Integrated Management of Chrysodeixis chalcites Esper (Lepidoptera: Noctuidae) Based on Trichogramma achaeae Releases in Commercial Banana Crops in the Canary Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Design
2.2. Ambient Conditions
2.3. Sampling
2.4. Data Analysis
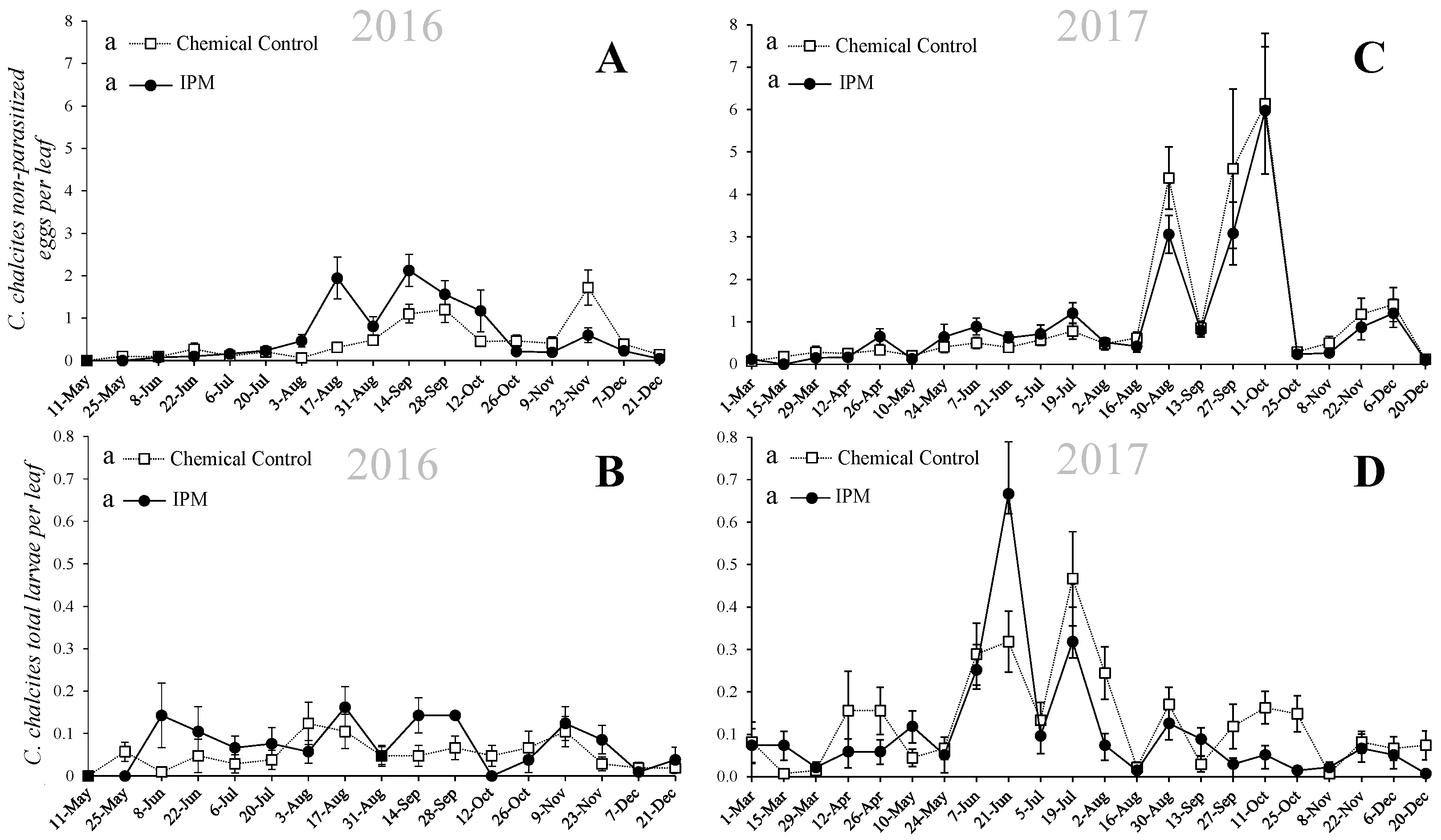
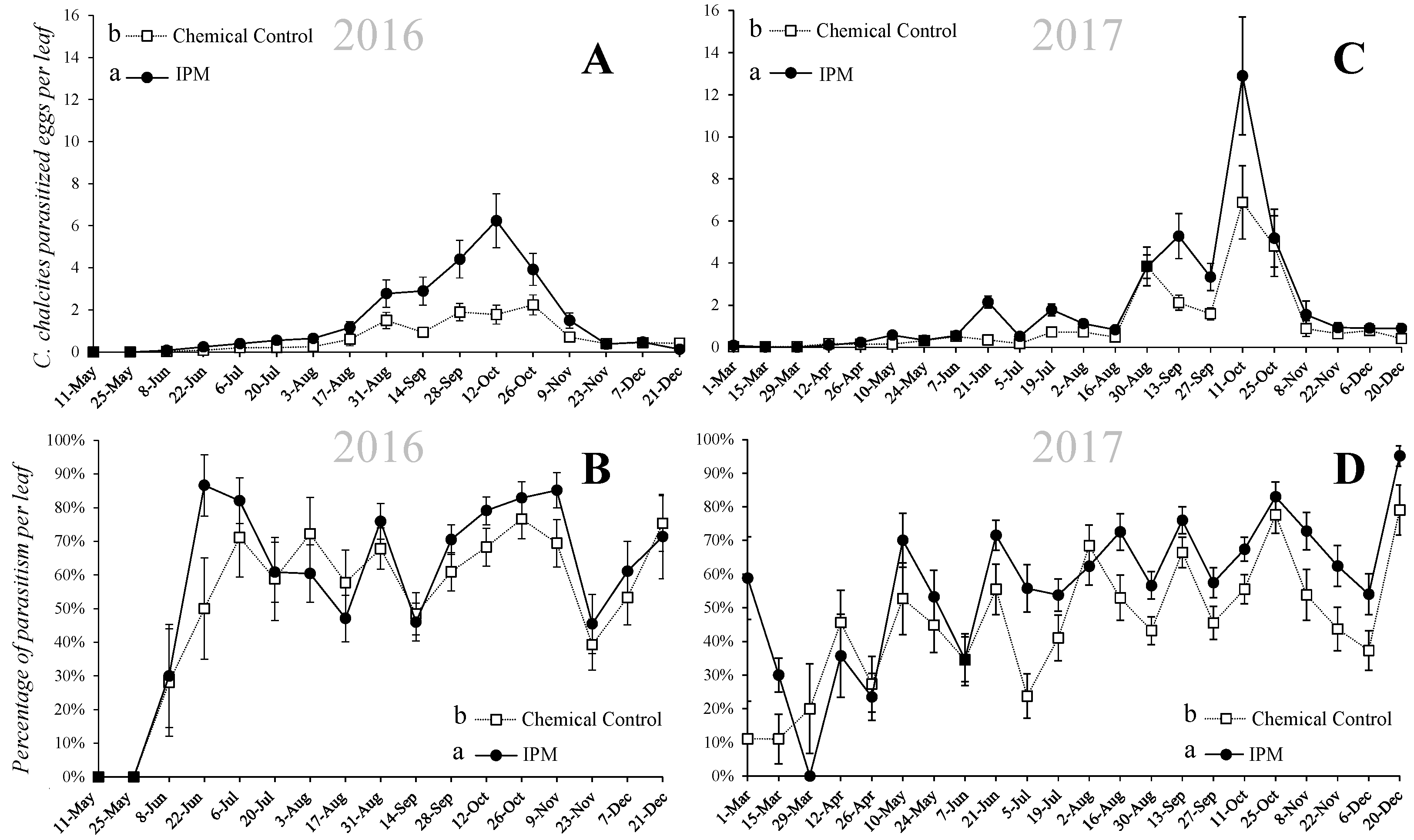
