The Influence of Flag Leaf Removal and Its Characteristics on Main Yield Components and Yield Quality Indices on Wheat
Abstract
:1. Introduction
2. Materials and Methods
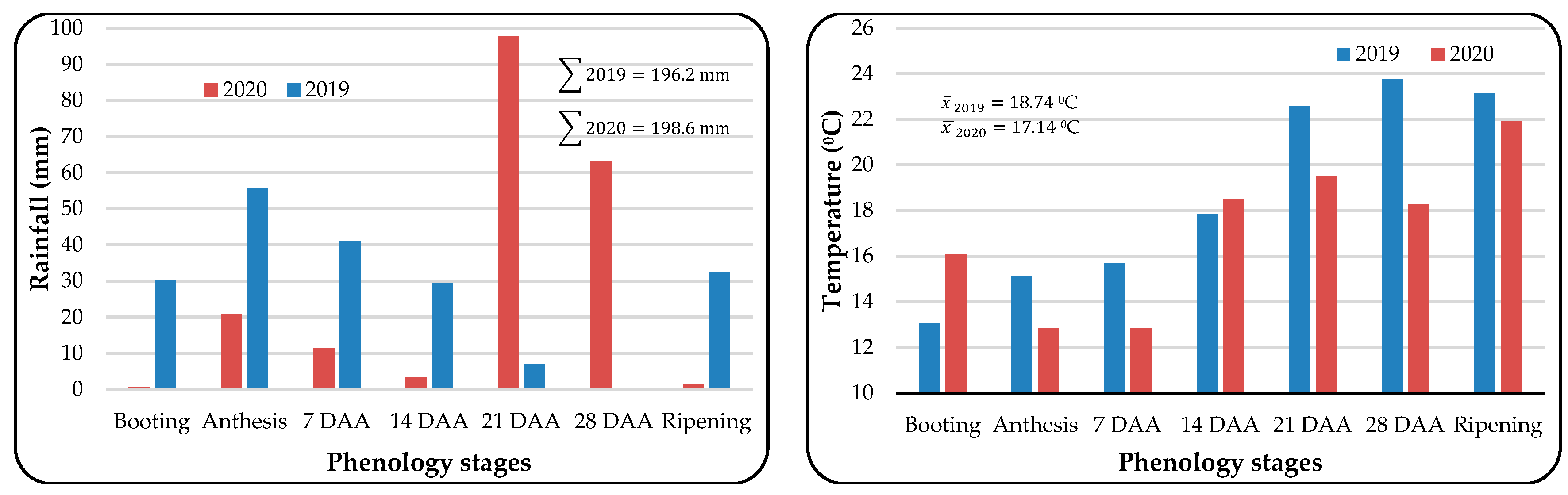
Climatic Conditions
3. Results
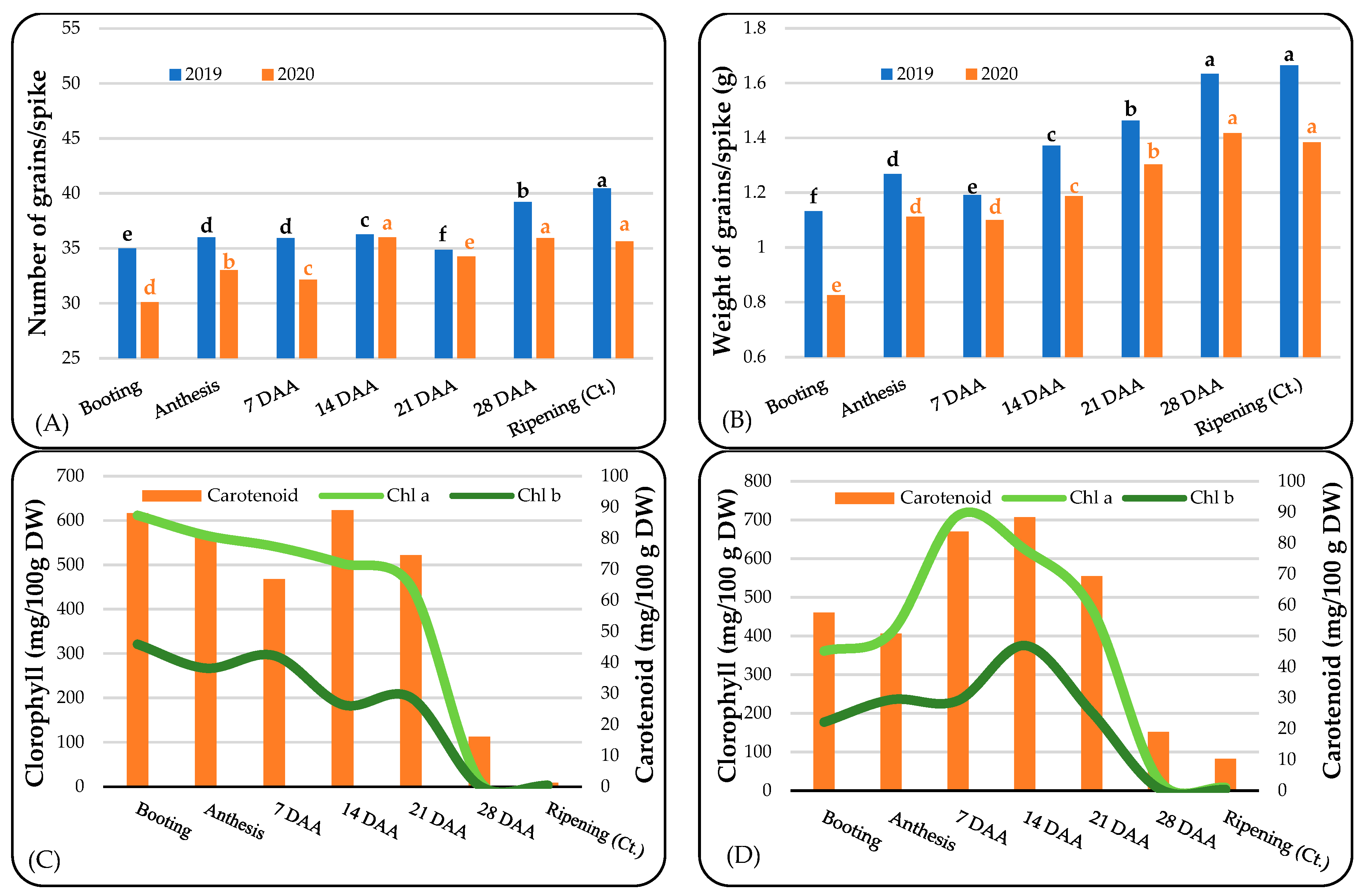
3.1. Yield Components and Photosynthetic Pigments
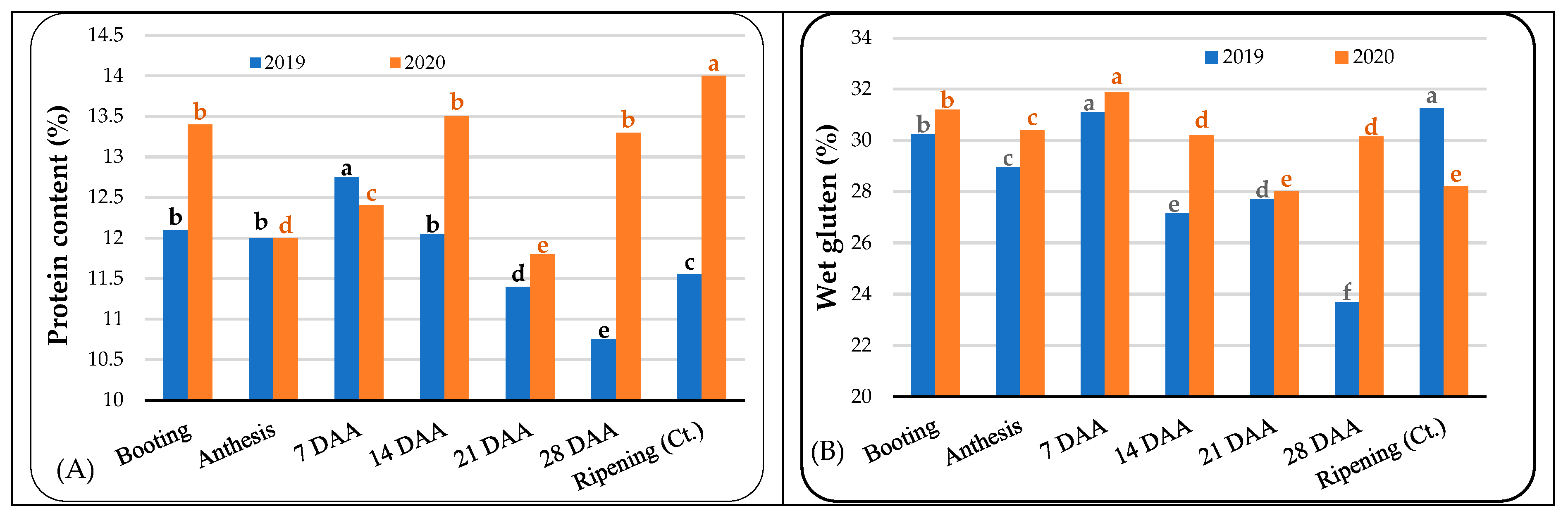
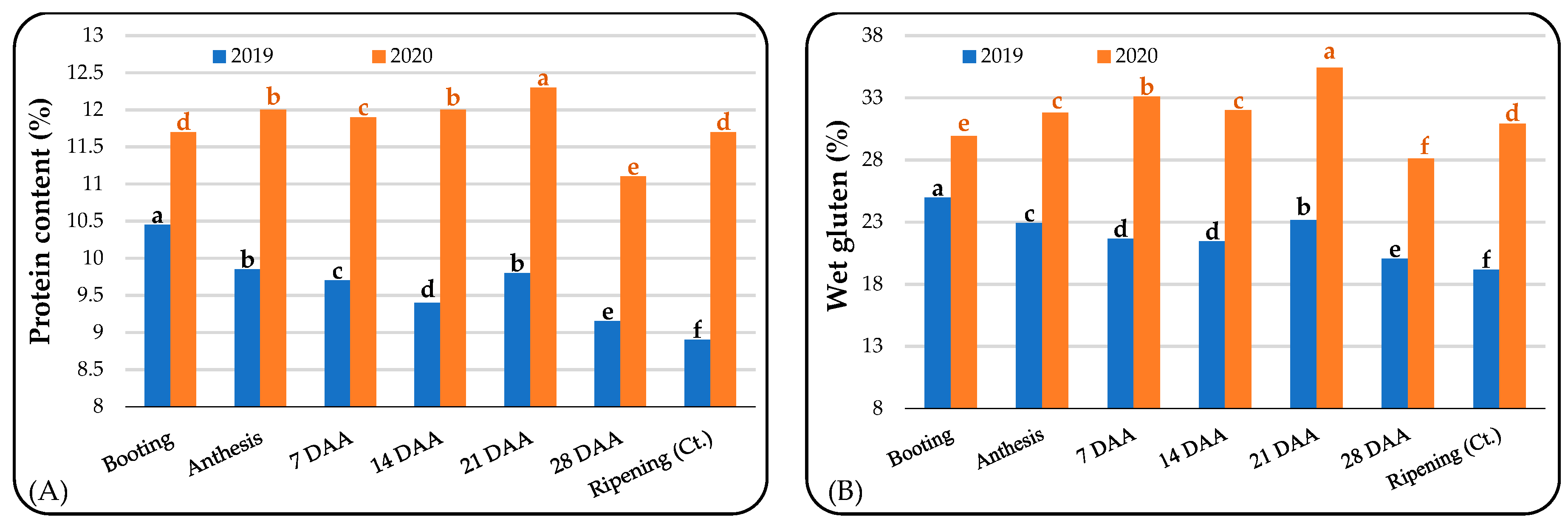
3.2. Grain Quality
3.3. Relationship between Analyzed Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mickky, B.; Aldesuquy, H.; Elnajar, M. Effect of Drought on Yield of Ten Wheat Cultivars Linked with Their Flag Leaf Water Status, Fatty Acid Profile and Shoot Vigor at Heading. Physiol. Mol. Biol. Plants 2020, 26, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food Security and the Dynamics of Wheat and Maize Value Chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic Gains in Wheat Breeding and Its Role in Feeding the World. Crop. Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar] [CrossRef] [Green Version]
- Balla, K.; Karsai, I.; Bónis, P.; Kiss, T.; Berki, Z.; Horváth, Á.; Mayer, M.; Bencze, S.; Veisz, O. Heat Stress Responses in a Large Set of Winter Wheat Cultivars (Triticum Aestivum L.) Depend on the Timing and Duration of Stress. PLoS ONE 2019, 14, e0222639. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Onyekachi, O.G.; Boniface, O.O.; Gemlack, N.F.; Nicholas, N. The Effect of Climate Change on Abiotic Plant Stress: A Review. In Abiotic and Biotic Stress in Plants; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, R. Understanding of Leaf Development—The Science of Complexity. Plants 2013, 2, 396–415. [Google Scholar] [CrossRef]
- Du, B.; Liu, L.; Wang, Q.; Sun, G.; Ren, X.; Li, C.; Sun, D. Identification of QTL underlying the leaf length and area of different leaves in barley. Sci. Rep. 2019, 9, 4431. [Google Scholar] [CrossRef] [Green Version]
- Biswal, A.K.; Kohli, A. Cereal flag leaf adaptations for grain yield under drought: Knowledge status and gaps. Mol. Breed. 2013, 31, 749–766. [Google Scholar] [CrossRef]
- Liu, L.; Sun, G.; Ren, X.; Li, C.; Sun, D. Identification of QTL Underlying Physiological and Morphological Traits of Flag Leaf in Barley. BMC Genet. 2015, 16, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towfiq, S.I.; Abdulqader, S.H.; Ahmad, K.R.; Hama, S.J. Response of grain yield and its components to organic matter and removal of some photosynthetic organs of durum wheat (Triticum aestivum L.) in two years of Sulaimani—Iraq region. Int. J. Plant Anim. Environ. Sci. 2015, 5, 134–140. [Google Scholar] [CrossRef]
