ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Plants
2.2. Medium or Solution
2.3. Conidiation
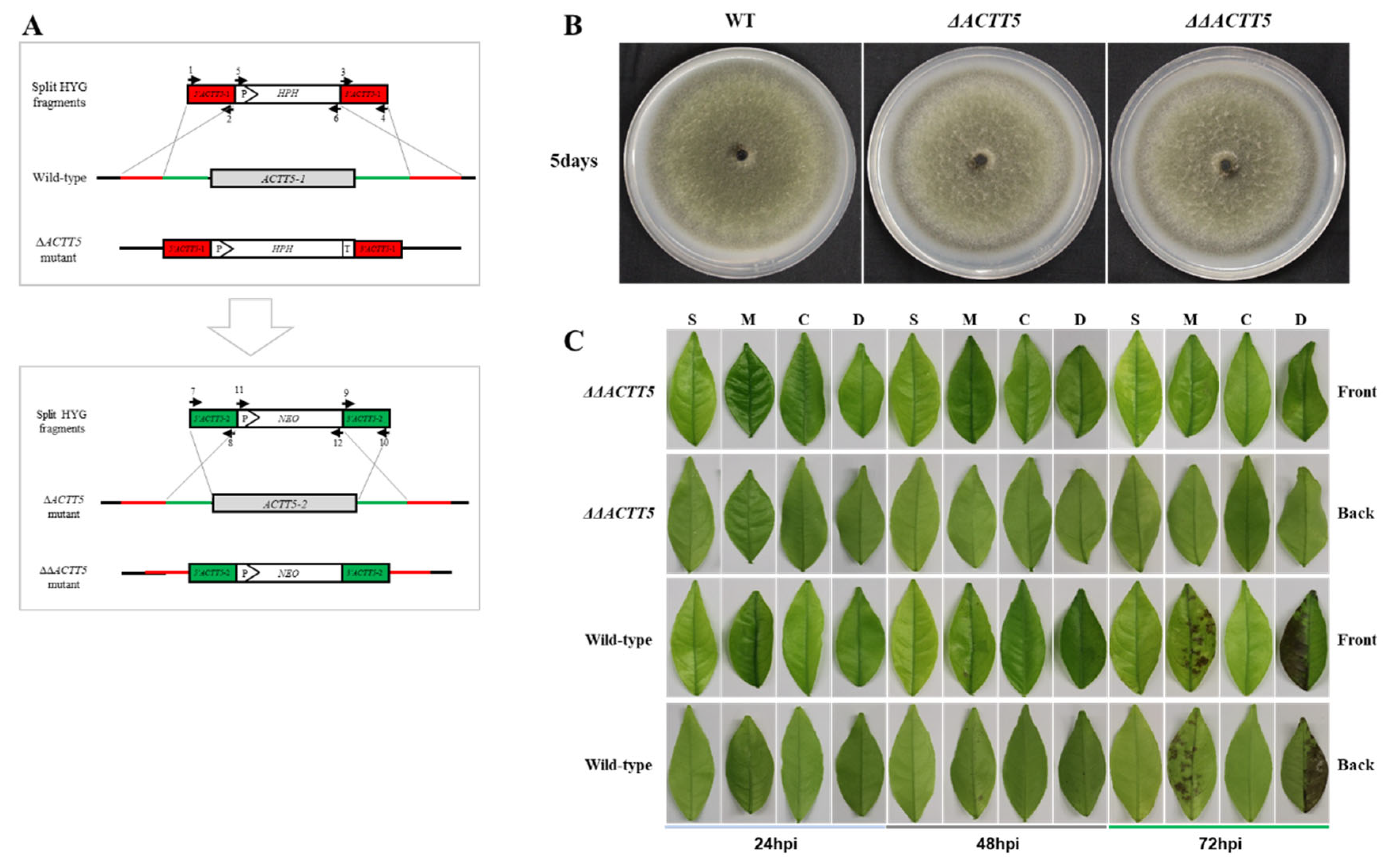
2.4. ACTT5 Knockout
2.5. Determination of Pathogenicity
2.6. ACT-Toxin Extraction and Virulence Assay
2.7. Phenotypic Analysis
2.8. Statistical Analysis
3. Results
3.1. ACT-Toxin Biosynthesis and ROS Detoxification-Related Genes Are Involved in the Pathogenicity of A. alternata
3.2. Different Citrus Species Display Distinct Sensitivity to ACT-Toxin
3.3. ACTT5 Is Required for the Pathogenicity of A. alternata
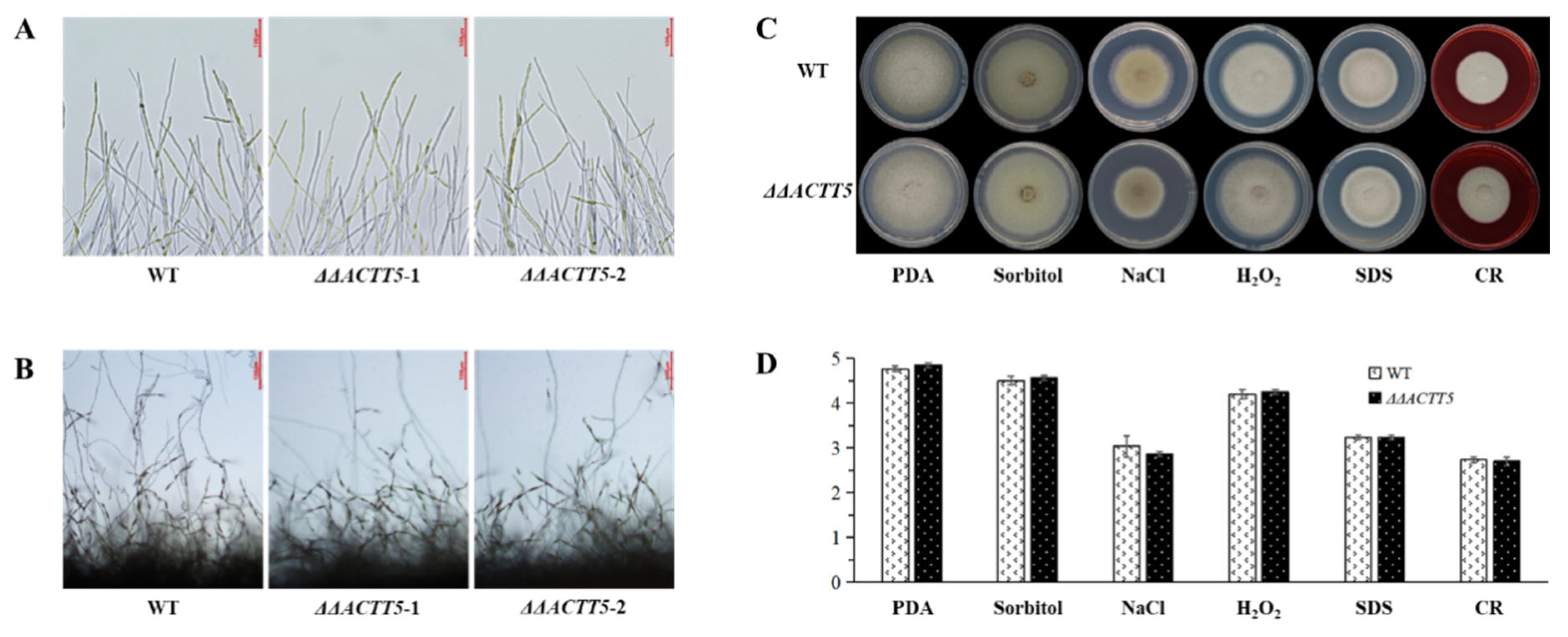
3.4. ACTT5 Is Not Involved in Conidiation, Vegetative Growth, and Multi-Stress Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. J. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Shimomura, Y.; Kondoh, Y.; Otani, H.; Kodama, M.; Nishimura, S.; Nakatsuka, S. Isolation and biological activities of two host-bpecific toxins from the tangerine pathotype of Alternaria alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Parada, R.; Sakuno, E.; Mori, N.; Oka, K.; Egusa, M.; Kodama, M.; Otani, H. Alternaria brassicae produces a host-specific protein toxin from germinating spores on host leaves. Phytopathology 2008, 98, 458–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, T.; Kono, Y.; Takeuchi, S.; Daly, J.M. Structure of HV-toxin M, a host-specific toxin-related compound produced by Helminthosporium victoriae. Agric. Biol. Chem. 1989, 53, 1283–1290. [Google Scholar] [CrossRef]
- Holden, M.J.; Sze, H. Helminthosporium maydis T toxin increased membrane permeability to Ca2+ in susceptible corn mitochondria. Plant Physiol. 1984, 75, 235–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, S.; Kohmoto, K. Host-specific toxins and chemical structures from Alternaria species. Annu. Rev. Phytopathol. 1983, 21, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, R.P.; Livingston, R.S. Host-selective toxins and their role in plant diseases. Science 1984, 223, 17–21. [Google Scholar] [CrossRef]
- Walton, J.D. Host-selective toxins: Agents of compatibility. Plant Cell 1996, 8, 1723. [Google Scholar]
- Wolpert, T.J.; Dunkle, L.D.; Ciuffetti, L.M. Host-selective toxins and avirulence determinants: What’s in a name? Annu. Rev. Phytopathol. 2002, 40, 251–285. [Google Scholar] [CrossRef] [Green Version]
- Yoder, O. Toxins in pathogenesis. Annu. Rev. Phytopathol. 1980, 18, 103–129. [Google Scholar] [CrossRef]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef]
