SSR Genotypes of the Puccinia triticina in 15 Provinces of China Indicate Regional Migration in One Season from East to West and South to North
Abstract
1. Introduction
2. Materials and Methods
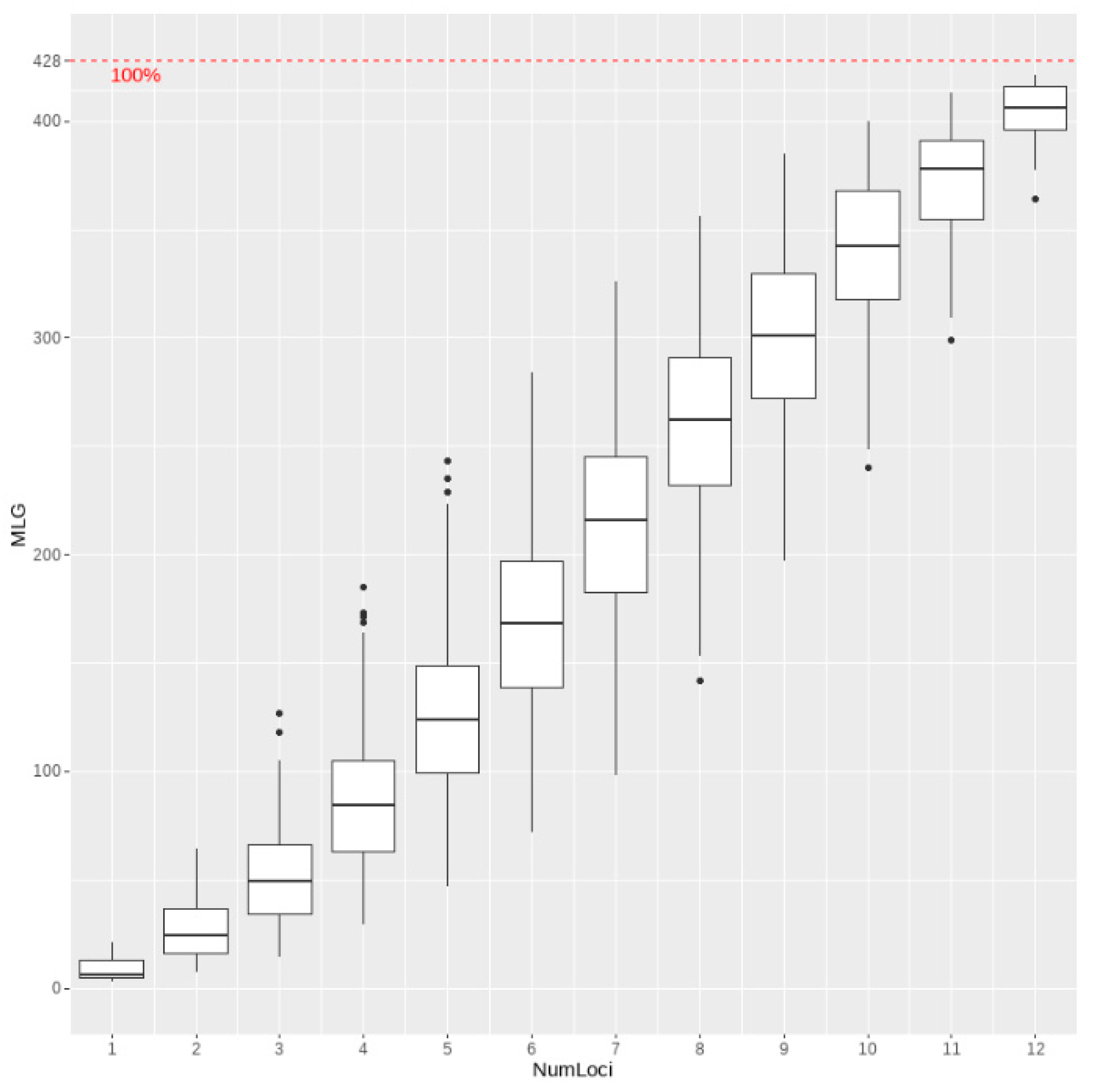
3. Results
4. Discussion
4.1. Population Genetic Structure of Pt Collections
4.2. Prediction of Pathogen Movement between Different Regions in China
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Province | City | Number | Longitude | Latitude |
|---|---|---|---|---|
| Beijing | Fangshan district | 21 | E115°58′ | N39°40′ |
| Hebei | Baoding | 48 | E115°37′ | N39°22′ |
| Zhangjiakou | E115°21′ | N41°20′ | ||
| Cangzhou | E116°57′ | N37°87′ | ||
| Handan | E114°53′ | N36°64′ | ||
| Xingtai | E114°60′ | N36°46′ | ||
| Shanxi | Yuncheng | 25 | E111°16′ | N35°19′ |
| Yunnan | Lincang | 56 | E100°08′ | N23°88′ |
| Jiangsu | Zhenjiang | 65 | E119°18′ | N34°58′ |
| Xuzhou | E117°32′ | N34°39′ | ||
| Gansu | Pingliang | 50 | E106°66′ | N35°54′ |
| Tianshui | E105°17′ | N34°45′ | ||
| Dingxi | E103°86′ | N35°37′ | ||
| Qinghai | Xining | 30 | E101°44′ | N36°43′ |
| Sichuan | Guangyuan | 14 | E105°23′ | N31°46′ |
| Mianyang | E105°06′ | N31°07′ | ||
| Hubei | Xiangyang | 51 | E111°83′ | N31°77′ |
| Shiyan | E110°99′ | N32°95′ | ||
| Suizhou | E113°38′ | N31°69′ | ||
| Shannxi | Ankang | 62 | E109°20′ | N32°44′ |
| Xianyang | E108°70′ | N34°32′ | ||
| Baoji | E107°40′ | N34°12′ | ||
| Weinan | E110°14′ | N35°19′ | ||
| Anhui | Lu’an | 80 | E116°52′ | N31°73′ |
| Anqing | E117°13′ | N31°28′ | ||
| Suzhou | E116°94′ | N33°64 | ||
| Hefei | E117°22′ | N31°82‘ | ||
| Fuyang | E115°81′ | N32°88′ | ||
| Bozhou | E115°97′ | N33°53′ | ||
| Huibei | E116°64′ | N34°02′ | ||
| Shangdong | Linyi | 66 | E118°24′ | N35°27′ |
| Zibo | E117°86′ | N35°85′ | ||
| Liaocheng | E116°26′ | N36°55′ | ||
| Rizhao | E119°22′ | N36°88′ | ||
| Jinan | E117°05′ | N36°84′ | ||
| Weifang | E119°04′ | N36°73′ | ||
| Zaozhuang | E117°54′ | N34°73′ | ||
| Dezhou | E116°26′ | N37°08′ | ||
| Henan | Anyang | 71 | E113°44′ | N36°05′ |
| Zhengzhou | E113°12′ | N34°04′ | ||
| Lingbao | E110°45′ | N34°21′ | ||
| Nanyang | E112°52′ | N32°99′ | ||
| Xinyang | E114°09′ | N32°14′ | ||
| Pingdingshan | E113°19′ | N33°76′ | ||
| Sanmenxia | E111°07′ | N34°44′ | ||
| Zhoukou | E114°41′ | N 33°97′ | ||
| Shangqiu | E116°53′ | N 33°98′ | ||
| Xinxiang | E113°92′ | N 35°08′ | ||
| Heilongjiang | Qiqihar | 32 | E126°26′ | N48°30′ |
| Inner Mongolia | Bayannur | 38 | E107°23′ | N40°44′ |
| Total | 709 |
| Level | Df | Sum Sq | Mean Sq | Est. Var | Variation (%) | p Value |
|---|---|---|---|---|---|---|