- Auriau, P.; Doussinault, G.; Jahier, J.; Lecompte, C.; Pierre, J.; Pluchard, P.; Rousset, M.; Saur, L.; Trottet, M. Le blé tendre. In Amélioration des Espèces Végétales Cultivées; Gallais, A., Bannerot, H., Eds.; INRA: Paris, France, 1992; pp. 22–38. [Google Scholar]
- Sylvester-Bradley, R.; Scott, R.K.; Wright, C.E. Physiology in the production and improvement of cereals. In HGCA Research Review No. 18; Soil Science Department: Cambridge, UK, 1990. [Google Scholar]
- Ma, J.; Xiao, Y.; Hou, L.; He, Y. Combining Protein Content and Grain Yield by Genetic Dissection in Bread Wheat under Low-Input Management. Foods 2021, 10, 1058. [Google Scholar] [CrossRef]
- Banitaba, A.; Naderi, M.; Javanmard, H.; Emami, B. Effect of flag leaf and awn removal on vegetative traits, grain yield and yield components of bread wheat (Triticum aestivum L.). Res. Agric. Sci. 2007, 2, 73–86. [Google Scholar]
- Alvaro, F.; Royo, C.; García del Moral, L.F.; Villegas, D. Grain filling and dry matter translocation responses to source–sink modifications in a historical series of durum wheat. Crop Sci. 2008, 48, 1523. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Aguado, J.; Reyes, F.; Rodes, R.; Perez, I.; Dorado, M. Effect of source-to-sink ratio on partitioning of dry matter and 14 C-photoassimilates in wheat during grain filling. Ann. Bot. 1999, 83, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Saeedipour, S.; Moradi, F. Comparison of the drought stress responses of tolerant and sensitive wheat cultivars during grain filling: Impact of invertase activity on carbon metabolism during kernel development. J. Agric. Res. 2011, 3, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Blake, N.K.; Lanning, S.P.; Martin, J.M.; Sherman, J.D.; Talbert, L.E. Relationship of Flag Leaf Characteristics to Economically Important Traits in Two Spring Wheat Crosses. Crop Sci. 2007, 47, 491. [Google Scholar] [CrossRef]
- Liu, X.; Yin, B.; Hu, Z.; Bao, X.; Wang, Y.; Zhen, W. Physiological response of flag leaf and yield formation of winter wheat under different spring restrictive irrigation regimes in the Haihe Plain, China. J. Integr. Agric. 2021, 20, 2343–2359. [Google Scholar] [CrossRef]