- Thomma, B.P. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in food, Ecophysiology, mycotoxin production and toxicology. Mycobiology 2015, 43, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Tanahashi, M.; Nakano, T.; Akamatsu, H.; Kodama, M.; Otani, H.; Osaki-Oka, K. Alternaria alternata apple pathotype (A. mali) causes black spot of European pear. Eur. J. Plant Pathol. 2016, 145, 787–795. [Google Scholar] [CrossRef]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Egusa, M.; Yamamoto, M.; Otani, H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Shiotani, H.; Yamamoto, M.; Tsuge, T. Insertional mutagenesis and cloning of the genes required for biosynthesis of the host-specific AK-toxin in the Japanese pear pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 1999, 12, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatsuka, S.; Ueda, K.; Goto, T.; Yamamoto, M.; Nishimura, S.; Kohmoto, K. Structure of AF-toxin II, one of the host-specific toxins produced by Alternaria alternata strawberry pathotype. Tetrahedron Lett. 1986, 27, 2753–2756. [Google Scholar] [CrossRef]
- Gilchrist, D.; Grogan, R. Production and nature of a host-specific toxin from Alternaria alternata f. sp. lycopersici. Phytopathology 1976, 66, 165–171. [Google Scholar] [CrossRef]
- Izumi, Y.; Ohtani, K.; Miyamoto, Y.; Masunaka, A.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Peever, T.L.; Akimitsue, K. A polyketide synthase gene, ACRTS2, is responsible for biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2012, 25, 1419–1429. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Akimitsu, K. Functional analysis of a multicopy host-selective ACT-toxin biosynthesis gene in the tangerine pathotype of Alternaria alternata using RNA silencing. Mol. Plant-Microbe Interact. 2008, 21, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Akimitsu, K.; Tsuge, T.; Kodama, M.; Yamamoto, M.; Otani, H. Alternaria host-selective toxins: Determinant factors of plant disease. J. Gen. Plant Pathol. 2014, 80, 109–122. [Google Scholar] [CrossRef]
- Ajiro, N.; Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Izumi, Y.; et al. Role of the host-selective ACT-toxin synthesis gene ACTTS2 encoding an enoyl-reductase in pathogenicity of the tangerine pathotype of Alternaria alternata. Phytopathology 2010, 100, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, Y.; Kamei, E.; Miyamoto, Y.; Ohtani, K.; Masunaka, A.; Fukumoto, T.; Gomi, K.; Tada, Y.; Ichimura, K.; Peever, T.L.; et al. Role of the pathotype-specific ACRTS1 gene encoding a hydroxylase involved in the biosynthesis of host-selective ACR-toxin in the rough lemon pathotype of Alternaria alternata. Phytopathology 2012, 102, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, Y.; Ishii, Y.; Honda, A.