| Among populations | 14 | 237.05 | 16.932 | 0.186 | 5.02 | 0.001 |
| Among individuals | 607 | 1062.82 | 1.751 | 0.000 | 0.00 | 0.001 |
| Within individuals | 622 | 2185.00 | 3.513 | 3.513 | 94.98 | 0.001 |
| Total | 1243 | 3484.87 | / | 3.698 | 100.00 |
| NO. | Beijing MLG = 11 | Hebei MLG = 30 | Shanxi MLG = 14 | Shaanxi MLG = 46 | Anhui MLG = 48 | Shandong MLG = 37 | Henan MLG = 50 | Heilongjiang MLG = 27 | Jangsu MLG = 51 | Hubei MLG = 38 | Yunnan MLG = 31 | Gansu MLG = 42 | Qinghai MLG = 21 | Sichuan MLG = 8 | Inner Mongolia MLG = 37 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLG.15 | 1 | 6 | |||||||||||||
| MLG.16 | 2 | 5 | 2 | ||||||||||||
| MLG.18 | 2 | 1 | |||||||||||||
| MLG.32 | 1 | 1 | |||||||||||||
| MLG.71 | 1 | 2 | |||||||||||||
| MLG.75 | 2 | 1 | |||||||||||||
| MLG.139 | 1 | 2 | |||||||||||||
| MLG.156 | 1 | 1 | |||||||||||||
| MLG.167 | 1 | 1 | |||||||||||||
| MLG.179 | 1 | 2 | 4 | 1 | 1 | ||||||||||
| MLG.183 | 1 | 2 | 1 | ||||||||||||
| MLG.192 | 1 | 1 | |||||||||||||
| MLG.193 | 2 | 1 | 1 | ||||||||||||
| MLG.196 | 3 | 2 | 3 | 1 | |||||||||||
| MLG.200 | 1 | 1 | |||||||||||||
| MLG.206 | 2 | 1 | 3 | 7 | 3 | 1 | |||||||||
| MLG.207 | 1 | 1 | |||||||||||||
| MLG.213 | 3 | 1 | |||||||||||||
| MLG.220 | 1 | 4 | 1 | 1 | 1 | ||||||||||
| MLG.237 | 3 | 1 | 1 | ||||||||||||
| MLG.242 | 1 | 1 | |||||||||||||
| MLG.243 | 1 | 1 | |||||||||||||
| MLG.281 | 1 | 1 | |||||||||||||
| MLG.282 | 4 | 2 | |||||||||||||
| MLG.283 | 2 | 1 | |||||||||||||
| MLG.284 | 1 | 8 | 4 | 5 | 1 | ||||||||||
| MLG.288 | 4 | 1 | 2 | ||||||||||||
| MLG.312 | 1 | 1 | |||||||||||||
| MLG.317 | 1 | 1 | |||||||||||||
| MLG.319 | 1 | 1 | |||||||||||||
| MLG.321 | 2 | 1 | |||||||||||||
| MLG.359 | 2 | 2 | 1 | ||||||||||||
| MLG.360 | 2 | 1 | 1 | 1 | |||||||||||
| MLG.383 | 1 | 1 | |||||||||||||
| MLG.384 | 1 | 1 | |||||||||||||
| MLG.394 | 2 | 1 | |||||||||||||
| MLG.488 | 1 | 2 | 1 | 1 | 1 | ||||||||||
| No. of shared MLGs | 5 | 10 | 7 | 8 | 14 | 14 | 12 | 6 | 5 | 7 | 5 | 8 | 0 | 0 | 0 |
| No. of samples of shared MLGs | 10 | 13 | 10 | 8 | 36 | 36 | 24 | 7 | 8 | 7 | 5 | 9 | 0 | 0 | 0 |
| Proportion of shared MLGs/% | 90.9 | 43.3 | 71.4 | 17.4 | 75.0 | 97.3 | 48.0 | 25.9 | 15.7 | 18.4 | 16.1 | 21.4 | 0.0 | 0.0 | 0.0 |
References
- Jin, S.B. Chinese Wheat Science; China Agriculture Press: Beijing, China, 1996. (In Chinese) [Google Scholar]
- Lu, Y.J.; Li, X.; Jiang, N. Development and trend analysis of world wheat industry in 2017. China Mark. 2017, 26, 63–65. (In Chinese) [Google Scholar]
- Peng, S.Y. Comprehensive control technology of wheat leaf rust. Mod. Agric. 2013, 2, 30–31. (In Chinese) [Google Scholar]
