Confirmation of the Mechanisms of Resistance to ACCase-Inhibiting Herbicides in Chinese Sprangletop (Leptochloa chinensis (L.) Nees) from South Sulawesi, Indonesia
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Herbicides Dose–Response Experiments
2.3. Statistical Analyses of the Dose–Response Experiments
2.4. Isolation of DNA and Gene Sequence
3. Results
3.1. Dose–Response Experiments
3.2. Isolation of DNA and Gene Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vrbničanin, S.; Pavlović, D.; Božić, D. Weed Resistance to Herbicides. In Herbicide Resistance in Weeds and Crops; Pacanoski, Z., Ed.; Intech Open: London, UK, 2017; pp. 7–35. [Google Scholar]
- Takano, H.K.; Ovejero, R.F.L.; Belchior, G.G.; Maymone, G.P.L.; Dayan, F.E. ACCase-inhibiting herbicides: Mecha-nism of action, resistance evolution and stewardship. Sci. Agric. 2020, 78, 1–11. [Google Scholar]
- Délye, C.; Zhang, X.Q.; Michel, S.; Matéjicek, A.; Powles, S.B. Molecular bases for sensitivity to acetyl-coenzyme a carboxylase-inhibitors in black-grass. Plant Physiol. 2005, 137, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.L. Lessons Learned from the History of Herbicide Resistance. Weed Sci. 2014, 62, 427–431. [Google Scholar] [CrossRef]
- Nandula, V.K.; Riechers, D.E.; Ferhatoglu, Y.; Barrett, M.; Duke, S.O.; Dayan, F.E.; Goldberg-Cavalleri, A. Herbicide Metabolism: Crop Selectivity, Bioactivation, Weed Resistance, and Regulation. Weed Sci. 2019, 67, 149–175. [Google Scholar] [CrossRef]
- Lancaster, Z.D.; Norsworthy, J.K.; Scott, R.C. Residual Activity of ACCase-Inhibiting Herbicides on Monocot Crops and Weeds. Weed Technol. 2018, 32, 364–370. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Zhang, Z.; Wang, J.; Fu, W.; Li, Y. Investigating the resistance levels and mechanisms to penox-sulam and cyhalofop-butyl in barnyardgrass (Echinochloa crus-galli) from Ningxia Province, China. Weed Sci. 2021, 69, 422–429. [Google Scholar]
- Chauhan, B.S.; Johnson, D.E. Germination Ecology of Chinese Sprangletop (Leptochloa chinensis) in the Philip-pines. Weed Sci. 2008, 56, 820–825. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, T.; Wang, W.; Cao, T.; Li, R.; Lou, Y. Silencing OsSLR1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Plant Cell Environ. 2017, 40, 2147–2159. [Google Scholar]
- Pane, H.; Mansor, M.; Watanabe, H. Yield Component Analysis of Direct Seeded Rice Under Several Densities of Red Sprangletop (Leptochloa chinensis L.) Nees in Peninsular Malaysia. J. Weed Sci. Technol. 1996, 41, 216–224. [Google Scholar] [CrossRef]
- Webster, E.P.; Bergeron, E.A.; Blouin, D.C.; McKnight, B.M.; Osterholt, M.J. Impact of Nealley’s Sprangletop on Rough Rice Yield. Weed Technol. 2018, 32, 532–536. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. 2022. Available online: www.weedscience.org (accessed on 20 September 2022).