- Vicente, R.; Vergara-Díaz, O.; Medina, S.; Chairi, F.; Kefauver, S.C.; Bort, J.; Serret, M.D.; Aparicio, N.; Araus, J.L. Durum wheat ears perform better than the flag leaves under water stress: Gene expression and physiological evidence. Environ. Exp. Bot. 2018, 153, 271–285. [Google Scholar] [CrossRef]
- Wazziki, E.H.; Yousfi, B.E.; Serghat, S. Contributions of three upper leaves of wheat, either healthy or inoculated by Bipolaris sorokiniana, to yield and yield components. Aust. J. Crop Sci. 2015, 9, 629–637. [Google Scholar]
- Kong, L.; Sun, M.; Xie, Y.; Wang, F.; Zhao, Z. Photochemical and antioxidative responses of the glume and flag leaf to seasonal senescence in wheat. Front. Plant Sci. 2015, 6, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliç, H.; Yağbasanlar, T. The Effect of Drought Stress on Grain Yield, Yield Components and Some Quality Traits of Durum Wheat (Triticum turgidum ssp. durum) Cultivars. Not. Bot. Horti Agrobot. 2010, 38, 164–170. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, F.; Wang, W.; Zhou, Y.; Fu, B.; Li, Z. Comparative transcriptome sequencing of tolerant rice introgression line and its parents in response to drought stress. BMC Genom. 2014, 15, 1026. [Google Scholar] [CrossRef] [Green Version]
- Mou, H.M.; He, J.Q.; Xing, J.J.; Yao, S.J.; Tang, Y.; Wang, Z.Q.; Du, G.Y. Water changes in wheat spike during grain filling stage investigated by nuclear magnetic resonance. J. Agric. Eng. 2016, 32, 98–104. [Google Scholar]
- Zhang, L.; Zhang, C.; Du, B.; Lu, B.; Zhou, D.; Zhou, J.; Zhou, J. Effects of node restriction on cadmium accumulation in eight Chinese wheat (Triticum turgidum) cultivars. Sci. Total Environ. 2020, 725, 138358. [Google Scholar] [CrossRef]
- Liu, S.N.; Yu, F.Y.; Fu, Z.H.; Yang, J.Y.; Chen, M.L.; Fu, Y.H.; Li, Y.J.; Chang, H.Q.; Zhou, W.L.; Wang, X.G.; et al. Key factors affect selenite absorption in wheat leaf blades: pH, temperature, light intensity and leaf position. Plant Soil Environ. 2020, 66, 431–436. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Shi, Y.; Yu, Z. Optimized split nitrogen fertilizer increase photosynthesis, grain yield, nitrogen use efficiency and water use efficiency under water-saving irrigation. Sci. Rep. 2020, 10, 20310. [Google Scholar] [CrossRef]