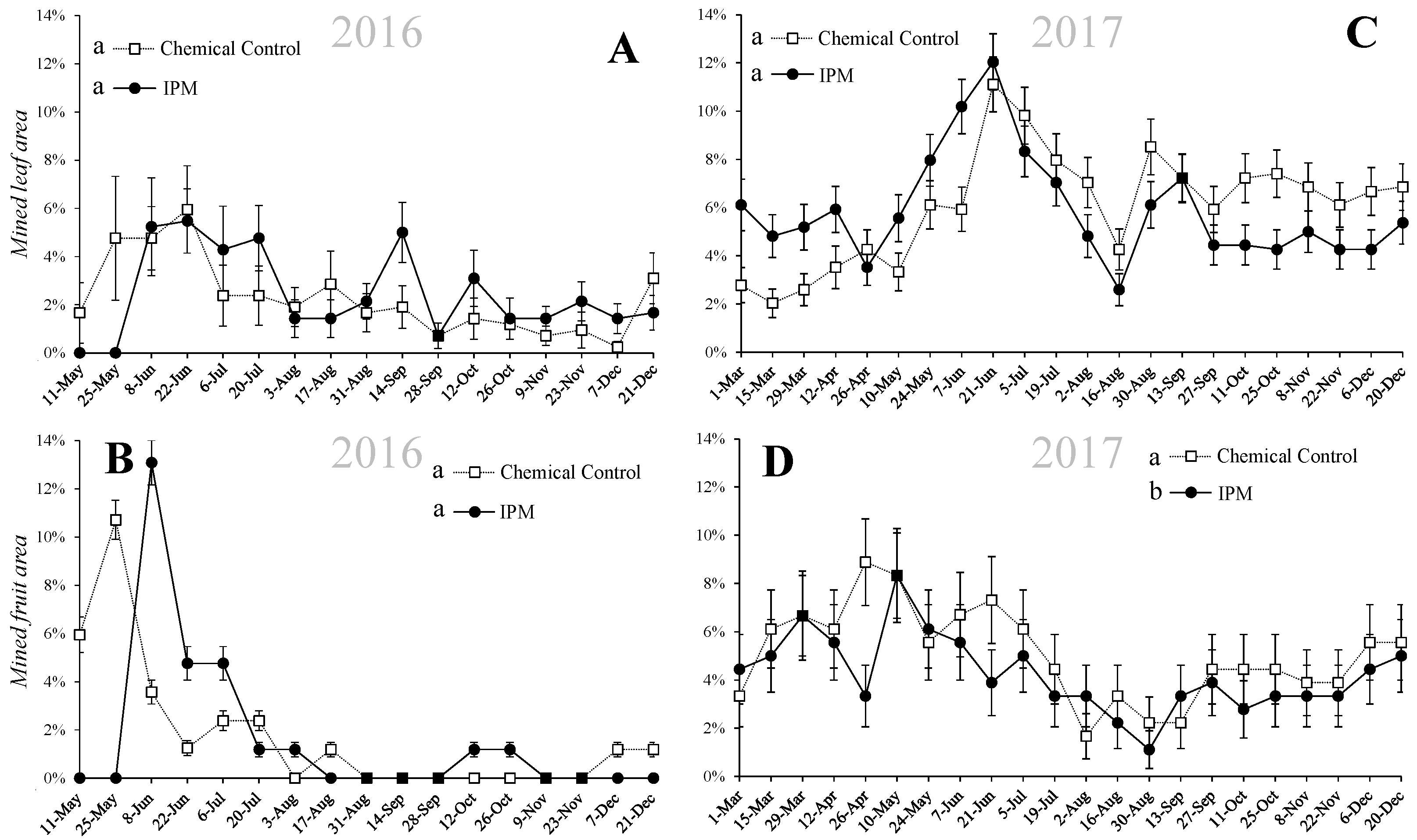
3. Results
3.1. Population Dynamics of Chrysodeixis chalcites
3.2. Incidence of Parasitism
3.3. Leaf and Fruit Damage
3.4. Relative Abundance of Egg Parasitoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data (accessed on 5 September 2022).
- ISTAC. Canarian Institute of Statistics. Available online: http://www.gobiernodecanarias.org/istac/ (accessed on 5 September 2022).
- European Commission. Agriculture and Rural Development, Statistics on Banana Production, Prices and Trade. Available online: https://agriculture.ec.europa.eu/data-and-analysis/markets/overviews/market-observatories/fruit-and-vegetables/bananas-statistics_en (accessed on 5 September 2022).