- Kurniadie, D.; Widianto, R.; Widayat, D.; Umiyati, U.; Nasahi, C. Confirmation of resistance Monochoria vaginalis (Burm. f.) C. Presl from West Java and Lampung Indonesia to bensulfuron-methyl herbicide. J. Plant Prot. Res. 2021, 61, 139–144. [Google Scholar]
- Lee, S.M.; Radhakrishnan, R.; Kang, S.M.; Kim, J.H.; Lee, I.Y.; Moon, B.K.; Yoon, B.W. Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Saf. 2015, 122, 230–237. [Google Scholar] [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory Smith, C.; Burgos, N.R.; Johnson, W.G. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Feurst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Yu, J.; Gao, H.; Pan, L.; Yao, Z.; Dong, L. Mechanism of resistance to cyhalofop-butyl in Chinese sprangletop (Leptochloa chinensis (L). Nees). Pestic. Biochem. Physiol. 2017, 143, 306–311. [Google Scholar] [CrossRef]
- Ahmad-Hamdani, M.S.; Owen, M.J.; Yu, Q.; Powles, S.B. ACCase-Inhibiting Herbicide-Resistant Avena spp. Populations from the Western Australian Grain Belt. Weed Technol. 2012, 26, 130–136. [Google Scholar] [CrossRef]
- Xia, X.; Tang, W.; He, S.; Kang, J.; Ma, H.; Li, J. Mechanism of metamifop inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase in Echinochloa crus-galli. Sci. Rep. 2016, 6, 34066. [Google Scholar] [CrossRef]
- Widianto, R.; Kurniadie, D.; Widayat, D.; Umiyati, U.; Nasahi, C.; Sari, S.; Juraimi, A.S. Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia. Plants 2022, 11, 400. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Herbicides, Carotenoid Biosynthesis Inhibitors. In Encyclopedia of Agrochemicals; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Beckie, H.J.; Reboud, X. Selecting for Weed Resistance: Herbicide Rotation and Mixture. Weed Technol. 2009, 23, 363–370. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Z.; Li, T.; Qian, Z.; Guo, W.; Shen, G. Cross-resistance pattern to ACCase-inhibiting herbicides in a rare Trp-2027-Ser mutation chinese sprangletop (Leptochloa chinensis) population. Chil. J. Agric. Res. 2021, 81, 62–69. [Google Scholar] [CrossRef]
- Won, O.J.; Lee, J.J.; Eom, M.Y.; Suh, S.J.; Park, S.H.; Hwang, K.S.; Pyon, J.Y. Identification of Herbicide-Resistant Barnyardgrass (Echinochloa crus-galli var. crus-galli) Biotypes in Korea. Weed Turfgrass Sci. 2014, 3, 110–113. [Google Scholar] [CrossRef][Green Version]