- Quirino, B.F.; Noh, Y.S.; Himelblau, E.; Amasino, R.M. Molecular aspects of leaf senescence. Trends Plant Sci. 2000, 5, 278–282. [Google Scholar] [CrossRef]
- Fornari, E.Z.; Gaviraghi, L.; Basso, C.J.; Pinheiro, M.V.M.; Vian, A.L.; Santi, A.L. Relationship between photosynthetic pigments and corn production under nitrogen sources. Pesqui. Agropecu. Trop. 2020, 50, e63661. [Google Scholar] [CrossRef]
- Bojović, B.; Stojanović, J. Chlorophyll and carotenoid content in wheat cultivars as a function of mineral nutrition. Arch. Biol. Sci. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Timilsina, R.; Khadka, D.; Khadka, A.; Ojha, P. Profile of chlorophylls and carotenoids of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) microgreens. J. Food Sci. Technol. 2019, 56, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2013, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Structures, nomenclature and general chemistry of carotenoids and their esters. In Foods: Physical, Chemical and Biological Properties; The Royal Society of Chemistry: London, UK, 2019; pp. 1–50. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ross, J. The Radiation Regime and Architecture of Plant Stands; Dr W. Junk Publishers: London, UK, 1981. [Google Scholar]
- Feng, B.; Liu, B.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, J.W. Effect of Heat Stress on the Photosynthetic Characteristics in Flag Leaves at the Grain-Filling Stage of Different Heat-Resistant Winter Wheat Varieties. J. Agron. Crop Sci. 2013, 200, 143–155. [Google Scholar] [CrossRef]
- Tashiro, T.; Wardlaw, I.F. The response of high temperature shock and humidity changes prior to and during early stages of grain development in wheat. Funct. Plant Biol. 1990, 17, 551–561. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M. Response of floret fertility and individual grain weight of wheat to high temperature stress: Sensitive stages and thresholds for temperature and duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Sears, R.G.; Gill, B.S.; Paulsen, G.M. Growth and senescence characteristics associated with tolerance of wheat alien amphiploids to high temperature under controlled conditions. Euphytica 2002, 126, 185–193. [Google Scholar] [CrossRef]