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; et al. Function of genes encoding acyl-CoA synthetase and enoyl-CoA hydratase for host-selective ACT-toxin biosynthesis in the tangerine pathotype of Alternaria alternata. Phytopathology 2009, 99, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Masunaka, A. Studies on genes for biosynthesis of host-selective ACT-toxin in the tangerine pathotype of Alternaria alternata. J. Gen. Plant Pathol. 2007, 73, 424–425. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Yu, D.; Xu, J.; Chung, K.; Li, H. Genomic and transcriptomic analyses of the tangerine pathotype of Alternaria alternata in response to oxidative stress. Sci. Rep. 2016, 6, 32437. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Tada, Y.; Ichimura, K.; et al. ACTTS3 encoding a polyketide synthase is essential for the biosynthesis of ACT-toxin and pathogenicity in the tangerine pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2010, 23, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ma, H.; Zheng, F.; Chen, Y.; Wang, M.; Jiao, C.; Li, H.; Gai, Y. The transcription regulator ACTR controls ACT-toxin biosynthesis and pathogenicity in the tangerine pathotype of Alternaria alternata. Microbiol. Res. 2021, 248, 126747. [Google Scholar] [CrossRef]
- Gai, Y.; Ma, H.; Chen, Y.; Li, L.; Cao, Y.; Wang, M.; Sun, X.; Jiao, C.; Riely, B.K.; Li, H.; et al. Chromosome-scale genome sequence of Alternaria alternata causing Alternaria brown spot of citrus. Mol. Plant-Microbe Interact. 2021, 34, 726–732. [Google Scholar] [CrossRef]
- Akimitsu, K.; Kohmoto, K.; Otani, H.; Nishimura, S. Host-specific effects of toxin from the rough lemon pathotype of Alternaria alternata on mitochondria. Plant Physiol. 1989, 89, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, D.; Akamatsu, H.; Otani, H.; Kodama, M. Pathological evaluation of host-specific AAL-toxins and fumonisin mycotoxins produced by Alternaria and Fusarium species. J. Gen. Plant Pathol. 2006, 72, 323–327. [Google Scholar] [CrossRef]
- Yang, S.L.; Chung, K.R. Similar and distinct roles of NADPH oxidase components in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2013, 14, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.R. Reactive oxygen species in the citrus fungal pathogen Alternaria alternata: The roles of NADPH-dependent oxidase. Physiol. Mol. Plant Pathol. 2014, 88, 10–17. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Wang, N.Y.; Chung, K.R. The FUS3 MAPK signaling pathway of the citrus pathogen Alternaria alternata functions independently or cooperatively with the fungal redox-responsive AP1 regulator for diverse developmental, physiological and pathogenic processes. Fungal Genet. Biol. 2010, 47, 381–391. [Google Scholar] [CrossRef]