- Bernal, A.; Williams, T.; Hernández, E.; Carnero, A.; Caballero, P.; Simón, O. A native variant of Chrysodeixis chalcites nucleopolyhedrovirus: The basis for a promising bioinsecticide for control of C. chalcites on Canary Islands’ banana crops. Biol. Control 2013, 67, 101–110. [Google Scholar] [CrossRef][Green Version]
- Fuentes, E.G.; Hernández, E.; Simón, O.; Williams, T.; Caballero, P. Chrysodeixis chalcites, a pest of banana crops on the Canary Islands: Incidence, economic losses and current control measures. Crop Prot. 2018, 108, 137–145. [Google Scholar] [CrossRef]
- Domínguez, E.; López-Cepero, J.; Nogueroles, C. Identificación y control de plagas y enfermedades. In Calidad y Sostenibilidad en el Cultivo de la Platanera en Canarias; Nogueroles, C., Ed.; Asociación de Organizaciones de Productores de Plátanos de Canarias (ASPROCAN): Santa Cruz de Tenerife, Spain, 2012; pp. 145–170. [Google Scholar]
- Del Pino, M.; Carnero, A.; Hernández, E.; Cabello, T. Bases para la gestión integrada de Chrysodeixis chalcites (Lep. Noctuidae) en cultivos de platanera de Canarias. Phytoma 2015, 271, 40–47. [Google Scholar]
- Çakmak, T. Advances in the Integrated Pest Management of the Banana Pest Chrysodeixis chalcites (Esper) in the Canary Islands. Ph.D. Thesis, University of La Laguna, Tenerife, Spain, 2019. [Google Scholar]
- Del Pino, M.; Gallego, J.R.; Hernández, E.; Cabello, T. Effect of Temperature on Life History and Parasitization Behavior of Trichogramma achaeae Nagaraja and Nagarkatti (Hym.: Trichogrammatidae). Insects 2020, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Pimentel, D.; Burgess, M. Environmental and economic costs of the application of pesticides primarily in the United States. In Integrated Pest Management, Pimentel, D., Peshin, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 47–71. [Google Scholar]
- Perera, S.; Molina, M. Plagas y enfermedades en el cultivo ecológico de la platanera. In El Cultivo Ecológico de la Platanera en Canarias, Nogueroles, C., Líbano, J., Eds.; Gabinete de Proyectos Agroecológicos SL: Tenerife, Spain, 2007; pp. 77–118. [Google Scholar]
- Cabrera, J.; Hernández, E.; Cubas, Á.P.; Vega, M.d.C.; Cepero, J. Banana production under Integrated Pest Management and organic production criteria: The Canary Islands case study. Inst. Canar. Investig. Agrar. 2010, 5. Available online: http://www.endure-network.eu/content/download/6427/47396/file/Banana%20Case%20Study%20Guide%20Number%205.pdf (accessed on 5 September 2022).