- Castro, M.; Peterson, C.J.; Rizza, M.D.; Dellavalle, P.D.; Vazquez, D.; Ibanez, V.; Ross, A. Influence of heat stress on wheat grain characteristics and protein molecular weight distribution. In Wheat Production in Stressed Environments; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 365–371. [Google Scholar]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and Responses of Chloroplasts to Heat Stress in Plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Bragado, R.; Elazab, A.; Zhou, B.; Serret, M.D.; Bort, J.; Nieto-Taladriz, M.T.; Araus, J.L. Contribution of the ear and the flag leaf to grain filling in durum wheat inferred from the carbon isotope signature: Genotypic and growing conditions effects. J. Integr. Plant Biol. 2014, 56, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Evans, L.T.; Wardlaw, I.F.; Fischer, R.A. Wheat. In Crop Physiology: Some Case Histories; Evans, L.T., Ed.; Cambridge University Press: Cambridge, UK, 1980; pp. 101–149. [Google Scholar]
- Araus, J.L.; Tapia, L. Photosynthetic gas exchange characteristics of wheat flag leaf blades and sheaths during grain filling. Plant Physiol. 1987, 85, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, P.K.; Fischer, R.A.; Liboon, S.P. Source-sink relations and effects of post-anthesis canopy defoliation in wheat at low latitudes. J. Agric. Sci. 1990, 114, 93–99. [Google Scholar] [CrossRef]
- Mandea, V.; Mustăṭea, P.; Marinciu, C.M.; Serban, G.; Meluca, C.; Păunescu, G.; Isticioaia, S.F.; Dragomir, C.; Bunta, G.; Filiche, E.; et al. Yield components compensation in winter wheat (Triticum aestivum L.) is cultivar dependent. Rom. Agric. Res. 2019, 36, 7. [Google Scholar]
- Jebbouj, R.; El Yousfi, B. Barley yield losses due to defoliation of upper three leaves either healthy or infected at boot stage by Pyrenophora teres f. teres. Eur. J. Plant Pathol. 2009, 125, 303–315. [Google Scholar] [CrossRef]
- Xu, C.; Tao, H.; Wang, P.; Wang, Z. Slight shading after anthesis increases photosynthetic productivity and grain yield of winter wheat (Triticum aestivum L.) due to the delaying of leaf senescence. J. Integr. Agric. 2016, 15, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Fokar, M.; Blum, A.; Nguyen, H.T. Heat tolerance in spring wheat. II. Grain filling. Euphytica 1998, 104, 9–15. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Li, J.; Li, X.; Yang, X.; Tong, Y.; Zhang, A.; Li, B.; Lin, J.; Kuang, T.; et al. Dynamic changes in flag leaf angle contribute to high photosynthetic capacity. Chin. Sci. Bull. 2009, 54, 3045–3052. [Google Scholar] [CrossRef]