- Lin, C.H.; Chung, K.R. Specialized and shared functions of the histidine kinase-and HOG1 MAP kinase-mediated signaling pathways in Alternaria alternata, a filamentous fungal pathogen of citrus. Fungal Genet. Biol. 2010, 47, 818–827. [Google Scholar] [CrossRef]
- Chen, L.H.; Lin, C.H.; Chung, K.R. Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet. Biol. 2012, 49, 802–813. [Google Scholar] [CrossRef]
- Ma, H.; Wang, M.; Gai, Y.; Fu, H.; Zhang, B.; Ruan, R.; Chung, K.-R.; Li, H. Thioredoxin and glutaredoxin systems required for oxidative stress resistance, fungicide sensitivity, and virulence of Alternaria alternata. Appl. Environ. Microbiol. 2018, 84, e00086-18. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhang, B.; Gai, Y.; Sun, X.; Chung, K.R.; Li, H. Cell-wall-degrading enzymes required for virulence in the host selective toxin-producing necrotroph Alternaria alternata of Citrus. Front. Microbiol. 2019, 10, 2514. [Google Scholar] [CrossRef]
- Fu, H.; Chung, K.R.; Gai, Y.; Mao, L.; Li, H. The basal transcription factor II H subunit Tfb5 is required for stress response and pathogenicity in the tangerine pathotype of Alternaria alternata. Mol. Plant Pathol. 2020, 21, 1337–1352. [Google Scholar] [CrossRef]
- Huang, F.; Fu, Y.; Nie, D.; Stewart, J.E.; Peever, T.L.; Li, H. Identification of a novel phylogenetic lineage of Alternaria alternata causing citrus brown spot in China. Fungal Biol. 2015, 119, 320–330. [Google Scholar] [CrossRef]
- Chung, K.-R.; Shilts, T.; Li, W.; Timmer, L.W. Engineering a genetic transformation system for Colletotrichum acutatum, the causal fungus of lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 2002, 213, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kohmoto, K.; Scheffer, R.P.; Whiteside, J.O. Host-selective toxins from Alternaria citri. Phytopathology 1979, 69, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Masunaka, A.; Tanaka, A.; Tsuge, T.; Peever, T.L.; Timmer, L.W.; Yamamoto, M.; Yamamoto, H.; Akimitsu, K. Distribution and characterization of AKT homologs in the tangerine pathotype of Alternaria alternata. Phytopathology 2000, 90, 762–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, T.; Ueno, T.; Fukami, H.; Taga, T.; Masuda, H.; Osaki, K.; Otani, H.; Kohmoto, K.; Nishimura, S. Isolation and structures of AK-Toxin I and II, host-specific phytotoxic metabolites produced by Alternaria alternata Japanese pear pathotype. Agric. Biol. Chem. 1985, 49, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Kohmoto, K.; Otani, H.; Kodama, M.; Nishimura, S. Host recognition: Can accessibility to fungal invasion be induced by host-specific toxins without necessitating necrotic cell death? Phytotoxins Plant Pathog. 1989, 27, 249–265. [Google Scholar]