- Yang, Q.; Deng, W.; Li, X.; Yu, Q.; Bai, L.; Zheng, M. Target-site and non-target-site based resistance to the herbicide tribenuron-methyl in flixweed (Descurainia sophia L.). BMC Genom. 2016, 171, 551. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Anita, K.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yang, M.; Li, Y.; Xia, Z.; Chen, Y.; Yuan, S.; Yang, Q. Enhanced metabolism confers a high level of cyhalo-fop-butyl resistance in a Chinese sprangletop (Leptochloa chinensis (L.) Nees) population. Pest Manag. Sci. 2021, 77, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Jiang, M.; Li, Q.; Gao, Q.; Zhang, J.; Liao, M.; Cao, H. Cyhalofop-butyl resistance conferred by a novel Trp-2027-Leu mutation of acetyl-CoA carboxylase and enhanced metabolism in Leptochloa chinensis. Pest Manag. Sci. 2022, 78, 1176–1186. [Google Scholar] [CrossRef]
- Panozzo, S.; Mascanzoni, E.; Scarabel, L.; Milani, A.; Dalazen, G.; Merotto, A.J.; Tranel, P.J.; Satting, M. Target-Site Mutations and Expression of ALS Gene Copies Vary According to Echinochloa Species. Genes 2021, 12, 1841. [Google Scholar] [CrossRef]
- Yanniccari, M.; Gigon, M.; Larsen, A. Cytochrome P450 Herbicide Metabolism as the Main Mechanism of Cross-Resistance to ACCase- and ALS-Inhibitors in Lolium spp. Populations from Argentina: A Molecular Approach in Characterization and Detection. Front. Plant Sci. 2020, 11, 600301. [Google Scholar] [CrossRef]
- Kaundun, S.S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 2014, 70, 1405–1417. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Powles, S.B. The molecular bases for resistance to acetyl co-enzyme A carboxylase (ACCase) inhibiting herbicides in two target-based resistant biotypes of annual ryegrass (Lolium rigidum). Planta 2006, 223, 550–557. [Google Scholar] [CrossRef]
- Sosnoskie, L.M.; Webster, T.M.; Kichler, J.M.; MacRae, A.W.; Grey, T.L.; Culpepper, A.S. Pollen-Mediated Dispersal of Glyphosate-Resistance in Palmer Amaranth under Field Conditions. Weed Sci. 2012, 60, 366–373. [Google Scholar] [CrossRef]
- Geddes, C.M.; Ostendorf, T.E.; Gulden, R.H.; Jones, T.; Leeson, J.Y.; Sharpe, S.M.; Shirriff, S.W. Rapid spread of glyphosate-resistant kochia [Bassia scoparia (L.) A.J. Scott] in Manitoba. In Proceedings of the 2019 Canadian Weed Science Society Annual Meeting, Pinawa, MB, Canada, 10–13 December 2019; pp. 2–3. [Google Scholar]
- Yuan, G.; Tian, Z.; Gao, Y.; Shen, G. Resistance status of Leptochloa chinensis to three acetyl-CoA carboxylase (ACCase) inhibitors in rice fields in Shanghai and involved ACCase gene mutations. Chin. J. Pestic. Sci. 2022, 24, 492–500. [Google Scholar]
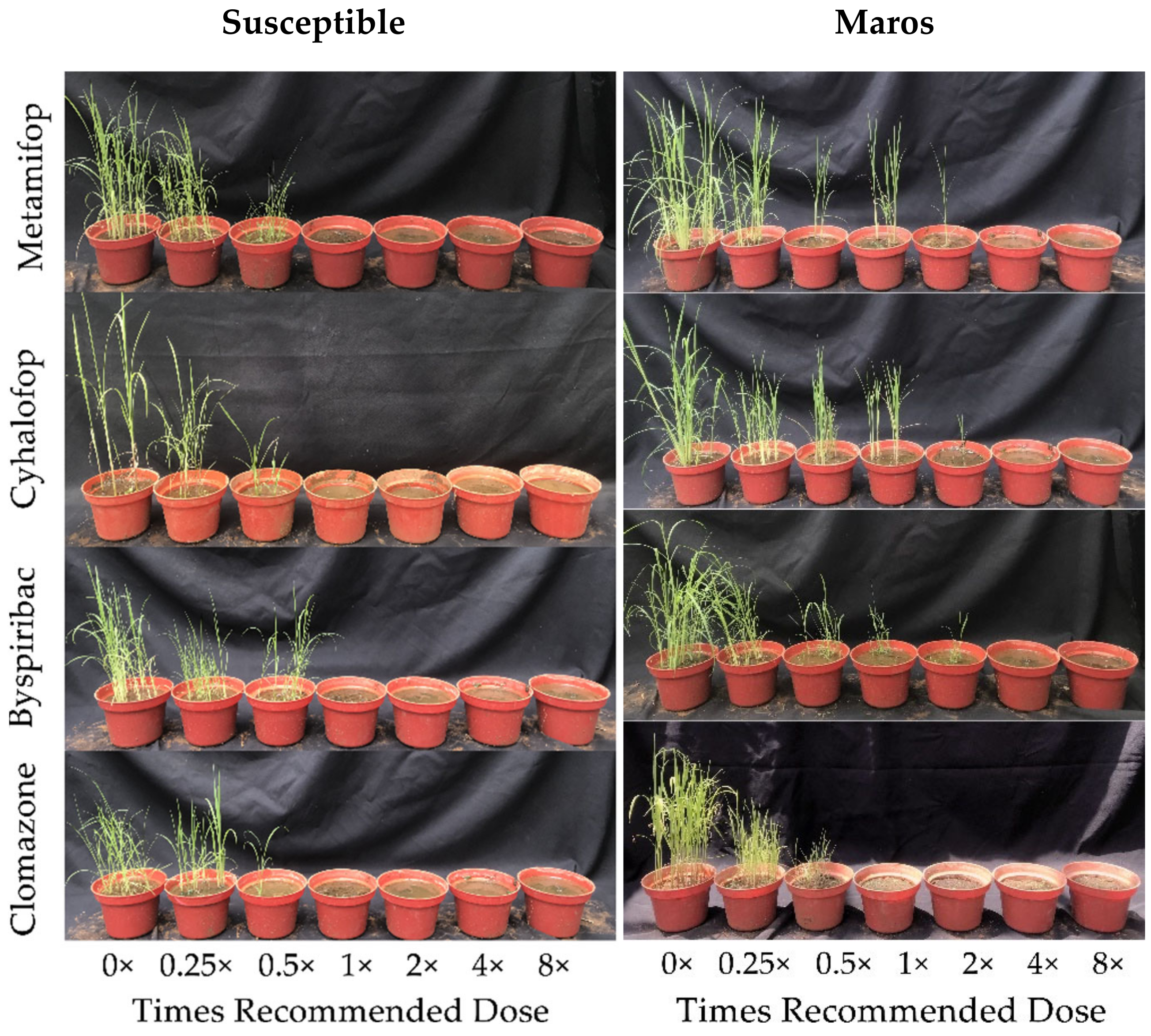


| Herbicide | Biotypes | Times Recommended Dose (g a.i. ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||
| Metamifop | Susceptible | 0 a,C | 69.02 a,B | 69.74 a,B | 93.67 a,A | 100 a,A | 100 a,A | 100 a,A |