- Martinez, D.E.; Luquez, V.M.; Bartoli, C.G.; Guiamet, J.J. Persistence of photosynthetic components and photochemical efficiency in ears of water-stressed wheat (Triticum aestivum). Physiol. Plant 2003, 119, 519–525. [Google Scholar] [CrossRef]
- Fan, Y.; Lv, Z.; Ge, T.; Li, Y.; Yang, W.; Zhang, W.; Ma, S.; Dai, T.; Huang, Z. Night-Warming Priming at the Vegetative Stage Alleviates Damage to the Flag Leaf Caused by Post-anthesis Warming in Winter Wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 706567. [Google Scholar] [CrossRef]
- Navabpour, S.; Jalali, P. Expression profiling of two sucrose transporter genes during post-anthesis in wheat. Zemdirb. Agric. 2019, 106, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Ommen, O.E.; Donnelly, A.; Vanhoutvin, S.; van Oijen, M.; Manderscheid, R. Chlorophyll content of spring wheat flag leaves grown under elevated CO2 concentrations and other environmental stresses within the ESPACE-wheat project. Eur. J. Agron. 1999, 10, 197–203. [Google Scholar] [CrossRef]
- Manivannan, P.; Abdul Jaleel, C.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.M.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.T.; Rizwan, M.; Khaliq, R.; Shahid, S.; Perveen, R.; Alamri, S.A.; Alyemeni, M.N.; et al. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 12924. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.K.; Gao, C.Y.; Zhang, R.B.; Liu, T.T.; Li, C.Y.; Guo, W.S.; Zhu, J.G.; Kobayashi, K. Effects of elevated atmospheric ozone concentration on flag leaf photosynthetic pigment contents of wheat. Ying Yong Sheng Tai Xue Bao 2012, 23, 2178–2184. (In Chinese) [Google Scholar]
- Deng, X.; Liu, Y.; Xu, X.; Liu, D.; Zhu, G.; Yan, X.; Wang, Z.; Yan, Y. Comparative Proteome Analysis of Wheat Flag Leaves and Developing Grains under Water Deficit. Front. Plant Sci. 2018, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Zhao, Y.; Yu, Z.; Zeng, J.; Xu, D.; Dong, J.; Ma, W. Wheat Quality Formation and Its Regulatory Mechanism. Front. Plant Sci. 2022, 13, 834654. [Google Scholar] [CrossRef]