- Huerta-Espino, J.; Germán, S.; Singh, R.P.; Mccallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, L.; Zhang, M.Y.; Yan, H.F.; Liu, D.Q. Development of specific molecular markers in Puccinia triticina. J. Agric. Univ. Hebei 2016, 39, 63–67. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, M.Y.; Meng, Q.F.; Zhang, L.; Gao, Y.; Yan, H.F.; Liu, D.Q. Analysis of genetic diversity of Puccinia triticina isolated from different wheat cultivars. J. Henan Agric. Sci. 2018, 47, 77–81. (In Chinese) [Google Scholar] [CrossRef]
- Chen, W.Q. The Occurrence and Control of Wheat Rust; China Agriculture Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused by Puccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Ordoñez, M.E.; Manisterski, J.; Anikster, Y. Genetic ifferentiation of Puccinia triticina opulations in Central Asia and the Caucasus. Phytopathology 2007, 97, 1141–1149. [Google Scholar] [CrossRef][Green Version]
- Xu, M.Q. Genetic Diversity of Puccinia triticina by SSR in Some Regions of China Hebei Agricultural University. Master’s Thesis, Hebei Agricultural University, Hebei, China, 2012. [Google Scholar]
- Ashley, M.V.; Dow, B.D. The use of microsatellite analysis in population biology: Background, methods and potential applications. EXS 1994, 69, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Enjalbert, J.; Vautrin, D.; Solignac, M.; Giraud, T. Isolation of 12 microsatellite loci, using an enrichment protocol, in the phytopathogenic fungus Puccinia triticina. Mol. Ecol. Notes 2003, 3, 65–67. [Google Scholar] [CrossRef]
- Szabo, L.J.; Kolmer, J.A. Development of simple sequence repeat markers for the plant pathogenic rust fungus Puccinia triticina. Mol. Ecol. Notes 2006, 7, 92–94. [Google Scholar] [CrossRef]
- Ordonez, M.E.; Kolmer, J.A. Simple sequence repeat diversity of a worldwide collection of Puccinia triticina from durum wheat. Phytopathology 2007, 97, 574–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolmer, J.A.; OrdoñEz, M.E.; Manisterski, J.; Anikster, Y. Genetic differentiation of Puccinia triticina populations in the Middle East and genetic similarity with populations in Central Asia. Phytopathology 2011, 101, 870–877. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Hanzalova, A.; Goyeau, H.; Bayles, R.; Morgounov, A. Genetic differentiation of the wheat leaf rust fungus Puccinia triticina in Europe. Plant Pathol. 2013, 62, 21–31. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Meng, Q.; Liu, D. Genetic Diversity of Puccinia triticina by SSR in Some Regions of China. J. Agric. Biotechnol. 2013, 21, 89–96. (In Chinese) [Google Scholar]