- Gardner, J.; Mansour, I.S.; Scheffer, R. Effects of the host-specific toxin of Periconia circinata on some properties of sorghum plasma membranes. Physiol. Plant Pathol. 1972, 2, 197–206. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Zhu, Z.; Wang, X. Expression of Ht2-related genes in response to the HT-Toxin of Exserohilum turcicum in Maize. Ann. Appl. Biol. 2010, 156, 111–120. [Google Scholar] [CrossRef]
- Okuno, T.; Ishita, Y.; Sawai, K.; Matsumoto, T. Characterization of alternariolide, a host-specific toxin produced by Alternaria mali roberts. Chem. Lett. 1974, 3, 635–638. [Google Scholar] [CrossRef]
- Akimitsu, K.; Peever, T.L.; Timmer, L.W. Molecular, ecological and evolutionary approaches to understanding Alternaria diseases of citrus. Mol. Plant Pathol. 2003, 4, 435–446. [Google Scholar] [CrossRef]
- Schröter, H.; Novacky, A.; Macko, V. Effect of Helminthosporium sacchari-toxin on cell membrane potential of susceptible sugarcane. Physiol. Plant Pathol. 1985, 26, 165–174. [Google Scholar] [CrossRef]
- Kohmoto, K.; Akimitsu, K.; Otani, H. Correlation of resistance and susceptibility of citrus to Alternaria alternata with sensitivity to host-specific toxins. Phytopathology 1991, 81, 719–722. [Google Scholar] [CrossRef]
- Maekawa, N.; Yamamoto, M.; Nishimura, S.; Kohmoto, K.; Kuwada, M.; Watanabe, Y. Studies on host-specific AF-toxins produced by Alternaria alternata strawberry pathotype causing Alternaria black spot of strawberry (1) production of host-specific toxins and their biological activities. Jpn. J. Phytopathol. 1984, 50, 600–609. [Google Scholar] [CrossRef]
- Slavov, S.; Mayama, S.; Atanassov, A. Toxin production of Alternaria alternata tobacco pathotype. Biotechnol. Biotechnol. Equip. 2004, 18, 90–95. [Google Scholar] [CrossRef]
- Walton, J.D.; Earle, E.D.; Gibson, B.W. Purification and structure of the host-specific toxin from Helminthosporium carbonum race 1. Biochem. Biophys. Res. Commun. 1982, 107, 785–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Betts, M.F.; Martinez, J.P. Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis–wheat interaction. New Phytol. 2010, 187, 911–919. [Google Scholar] [CrossRef]
- Liakopoulou-Kyriakides, M.; Lagopodi, A.; Thanassoulopoulos, C.; Stavropoulos, G.S.; Magafa, V. Isolation and synthesis of a host-selective toxin produced by Alternaria alternata. Phytochemistry 1997, 45, 37–40. [Google Scholar] [CrossRef]
- Bobylev, M.M.; Bobyleva, L.I.; Strobel, G.A. Synthesis and bioactivity of analogs of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). J. Agric. Food Chem. 1996, 44, 3960–3964. [Google Scholar] [CrossRef]