- Camacho, J. Estudio del Impacto Sobre Cultivo de Platanera de Especies del Orden Lepidóptera. Bachelor Thesis, Universidad de La Laguna, Tenerife, España, 2006. [Google Scholar]
- Del Pino, M.; Cabello, T.; Hernández, E. Biological Control Options for the Golden Twin-Spot Moth, Chrysodeixis chalcites (Esper) (Lepidoptera: Noctuidae) in Banana Crops of the Canary Islands. Insects 2022, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Santana, M. Seguimiento de Chrysodeixis chalcites y Spodoptera littoralis en Platanera en la isla de El Hierro; Búsqueda de Entomófagos y Entomopatógenos. Bachelor’s Thesis, University of La Laguna, Tenerife, Spain, 2007. [Google Scholar]
- Del Pino, M. Biología, Ecología y Control de Chrysodeixis chalcites (Esper, 1789) (Lepidoptera: Noctuidae) en Cultivos de Platanera de Canarias. Ph.D. Thesis, Universidad de La Laguna, Santa Cruz de Tenerife, Spain, 2011. [Google Scholar]
- Cabello, T. Lucha biológica contra Lepidópteros. In La Protección Fitosanitaria en Agricultura Ecológica. Estudio e Identificación de Plagas, Enfermedades, Desórdenes y Organismos Beneficiosos de Hortícolas en Invernadero; FIAPA, Ed.; Fundación para la Investigación Agraria de la Provincia de Almería: Almería, Spain, 2004; pp. 71–97. [Google Scholar]
- Cherif, A.; Mansour, R.; Grissa-Lebdi, K. The egg parasitoids Trichogramma: From laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol. 2021, 31, 661–693. [Google Scholar] [CrossRef]
- Parra, J.R.P. Mass Rearing of Egg Parasitoids for Biological Control Programs. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F.L., Parra, J.R.P., Zucchi, R.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 267–292. [Google Scholar]
- Singhamuni, S.; Hemachandra, K.; Sirisena, U. Potential for mass rearing of the egg parasitoids, Trichogramma chilonis and Tricogramma achaeae (Hymenoptera: Trichogrammatidae) on Corcyra cephalonica eggs. Trop. Agric. Res. 2015, 27, 1–12. [Google Scholar] [CrossRef]
- Polaszek, A.; Rugman-Jones, P.F.; Stouthamer, R.; Hernandez, E.; Cabello, T.; del Pino Pérez, M. Molecular and morphological diagnoses of five species of Trichogramma: Biological control agents of Chrysodeixis chalcites (Lepidoptera: Noctuidae) and Tuta absoluta (Lepidoptera: Gelechiidae) in the Canary Islands. BioControl 2012, 57, 21–35. [Google Scholar] [CrossRef]
- Del Pino, M.; Rugman-Jones, P.; Hernández, E.; Polaszek, A.; Stouthamer, R. Rapid molecular identification of five species of Trichogramma occurring in the Canary Islands with notes on their distribution in banana groves. BioControl 2013, 58, 515–524. [Google Scholar] [CrossRef]
- MAPA. Ministry of Agriculture, Fisheries and Food. Government of Spain. Available online: https://www.mapa.gob.es/es/ (accessed on 30 October 2022).
- Martín, A.; González, A. Guía de Gestión Integrada de Plagas: Platanera; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente (MAPAMA): Madrid, Spain, 2016; p. 104. [Google Scholar]
- Cabello, T.; Gallego, J.; Vila, E.; Soler, A.; Del Pino, M.; Carnero, A.; Hernández, E.; Polaszek, A. Biological control of the South American tomato pinworm, Tuta absoluta (Lep.: Gelechiidae), with releases of Trichogramma achaeae (Hym.: Trichogrammatidae) in tomato greenhouses of Spain. IOBC/WPRS Bull. 2009, 49, 225–230. [Google Scholar]
- Vila, E.; Parra, A.; Gallego, J.; Fernández, F.; Cabello, T. Biological control of the South American Tomato Pinworm, Tuta absoluta (Lep,: Gelechiidae) with releases of Trichogramma achaeae (Hym. Trichogrammatidae) on different tomato crop cycles in Spain. In Proceedings of the IXth European congress of entomology, Budapest, Hungary, 22–27 August 2010. [Google Scholar]