- Kolmer, J.A. Collections of Puccinia triticina in different provinces of China are highly related for virulence and molecular genotype. Phytopathology 2015, 105, 700–706. [Google Scholar] [CrossRef][Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, 281. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Reynolds, J.F.; Quartet, L.; Reynolds, J.F. Statistical Ecology: A Primer on Methods and Computing; John Wiley & Sons: Hoboken, NJ, USA, 1989; Volume 4, p. 38. [Google Scholar] [CrossRef]
- Grünwald, N.J.; Goodwin, S.B.; Milgroom, M.G.; Fry, W.E. Analysis of genotypic diversity data for populations of microorganisms. Phytopathology 2003, 93, 738–746. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Stoddart, J.A.; Taylor, J.F. Genotypic diversity: Estimation and prediction in samples. Genetics 1988, 118, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Belcher, P.R. Measurement of myocardial contractility. J. Cardiothorac. Vasc. Anesth. 1997, 11, 812. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- François, B.; Sébastien, D.; Thibaut, J. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Rosenberg, N.A. Distruct: A program for the graphical display of population structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, G.; Yang, W.; Liu, D. Analysis of the Virulence and Molecular Polymorphism of Puccinia recondita f.sp. tritici. J. Agric. Biotechnol. 2002, 10, 41–45. [Google Scholar]
- Whitlock, M.C.; Mccauley, D.E. Indirect measures of gene flow and migration: FST≠1/(4Nm+1). Heredity 1999, 82, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wu, X.H.; Cui, G.H.; Wang, X.B.; Yu, M.L. Present situation and existing problems of wheat production in Inner Mongolia. Inn. Mong. Agric. Sci. Technol. 2000, S1, 139–140. (In Chinese) [Google Scholar]
- Wu, X.L. Analysis of climatic conditions for wheat growth in Inner Mongolia. Beijing Agric. 2014, 33, 39–40. [Google Scholar]
- Ran, Y. Review of wheat rust control in Yunnan Province. Yunnan Agric. Sci. Technol. 1993, 6, 5–9. (In Chinese) [Google Scholar]



| Locus | Repeat Motif | Primer Sequences (5′-3′) | Tm (°C) | Florescence | Product Sizes (bp) |
|---|---|---|---|---|---|
| RB1 | (GT)5 | TTGTCGTTCTGGAATGATGC TGCCCACAACCCTCCTC | 56 | FAM | 126–134 |
| RB25 | (AT)4 + (GT)7 | ATGTCTGTAGTCGGCAGGGC GCCTCTGCGGGATCGGT | 58 | ROX | 226–228 |
| RB35 | (AC)9 + (TA)5 + (AG)5 | ACTGCGATATCCAGTACACACAC TGATGGGCTCGCAGTGG | 62 | FAM | 244–248 |
| RB29 | (CA)15 | CTCACCAAACATCAAGCACC GAGCCTAGCATCAGCATCC | 60 | HEX | 118–180 |