- Bottini, A.T.; Gilchrist, D.G. Phytotoxins. I. A 1-aminodimethylheptadecapentol from Alternaria alternata f. sp. lycopersici. Tetrahedron Lett. 1981, 22, 2719–2722. [Google Scholar] [CrossRef]
- Johnson, R.; Johnson, L.; Itoh, Y.; Kodama, M.; Otani, H.; Kohmoto, K. Cloning and characterization of a cyclic peptide synthetase gene from Alternaria alternata apple pathotype whose product is involved in AM-toxin synthesis and pathogenicity. Mol. Plant-Microbe Interact. 2000, 13, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Yakimova, E.T.; Yordanova, Z.P.; Slavov, S.; Kapchina-Toteva, V.M.; Woltering, E.J. Alternaria alternata AT toxin induces programmed cell death in tobacco. J. Phytopathol. 2009, 157, 592–601. [Google Scholar] [CrossRef]
- Stierle, A.C.; Cardellina, J.H.; Strobel, G.A. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternata. Proc. Natl. Acad. Sci. USA 1988, 85, 8008–8011. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Yang, S.L.; Chung, K.R. The YAP1 homolog–mediated oxidative stress tolerance is crucial for pathogenicity of the necrotrophic fungus Alternaria alternata in citrus. Mol. Plant-Microbe Interact. 2009, 22, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Yu, P.L.; Chung, K.R. The glutathione peroxidase-mediated reactive oxygen species resistance, fungicide sensitivity and cell wall construction in the citrus fungal pathogen Alternaria alternata. Environ. Microbiol. 2016, 18, 923–935. [Google Scholar] [CrossRef]
- Gai, Y.; Liu, B.; Ma, H.; Li, L.; Chen, X.; Moenga, S.; Riely, B.; Fayyaz, A.; Wang, M.; Li, H. The methionine biosynthesis regulator AaMetR contributes to oxidative stress tolerance and virulence in Alternaria alternata. Microbiol. Res. 2019, 219, 94–109. [Google Scholar] [CrossRef]
- Yu, P.L.; Chen, L.H.; Chung, K.R. How the pathogenic fungus Alternaria alternata copes with stress via the response regulators SSK1 and SHO1. PLoS ONE 2016, 11, e0149153. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.C.; Choo, C.Y.L.; Lu, H.Y.; Wei, X.Y.; Chen, Y.K.; Yago, J.I.; Chung, K.R. Pexophagy is critical for fungal development, stress response, and virulence in Alternaria alternata. Mol. Plant Pathol. 2022, 23, 1538–1554. [Google Scholar] [CrossRef]
- Chung, K.R.; Wu, P.C.; Chen, Y.K.; Yago, J.I. The siderophore repressor SreA maintains growth, hydrogen peroxide resistance, and cell wall integrity in the phytopathogenic fungus Alternaria alternata. Fungal Genet. Biol. 2020, 139, 103384. [Google Scholar] [CrossRef]