- Del Pino, M.; Carnero, A.; Hernández, E. Control biológico de la lagarta de la platanera Chrysodeixis chalcites (Esper) en Canarias. In Proceedings of the I Jornadas de Transferencia de I+D+i Para una Producción Sostenible del Plátano en las RUPs, Tenerife, Spain, 18–20 October 2010. [Google Scholar]
- Del pino, M.; Carnero, A.; Hernández, E. Curvas de vuelo de Chrysodeixis chalcites en Cultivos de Platanera de Canarias; Tecnical Report MAC/1/C054-BIOMUSA; Instituto Canario de Investigaciones Agrarias: Tenerife, Spain, 2012; p. 10. [Google Scholar]
- Messelink, G.; Maanen, R.; van Steenpaal, S.; Janssen, A. Biological control of thrips and whiteflies by a shared predator: Two pests are better than one. Biol. Control 2008, 44, 372–379. [Google Scholar] [CrossRef]
- Calvo, F.; Torres-Ruiz, A.; Velázquez-González, J.; Rodríguez-Leyva, E.; Lomeli-Flores, J. Evaluation of Dicyphus hesperus for biological control of sweet potato whitefly and potato psyllid on greenhouse tomato. BioControl 2016, 61, 415–424. [Google Scholar] [CrossRef]
- Townsend, G.; Heuberger, J. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Rep. 1943, 27, 340–343. [Google Scholar]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, J.; Frandon, J.; Ferran, A.; Marconi, A.; Migliore, O.; Bezert, J. Study of the dispersion of Trichogramma evanescens Westwood in protected tomato crops. In Proceedings of the Colloque International Tomate Sous Abri, Protection Intégrée-Agriculture Biologique, Avignon, France, 17–19 September 2003. [Google Scholar]
- Suverkropp, B.; Bigler, F.; van Lenteren, J. Dispersal behaviour of Trichogramma brassicae in maize fields. Bull. Insectol. 2009, 62, 113–120. [Google Scholar]
- Shashi; Kottala, S.Y.; Singh, R. A review of sustainability, deterrents, personal values, attitudes and purchase intentions in the organic food supply chain. Pac. Sci. Rev. B Humanit. Soc. Sci. 2015, 1, 114–123. [Google Scholar] [CrossRef]
- Ott, S.L.; Huang, C.L.; Misra, S.K. Consumers’ Perceptions of Risks from Pesticide Residues and Demand for Certification of Residue-Free Produce. In Economics of Food Safety; Caswell, J.A., Ed.; Springer: Dordrecht, The Netherlands, 1991; pp. 175–188. [Google Scholar]
- Lorenzo, J.M. Seguimiento de la dinámica poblacional de Dysmicoccus grassii (Leonardi) (Homoptera: Pseudococcidae) y Tetranichus urticae Koch (Acari: Tetranychidae) en Musa acuminata Colla. Subgrupo Cavendish, cvs. Pequeña Enana y Gran enana, al Aire Libre y en Invernadero Respectivamente. Ph.D. Thesis, University of La Laguna, Tenerife, Spain, 2005. [Google Scholar]
- Thubru, D.P.; Firake, D.M.; Behere, G.T. Assessing risks of pesticides targeting lepidopteran pests in cruciferous ecosystems to eggs parasitoid, Trichogramma brassicae (Bezdenko). Saudi J. Biol. Sci. 2018, 25, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lian, M.; Wang, J.; Qin, S. Toxicity and safety evaluation of five insecticides on egg parasitoid, Trichogramma evanescens Westwood. Chin. J. Eco-Agric. 2009, 17, 715–720. [Google Scholar] [CrossRef]
- Fontes, J.; Roja, I.S.; Tavares, J.; Oliveira, L. Lethal and sublethal effects of various pesticides on Trichogramma achaeae (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 2018, 111, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Amaro, J.T.; Bueno, A.F.; Pomari-Fernandes, A.F.; Neves, P.M.O.J. Selectivity of Organic Products to Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Neotrop. Entomol. 2015, 44, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Toscano, L.; Calado Filho, G.; Cardoso, A.; Maruyama, W.; Tomquelski, G. Impact of insecticides on Spodoptera frugiperda (Lepidoptera, Noctuidae) and its natural enemies on off-season maize in Cassilândia and Chapadão do Sul, State of Mato Grosso do Sul, Brazil. Arq. Inst. Biológico 2012, 79, 223–231. [Google Scholar] [CrossRef][Green Version]