| RB4 | (GT)8 | CAGTATTGTGGTGGTTGGATG ACTCAAGAATAATGGGGAACAC | 62 | ROX | 230–244 |
| RB17 | (TGC)5 + (TGG)6 | CTTCGGTAGGATTTCGAGCG CAGCTCCAAATCCTTTGCC | 60 | FAM | 89–92 |
| RB8 | (TGG)7 | CGCCGTTCCCATCGTTC TAAAACACTCCACCCACGCC | 64 | HEX | 138–147 |
| RB11 | (CA)17 | AGCAGTGAGCAGCAGCGTC ACTACTGTGAGTGTCGGCTTGG | 58 | ROX | 178–208 |
| RB26 | (CT)8+6 | TCGTCCTGCCTACCTCTGAC AAAGTGCATGATCTGCATGTG | 58 | FAM | 340–346 |
| RB27 | (CA)4+3 | CTATCGAGTCCAGAACCGAAC CAAGCCAAGACCTGAGCTATC | 60 | HEX | 170 |
| RB12 | (AG)5+3 | CCACAAGCAACCACATACCACC TGGTCCATGAAGAAGTCTCTGAAC | 60 | ROX | 288–298 |
| RB10 | (GT)7+4+4 | TAAGATTGGTGGTATGTGGTGGA TTGTCTTTCATCTCATCCAGCC | 53 | FAM | 218 |
| RB28 | (TGG)5 | CATCTGGCTGGTGAGGTCGC GAAGCCCGCCGAGCAGC | 58 | HEX | 315–318 |
| Locus | Allele | 1-D | Hexp | Evenness |
|---|---|---|---|---|
| RB1 | 3.000 | 0.495 | 0.495 | 0.959 |
| RB25 | 3.000 | 0.076 | 0.076 | 0.435 |
| RB35 | 3.000 | 0.530 | 0.530 | 0.829 |
| RB29 | 13.000 | 0.729 | 0.729 | 0.708 |
| RB4 | 6.000 | 0.560 | 0.560 | 0.711 |
| RB17 | 4.000 | 0.215 | 0.215 | 0.591 |
| RB8 | 5.000 | 0.508 | 0.509 | 0.856 |
| RB11 | 6.000 | 0.651 | 0.652 | 0.895 |
| RB26 | 2.000 | 0.113 | 0.113 | 0.499 |
| RB27 | 6.000 | 0.154 | 0.154 | 0.448 |
| RB12 | 2.000 | 0.498 | 0.499 | 0.997 |
| RB10 | 12.000 | 0.600 | 0.601 | 0.714 |
| RB28 | 3.000 | 0.231 | 0.231 | 0.505 |
| mean | 5.231 | 0.412 | 0.413 | 0.704 |
| Province | N | MLG | eMLG | H | G | lambda | Corrected Lambda | E.5 |
|---|---|---|---|---|---|---|---|---|
| Beijing | 18 | 11 | 7.65 | 2.29 | 9.00 | 0.889 | 0.909 | 0.898 |
| Hebei | 35 | 30 | 9.59 | 3.38 | 27.00 | 0.963 | 0.964 | 0.920 |
| Shanxi | 20 | 14 | 8.32 | 2.53 | 11.11 | 0.910 | 0.909 | 0.878 |
| Shaanxi | 48 | 46 | 9.92 | 3.81 | 44.31 | 0.977 | 0.974 | 0.977 |
| Anhui | 75 | 48 | 9.12 | 3.64 | 28.27 | 0.965 | 0.974 | 0.734 |
| Shandong | 61 | 37 | 8.72 | 3.33 | 20.33 | 0.951 | 0.955 | 0.718 |
| Henan | 63 | 50 | 9.53 | 3.80 | 37.09 | 0.973 | 0.976 | 0.830 |
| Heilongjiang | 28 | 27 | 9.88 | 3.28 | 26.13 | 0.962 | 0.955 | 0.980 |
| Jiangsu | 59 | 51 | 9.77 | 3.88 | 45.21 | 0.978 | 0.980 | 0.932 |
| Hubei | 42 | 38 | 9.67 | 3.57 | 31.50 | 0.968 | 0.971 | 0.881 |
| Yunnan | 54 | 31 | 7.64 | 2.92 | 9.11 | 0.890 | 0.966 | 0.464 |
| Gansu | 45 | 42 | 9.86 | 3.71 | 39.71 | 0.975 | 0.971 | 0.967 |
| Qinghai | 29 | 21 | 8.83 | 2.92 | 15.87 | 0.937 | 0.952 | 0.848 |
| Sichuan | 8 | 8 | 8.00 | 2.08 | 8.00 | 0.875 | 0.875 | 1.000 |
| Inner Mongolia | 37 | 37 | 10.00 | 3.61 | 37.00 | 0.973 | 0.973 | 1.000 |
| Total | 622 | 428 | 9.83 | 5.77 | 177.14 | 0.994 | 0.998 | 0.564 |