- Laidig, F.; Piepho, H.P.; Rentel, D.; Drobek, T.; Meyer, U.; Huesken, A. Breeding progress, environmental variation and correlation of winter wheat yield and quality traits in German official variety trials and on-farm during 1983–2014. Theor. Appl. Genet. 2017, 130, 223–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahar, B. Relationships among flag leaf chlorophyll content, agronomical traits, and some physiological traits of winter wheat genotypes. Dufed 2015, 4, 1–5. [Google Scholar]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, N.; Ahmed, J.; Hameed, A.; Ashraf, M.A.; Zafar, A.S.; Maqbool, A.; Al-Amrah, H.; Alatawi, H.A.; Al-Harbi, M.S.; et al. Grain yield, chlorophyll and protein contents of elite wheat genotypes under drought stress. J. King Saud Univ. Sci. 2022, 34, 102279. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Hernandez-Espinosa, N.; Pena, R.J. The influence of drought and heat stress on the expression of end-use quality parameters of common wheat. J. Cereal Sci. 2013, 57, 73–78. [Google Scholar] [CrossRef]



| Source of Variation | No of Grains/Spike | Weight of Grains/Spike | Chl a | Chl b | Carotenoids |
|---|---|---|---|---|---|
| Ciprian | |||||
| Year (A) | ** | ** | ns | ns | ns |
| Phenophasis (B) | ** | ** | ** | ** | ** |
| A × B | ** | ** | ** | ** | ** |
| Andrada | |||||
| Year (A) | ** | ** | ns | ns | ns |
| Phenophasis (B) | ** | ** | ** | ** | ** |
| A × B | ** | ** | ** | ** | ** |
| Codru | |||||
| Year (A) | ** | ** | ns | ns | ** |
| Phenophasis (B) | ** | ** | ** | ** | ** |
| A × B | ** | ** | ** | ** | ** |
| Source of Variation | Grain Protein Content | Wet Gluten Content |
|---|---|---|
| Ciprian | ||
| Year (A) | ** | ns |
| Phenophasis (B) | ** | ** |
| A × B | ** | ** |
| Andrada | ||
| Year (A) | ** | ns |
| Phenophasis (B) | ** | ** |
| A × B | ** | ** |
| Codru | ||
| Year (A) | ** | * |
| Phenophasis (B) | ** | ** |
| A × B | ** | ** |
| Ciprian Variety | ||||||||
| 2019 | 2020 | |||||||
| NGS | WGS | Chl a | Chl b | Carotenoids | Protein | Wet gluten | ||
| NGS | - | 0.84 *** | −0.11 | 0.02 | −0.11 | 0.28 | 0.14 | |
| WGS | 0.85 *** | - | −0.44 0 | −0.43 0 | −0.49 00 | 0.31 | −0.06 | |
| Chl a | −0.81 000 | −0.72 000 | - | 0.91 *** | 0.97 *** | −0.47 00 | 0.34 | |
| Chl b | −0.78 000 | −0.75 000 | 0.97 *** | - | 0.92 *** | −0.35 0 | 0.24 | |
| Carotenoids | −0.78 000 | −0.60 000 | 0.96 *** | 0.89 *** | - | −0.45 00 | 0.22 | |
| Protein | −0.10 | −0.17 | 0.62 *** | 0.65 *** | 0.54 ** | - | 0.24 | |
| Wet gluten | −0.34 | −0.60 000 | 0.25 | 0.34 | 0.04 | 0.18 | - | |
| Andrada Variety | ||||||||
| 2019 | NGS | WGS | Chl a | Chl b | Carotenoids | Protein | Wet gluten | |