- Chung, K.-R. Stress response and pathogenicity of the necrotrophic fungal pathogen Alternaria alternata. Scientifica 2012, 2012, 635431. [Google Scholar] [CrossRef] [Green Version]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, S.L.; Chung, K.R. Cellular responses required for oxidative stress tolerance, colonization, and lesion formation by the necrotrophic fungus Alternaria alternata in citrus. Curr. Microbiol. 2011, 62, 807–815. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Luo, Z.; Gao, L.; Li, R.; Zhang, Y.; Kalaji, H.M.; Qiang, S.; Chen, S. Recent advances in Alternaria phytotoxins: A review of their occurrence, structure, bioactivity, and biosynthesis. J. Fungi 2022, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.; Kono, Y.; Chandler, J. Bioassay and host-selectivity of Alternaria citri toxins affecting rough lemon and mandarins. Physiol. Mol. Plant Pathol. 1986, 29, 293–304. [Google Scholar] [CrossRef]
- Solel, Z.; Kimchi, M. Susceptibility and resistance of citrus genotypes to Alternaria alternata pv. citri. J. Phytopathol. 1997, 145, 389–391. [Google Scholar] [CrossRef]
- Peever, T.L.; Olsen, L.; Ibañez, A.; Timmer, L.W. Genetic differentiation and host specificity among populations of Alternaria spp. causing brown spot of grapefruit and tangerine × grapefruit hybrids in florida. Phytopathology 2000, 90, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Vicent, A.; Badal, J.; Asensi, M.J.; Sanz, N.; Armengol, J.; García-Jiménez, J. Laboratory evaluation of citrus cultivars susceptibility and influence of fruit size on fortune mandarin to infection by Alternaria alternata pv. citri. Eur. J. Plant Pathol. 2004, 110, 245–251. [Google Scholar] [CrossRef]

| No | Pathogen | Toxin | Host | Reference |
|---|---|---|---|---|
| 1 | A. alternata Japanese pear pathotype | AK | Pear | [44,45] |
| 2 | Helminthosporium victoriae | HV | Oat | [4] |
| 3 | Periconia circinata | PC | Sorghum | [46] |
| 4 | Helminthosporium turcicum | HT | Maize | [47] |
| 5 | A. alternata apple pathotype | AM | Apple | [48,49] |
| 6 | A. alternata tangerine pathotype | ACT | Citrus | [2,49] |
| 7 | Helminthosporium maydis | HMT | Maize | [5] |
| 8 | Helminthosporium sacchari | HS | Sugarcane | [50] |
| 9 | A. alternata tomato pathotype | AAL | Tomato | [18] |
| 10 | A. alternata rough lemon pathotype | ACR | Rough lemon | [42,51] |
| 11 | A. alternata strawberry pathotype | AF | Strawberry | [52] |
| 12 | A. alternata tobacco pathotype | AT | Tobacco | [53] |
| 13 | Helminthosporium carbonum | HC | Maize | [54] |
| 14 | Pyrenophora tritici-repentis | PTR | Wheat | [55] |
| 15 | A. alternata sunflower pathotype | AS-I | Sunflower | [56] |
| 16 | A. alternata spotted knapweed pathotype | Maculosin | Knapweed | [57] |
| 17 | A. brassicae | ABR | Brassica spp. | [3] |
| No | Pathotype | HSTs | Disease | Chemical Characteristics | Reference |