| Beijing | Hebei | Shanxi | Shaanxi | Anhui | Shandong | Henan | Heilongjiang | Jiangsu | Hubei | Yunnan | Gansu | Qinghai | Sichuan | Inner Mongolia | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beijing | **** | 22.804 | 10.592 | 11.137 | 13.288 | 14.439 | 16.472 | 12.661 | 6.081 | 5.542 | 7.218 | 6.499 | 3.295 | 2.980 | 2.392 |
| Hebei | **** | 8.852 | 9.777 | 10.670 | 19.790 | 15.516 | 9.381 | 6.354 | 6.145 | 6.792 | 6.624 | 3.128 | 3.557 | 2.556 | |
| Shanxi | **** | 27.330 | 17.675 | 15.738 | 15.850 | 10.297 | 11.384 | 7.397 | 7.197 | 7.888 | 3.633 | 3.669 | 2.629 | ||
| Shaanxi | **** | 29.722 | 17.275 | 22.931 | 10.971 | 9.710 | 10.638 | 7.814 | 8.471 | 3.731 | 4.590 | 3.259 | |||
| Anhui | **** | 17.000 | 34.712 | 12.817 | 8.281 | 11.811 | 7.592 | 7.944 | 3.674 | 4.216 | 2.811 | ||||
| Shandong | **** | 20.344 | 10.567 | 9.615 | 7.944 | 6.271 | 7.373 | 3.099 | 3.574 | 2.913 | |||||
| Henan | **** | 26.657 | 7.339 | 8.747 | 7.612 | 8.440 | 3.411 | 4.212 | 2.784 | ||||||
| Heilongjiang | **** | 5.882 | 5.678 | 5.383 | 7.486 | 3.065 | 3.149 | 2.232 | |||||||
| Jiangsu | **** | 7.540 | 6.331 | 20.202 | 7.553 | 7.970 | 5.204 | ||||||||
| Hubei | **** | 5.029 | 7.308 | 3.159 | 4.715 | 3.148 | |||||||||
| Yunnan | **** | 8.500 | 4.750 | 4.868 | 2.968 | ||||||||||
| Gansu | **** | 8.324 | 12.984 | 4.363 | |||||||||||
| Qinghai | **** | 6.420 | 4.213 | ||||||||||||
| Sichuan | **** | 3.991 | |||||||||||||
| Inner Mongolia | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Li, H.; Xia, X.; Liu, B.; Gao, L.; Chen, W.; Liu, T. SSR Genotypes of the Puccinia triticina in 15 Provinces of China Indicate Regional Migration in One Season from East to West and South to North. Agronomy 2022, 12, 3068. https://doi.org/10.3390/agronomy12123068
Xu Z, Li H, Xia X, Liu B, Gao L, Chen W, Liu T. SSR Genotypes of the Puccinia triticina in 15 Provinces of China Indicate Regional Migration in One Season from East to West and South to North. Agronomy. 2022; 12(12):3068. https://doi.org/10.3390/agronomy12123068
Chicago/Turabian StyleXu, Zhe, Hongfu Li, Xiaoshuang Xia, Bo Liu, Li Gao, Wanquan Chen, and Taiguo Liu. 2022. "SSR Genotypes of the Puccinia triticina in 15 Provinces of China Indicate Regional Migration in One Season from East to West and South to North" Agronomy 12, no. 12: 3068. https://doi.org/10.3390/agronomy12123068
APA StyleXu, Z., Li, H., Xia, X., Liu, B., Gao, L., Chen, W., & Liu, T. (2022). SSR Genotypes of the Puccinia triticina in 15 Provinces of China Indicate Regional Migration in One Season from East to West and South to North. Agronomy, 12(12), 3068. https://doi.org/10.3390/agronomy12123068