| NGS | - | 0.54 ** | 0.19 | 0.18 | −0.04 | 0.59 *** | 0.59 *** | |
| WGS | 0.54 ** | - | −0.35 0 | −0.42 0 | −0.10 | 0.00 | 0.14 | |
| Chl a | 0.42 * | 0.22 | - | 0.96 *** | 0.81 *** | 0.64 *** | 0.34 | |
| Chl b | 0.23 | 0.08 | 0.90 *** | - | 0.67 *** | 0.65 *** | 0.36 * | |
| Carotenoids | 0.51 ** | 0.38 * | 0.88 *** | 0.60 *** | - | 0.31 | 0.04 | |
| Protein | 0.09 | 0.17 | −0.03 | 0.19 | −0.21 | - | 0.86 *** | |
| Wet gluten | −0.58 000 | −0.83 000 | −0.07 | 0.09 | −0.25 | 0.20 | - | |
| Codru Variety | ||||||||
| 2019 | NGS | WGS | Chl a | Chl b | Carotenoids | Protein | Wet gluten | |
| NGS | - | 0.67 *** | 0.02 | −0.19 | −0.38 0 | 0.41 * | 0.11 | |
| WGS | 0.92 *** | - | −0.58 000 | −0.69 000 | −0.70 000 | 0.21 | 0.06 | |
| Chl a | −0.42 0 | −0.30 | - | 0.90 *** | 0.52 ** | 0.34 | 0.34 | |
| Chl b | −0.42 0 | −0.38 0 | 0.95 *** | - | 0.74 *** | 0.46 ** | 0.54 ** | |
| Carotenoids | −0.44 0 | −0.21 | 0.93 *** | 0.79 *** | - | 0.19 | 0.29 | |
| Protein | −0.13 | −0.05 | 0.54 ** | 0.66 *** | 0.47 ** | - | 0.84 | |
| Wet gluten | −0.47 00 | −0.60 000 | 0.50 ** | 0.64 *** | 0.30 | 0.12 | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Racz, I.; Hirişcău, D.; Berindean, I.; Kadar, R.; Muntean, E.; Tritean, N.; Russu, F.; Ona, A.; Muntean, L. The Influence of Flag Leaf Removal and Its Characteristics on Main Yield Components and Yield Quality Indices on Wheat. Agronomy 2022, 12, 2545. https://doi.org/10.3390/agronomy12102545
Racz I, Hirişcău D, Berindean I, Kadar R, Muntean E, Tritean N, Russu F, Ona A, Muntean L. The Influence of Flag Leaf Removal and Its Characteristics on Main Yield Components and Yield Quality Indices on Wheat. Agronomy. 2022; 12(10):2545. https://doi.org/10.3390/agronomy12102545
Chicago/Turabian StyleRacz, Ionuṭ, Diana Hirişcău, Ioana Berindean, Rozalia Kadar, Edward Muntean, Nicolae Tritean, Florin Russu, Andreea Ona, and Leon Muntean. 2022. "The Influence of Flag Leaf Removal and Its Characteristics on Main Yield Components and Yield Quality Indices on Wheat" Agronomy 12, no. 10: 2545. https://doi.org/10.3390/agronomy12102545
APA StyleRacz, I., Hirişcău, D., Berindean, I., Kadar, R., Muntean, E., Tritean, N., Russu, F., Ona, A., & Muntean, L. (2022). The Influence of Flag Leaf Removal and Its Characteristics on Main Yield Components and Yield Quality Indices on Wheat. Agronomy, 12(10), 2545. https://doi.org/10.3390/agronomy12102545








