97, 87–100. [Google Scholar] [CrossRef]
- Impe, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A.; Nagel, M. Assessment of pollen viability for wheat. Front. Plant Sci. 2020, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.J.; Velten, J.; Oliver, M.J. In-vitro analysis of cotton pollen germination. Agron. J. 2004, 96, 359–368. [Google Scholar] [CrossRef]
- Mbogning, J.; Youmbi, E.; Nkongmeneck, B. Morphological and in-vitro germination studies of pollen grains in kola tree (Cola sp.). Akdeniz Üniv. Ziraat Fakült. Derg. 2007, 20, 311–318. [Google Scholar]
- Ahmad, N.M.; Martin, P. Pollen morphology and physiology of Poa labillardieri (Poaceae). Int. J. Plant Reprod. Biol. 2017, 2, 139–147. [Google Scholar]
- Cheng, C.; Rerkasem, B. Effect of boron on pollen viability in wheat. Plant Soil 1993, 155, 313–315. [Google Scholar] [CrossRef]
- Tuinstra, M.R.; Wedel, J. Estimation of pollen viability in grain sorghum. Crop Sci. 2000, 40, 968–970. [Google Scholar]
- Steinhorst, L.; Kudla, J. Calcium-a central regulator of pollen germination and tube growth. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.G.; Mankad, A.U. In-vitro pollen germination—A review. Int. J. Sci. 2014, 3, 304–307. [Google Scholar]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- La Porta, N.; Roselli, G. Relationship between pollen germination in-vitro and fluorochromatic reaction in cherry clone F12/1 (Prunus avium L.) and some of its mutants. J. Hortic. Sci. 1991, 66, 171–175. [Google Scholar] [CrossRef]
- Trognitz, B.R. Comparison of different pollen viability assays to evaluate pollen fertility of potato dihaploids. Euphytica 1991, 56, 143–148. [Google Scholar] [CrossRef]
- Sedgley, M.; Harbard, J. Pollen storage and breeding system in relation to controlled pollination of four species of Acacia (Leguminosae: Mimosoideae). Aust. J. Bot. 1993, 41, 601–609. [Google Scholar] [CrossRef]
- Heidmann, I.; Schade-Kampmann, G.; Lambalk, J.; Ottiger, M.; Di Berardino, M. Impedance flow cytometry: A novel technique in pollen analysis. PLoS ONE 2016, 11, e0165531. [Google Scholar] [CrossRef]
- De Vries, A.P. Flowering biology of wheat, particularly in view of hybrid seed production—A review. Euphytica 1971, 20, 152–170. [Google Scholar] [CrossRef]
- Stanley, R.G.; Linskens, H.F. Pollen: Biology Biochemistry Management; Business Media; Springer Science: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Baninasab, B.; Tabori, M.; Yu, J.; Zhang, Y.; Wang, X.; Deschiffart, I.; Khanizadeh, S. Low temperature storage and in-vitro pollen germination of selected spring wheat accessions. J. Agric. Sci. 2017, 9, 9. [Google Scholar] [CrossRef]
- Stöger, E.; Moreno, R.M.B.; Ylstra, B.; Vicente, O.; Heberle-Bors, E. Comparison of different techniques for gene transfer into mature and immature tobacco pollen. Transgenic Res. 1992, 1, 71–78. [Google Scholar] [CrossRef]
- Schreiber, D.N.; Dresselhaus, T. In-vitro pollen germination and transient transformation of Zea mays and other plant species. Plant Mol. Biol. 2003, 21, 31–41. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Yang, C.; Li, Y.; Liu, L.; Xu, J. Pollen-mediated transformation of Sorghum bicolor plants. Biotechnol. Appl. Biochem. 2007, 48, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.E.; Lukaszewski, A.J. Pollen longevity in wheat, rye and triticale. Plant Breed. 1989, 102, 31–34. [Google Scholar] [CrossRef]
- McCormick, S. Control of male gametophyte development. Plant Cell 2004, 16, S142–S153. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).