- Prischmann, D.A.; James, D.G.; Wright, L.C.; Teneyck, R.D.; Snyder, W.E. Effects of chlorpyrifos and sulfur on spider mites (Acari: Tetranychidae) and their natural enemies. Biol. Control 2005, 33, 324–334. [Google Scholar] [CrossRef]
- Maia, J.B.; Carvalho, G.A.; Medina, P.; Garzón, A.; Gontijo, P.d.C.; Viñuela, E. Lethal and sublethal effects of pesticides on Chrysoperla carnea larvae (Neuroptera: Chrysopidae) and the influence of rainfastness in their degradation pattern over time. Ecotoxicology 2016, 25, 845–855. [Google Scholar] [CrossRef]
- Ruberson, J.; Nemoto, H.; Hirose, Y. Pesticides and conservation of natural enemies in pest management. In Conservation Biological Control; Barbosa, P., Ed.; Academic Press: San Diego, CA, USA, 1998; pp. 207–220. [Google Scholar]
- Studebaker, G.; Kring, T. Lethal and sub-lethal effects of selected insecticides on Orius insidiosus. In Proceedings of the Beltwide Cotton Conferences, Orlando, FL, USA, 3–7 January 1999. [Google Scholar]
- Hewa-Kapuge, S.; McDougall, S.; Hoffmann, A.A. Effects of Methoxyfenozide, Indoxacarb, and Other Insecticides on the Beneficial Egg Parasitoid Trichogramma nr. brassicae (Hymenoptera: Trichogrammatidae) Under Laboratory and Field Conditions. J. Econ. Entomol. 2003, 96, 1083–1090. [Google Scholar] [CrossRef]
- Liu, T.-X.; Zhang, Y. Side effects of two reduced-risk insecticides, indoxacarb and spinosad, on two species of Trichogramma (Hymenoptera: Trichogrammatidae) on cabbage. Ecotoxicology 2012, 21, 2254–2263. [Google Scholar] [CrossRef]
- Gallego, J.R.; Guerrero-Manzano, J.; Fernández-Maldonado, F.J.; Cabello, T. Susceptibility of the egg parasitoid Trichogramma achaeae (Hymenoptera: Trichogrammatidae) to selected insecticides used in tomato greenhouses. Span. J. Agric. Res. 2019, 17, e1009. [Google Scholar] [CrossRef]
- Chen, Y.; Bird, L.; Woolley, L.; Walsh, T.; Gordon, K.; Herron, G. Linkage mapping an indoxacarb resistance locus in Helicoverpa armigera (Lepidoptera: Noctuidae) by genotype-by-sequencing. Pest Manag. Sci. 2020, 76, 617–627. [Google Scholar] [CrossRef]
- Pang, S.; You, W.; Duan, L.; Song, X.; Li, X.; Wang, C. Resistance selection and mechanisms of oriental tobacco budworm (Helicoverpa assulta Guenee) to indoxacarb. Pestic. Biochem. Physiol. 2012, 103, 219–223. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Zhang, Y.; Liao, X. A systemic study of indoxacarb resistance in Spodoptera litura revealed complex expression profiles and regulatory mechanism. Sci. Rep. 2019, 9, 14997. [Google Scholar] [CrossRef] [PubMed]
- Mills, N. Egg parasitoids in biological control and integrated pest management. In Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma; Consoli, F., Parra, J., Zucchi, R., Eds.; Springer: Dordrecht, The Netherland, 2009; Volume 9, pp. 389–411. [Google Scholar]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Keçeci, M.; Öztop, A. Possibilities for biological control of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) in the western Mediterranean Region of Turkey. Turk. J. Entomol. 2017, 41, 219–230. [Google Scholar] [CrossRef][Green Version]
| 2016 Trials | 2017 Trials | |||||
|---|---|---|---|---|---|---|
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | |
| Municipality | Arona | San Miguel | Adeje | Arona | Arona | San Miguel |
| Latitude | 28°1′25.00″ N | 28°2′15.19″ N | 28°6′7.27″ N | 28°1′25.00″ N | 28°2′15.81″ N | 28°3′4.32″ N |