| Maros | 0 a,E | 39.71 a,D | 67.39 a,C | 76.66 b,B | 89.49 b,B | 100 a,A | 100 a,A | |
| Butyl-Cyhalofop | Susceptible | 0 a,C | 54.35 a,B | 65.73 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Maros | 0 a,D | 19.22 b,C | 46.71 b,B | 61.16 b,B | 90.68 a,A | 100 a,A | 100 a,A | |
| Byspribac-Sodium | Susceptible | 0 a,D | 56.50 a,C | 70.70 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Maros | 0 a,E | 33.25 b,D | 50.54 b,C | 82.29 b,B | 100 a,A | 100 a,A | 100 a,A | |
| Clomazone | Susceptible | 0 a,D | 43.79 a,C | 80.20 a,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A |
| Maros | 0 a,C | 53.26 a,B | 59.39 b,B | 100 a,A | 100 a,A | 100 a,A | 100 a,A | |
| Herbicides | b | r2 | Biotypes | GR50 (g a.i ha−1) | R/S | Resistant Category [18] |
|---|---|---|---|---|---|---|
| Metamifop | 1.11 | 0.83 | Susceptible | 17.91 ± 6.09 | 1.00 | - |
| 1.29 | 0.98 | Maros | 66.82 ± 5.54 | 3.73 | Low | |
| Cyhalofop-butyl | 1.64 | 0.90 | Susceptible | 30.97 ± 9.65 | 1.00 | - |
| 1.75 | 0.99 | Maros | 75.83 ± 26.94 | 2.44 | Low | |
| Bsypribac-sodium | 1.68 | 0.92 | Susceptible | 3.67 ± 0.62 | 1.00 | - |
| 1.27 | 0.94 | Maros | 6.83 ± 0.73 | 1.86 | Susceptible | |
| Clomazone | 2.57 | 0.83 | Susceptible | 13.33 ± 1.11 | 1.00 | - |
| 2.36 | 0.86 | Maros | 12.79 ± 1.72 | 0.96 | Susceptible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniadie, D.; Widianto, R.; Aprilia, A.N.; Damayanti, F. Confirmation of the Mechanisms of Resistance to ACCase-Inhibiting Herbicides in Chinese Sprangletop (Leptochloa chinensis (L.) Nees) from South Sulawesi, Indonesia. Agronomy 2022, 12, 3152. https://doi.org/10.3390/agronomy12123152
Kurniadie D, Widianto R, Aprilia AN, Damayanti F. Confirmation of the Mechanisms of Resistance to ACCase-Inhibiting Herbicides in Chinese Sprangletop (Leptochloa chinensis (L.) Nees) from South Sulawesi, Indonesia. Agronomy. 2022; 12(12):3152. https://doi.org/10.3390/agronomy12123152
Chicago/Turabian StyleKurniadie, Denny, Ryan Widianto, Annisa Nadiah Aprilia, and Farida Damayanti. 2022. "Confirmation of the Mechanisms of Resistance to ACCase-Inhibiting Herbicides in Chinese Sprangletop (Leptochloa chinensis (L.) Nees) from South Sulawesi, Indonesia" Agronomy 12, no. 12: 3152. https://doi.org/10.3390/agronomy12123152
APA StyleKurniadie, D., Widianto, R., Aprilia, A. N., & Damayanti, F. (2022). Confirmation of the Mechanisms of Resistance to ACCase-Inhibiting Herbicides in Chinese Sprangletop (Leptochloa chinensis (L.) Nees) from South Sulawesi, Indonesia. Agronomy, 12(12), 3152. https://doi.org/10.3390/agronomy12123152