| Longitude | 16°40′45.08″ W | 16°37′54.13″ W | 16°44′50.31″ W | 16°40′45.08″ W | 16°38′58.14″ W | 16°37′55.84″ W |
| Altitude | 40 MASL | 99 MASL | 31 MASL | 40 MASL | 85 MASL | 158 MASL |
| Crop Age | >10 years | >10 years | >10 years | >10 years | >10 years | >10 years |
| Variety | Grand Nain | Gruesa Palmera | Gruesa Palmera | Grand Nain | Gruesa Palmera | Grand Nain |
| Chemical C | ||||||
| Size (m2) | 1895 | 11,400 | 4600 | 1895 | 5850 | 2000 |
| Plants | 379 | 2111 | 767 | 379 | 936 | 360 |
| IPM | ||||||
| Size (m2) | 1300 | 5700 | 4000 | 1300 | 3750 | 3000 |
| Plants | 260 | 1056 | 733 | 260 | 600 | 540 |
| Total Size | 3195 | 17,100 | 9000 | 3195 | 9600 | 5000 |
| Total Plants | 639 | 3167 | 1500 | 639 | 1536 | 900 |
| No Trap Catches in Pheromone Trap | Trap Catches in Pheromone Traps > 0 | ||
|---|---|---|---|
| No new pest damage & <10% defoliation | 10 wasps/m2 × week | No new pest damage & <10% defoliation | 75 wasps/m2 × week |
| <10% defoliation with larvae on crop | 75 wasps/m2 × week | <10% defoliation with larvae on crop | 75 wasps/m2 × week |
| ≥10% defoliation with larvae on crop | Selective pesticide | ≥10% defoliation with larvae on crop | Selective pesticide |
| Fresh fruit damage and/or larvae on fruits | Selective pesticide | Fresh fruit damage and/or larvae on fruits | Selective pesticide |
| Dates | Replicate 1 | Replicate 2 | Replicate 3 | |||
|---|---|---|---|---|---|---|
| IPM | Chemical C. | IPM | Chemical C. | IPM | Chemical C. | |
| 08 June | 75 i/m2 | IND | 75 i/m2 | IND | 75 i/m2 | |
| 22 June | 75 i/m2 | CLP | 75 i/m2 | 75 i/m2 | ||
| 06 July | 75 i/m2 | |||||
| 20 July | 75 i/m2 | |||||
| 03 August | 75 i/m2 | CLP | 75 i/m2 | |||
| 17 August | 75 i/m2 | 75 i/m2 | 75 i/m2 | |||
| 31 August | 75 i/m2 | 75 i/m2 | ||||
| 14 September | 75 i/m2 | |||||
| 28 September | 75 i/m2 | 75 i/m2 | 75 i/m2 | |||
| 12 October | 75 i/m2 | |||||
| 26 October | 75 i/m2 | 75 i/m2 | 75 i/m2 | |||
| 09 November | 75 i/m2 | 75 i/m2 | 75 i/m2 | |||
| 23 November | 75 i/m2 | 75 i/m2 | 75 i/m2 | |||
| Total | 750 i/m2 (258 i/m2) | 750 i/m2 (195 i/m2) | 675 i/m2 (193 i/m2) | |||
| Dates | Replicate 1 | Replicate 2 | Replicate 3 | |||
|---|---|---|---|---|---|---|
| IPM | Chemical C. | IPM | Chemical C. | IPM | Chemical C. | |
| 24 May | Bt | Bt | ||||
| 21 June | ||||||
| 19 July | Bt | Bt, IND | ||||
| 27 September | Bt | Bt | ||||
| 11 October | IND | |||||
| 08 November | CLP | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionisio, M.A.; Calvo, F.J. Integrated Management of Chrysodeixis chalcites Esper (Lepidoptera: Noctuidae) Based on Trichogramma achaeae Releases in Commercial Banana Crops in the Canary Islands. Agronomy 2022, 12, 2982. https://doi.org/10.3390/agronomy12122982
Dionisio MA, Calvo FJ. Integrated Management of Chrysodeixis chalcites Esper (Lepidoptera: Noctuidae) Based on Trichogramma achaeae Releases in Commercial Banana Crops in the Canary Islands. Agronomy. 2022; 12(12):2982. https://doi.org/10.3390/agronomy12122982
Chicago/Turabian StyleDionisio, Miguel A., and Francisco J. Calvo. 2022. "Integrated Management of Chrysodeixis chalcites Esper (Lepidoptera: Noctuidae) Based on Trichogramma achaeae Releases in Commercial Banana Crops in the Canary Islands" Agronomy 12, no. 12: 2982. https://doi.org/10.3390/agronomy12122982
APA StyleDionisio, M. A., & Calvo, F. J. (2022). Integrated Management of Chrysodeixis chalcites Esper (Lepidoptera: Noctuidae) Based on Trichogramma achaeae Releases in Commercial Banana Crops in the Canary Islands. Agronomy, 12(12), 2982. https://doi.org/10.3390/agronomy12122982