|---|---|---|---|---|---|
| 1 | Tomato pathotype | AAL | Alternaria stem canker of tomato | Aminopentol esters | [1,18,58] |
| 2 | Tangerine pathotype | ACT | Citrus brown spot | Epoxy-decatrienoic esters | [2] |
| 3 | Rough lemon pathotype | ACR | Leaf spot of rough lemon | Terpenoid | [19] |
| 4 | Strawberry pathotype | AF | Black spot of strawberry | Epoxy-decatrienoic esters | [17] |
| 5 | Japanese pear pathotype | AK | Black spot of Japanese pear | Epoxy-decatrienoic esters | [16] |
| 6 | Apple pathotype | AM | Alternaria blotch of apple | Cyclic peptide | [59] |
| 7 | Sunflower pathotype | AS-I | Leaf spot of sunflower | Tetrapeptide | [56] |
| 8 | Tobacco pathotype | AT | Brown spot of tobacco | --- | [60] |
| 9 | Spotted knapweed pathotype | Maculosin | Black leaf blight of knapweed | Tetrapeptide | [61] |
| No | Gene | Name | Copy Number | Accession Number | Reference |
|---|---|---|---|---|---|
| 1 | ACTT2 | Hydrolase | ≥2 | AALT_g11743 | [20] |
| 2 | ACTTS2 | Enoyl-reductase | ≥2 | AALT_g12031 | [22] |
| 3 | ACTT3 | HMG-CoA hydrolase | ≥2 | AALT_g11755 | [25] |
| 4 | ACTTS3 | Polyketide synthase | ≥3 | AALT_g11750 | [27] |
| 5 | ACTT5 | Acyl-CoA synthetase | ≥3 | AALT_g11751 | [24] |
| 6 | ACTT6 | Enoyl-CoA hydratase | 2 | AALT_g12047 | [24] |
| 7 | ACTTR | Zn(II)2Cys6 transcription factor | ≥2 | AALT_g11754 | [28] |
| No | Gene | Vegetative Growth | Conidiation | Pathogenicity | Accession Number | Reference |
|---|---|---|---|---|---|---|
| 1 | Hog1 | Required | Required | Required | GQ414509 | [35] |
| 2 | Skn7 | Required | Required | Required | JQ716919 | [36] |
| 3 | Ap1 | Required | --- | Required | FJ376607 | [62] |
| 4 | Gpx3 | Required | Required | Required | ACY73852 | [63] |
| 5 | Tsa1 | Not required | Not required | Required | MG593564 | [37] |
| 6 | Trr1 | Required | Required | Required | MG593563 | [37] |
| 7 | Glr1 | Required | Required | Required | MG593559 | [37] |
| 8 | NoxA | Required | Required | Required | JN900389 | [32] |
| 9 | NoxB | Required | Required | Required | JX136700 | [32] |
| 10 | NoxR | Required | Required | Required | JX207117 | [32] |
| 11 | MetR | Required | Required | Required | Aa03030 | [64] |
| 12 | SSK1 | Required | Required | Required | KU170060 | [65] |
| 13 | Tbf1 | Required | Required | Required | MT184174 | [39] |
| 14 | Atg8 | Required | Required | Required | OK617334 | [66] |
| 15 | SreA | Required | Required | Required | OWY49902.1 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Jia, Z.; Li, H.; Zhang, S.; Shen, J.; Gai, Y.; Jiao, C.; Sun, X.; Duan, S.; Wang, M.; et al. ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species. Agronomy 2022, 12, 3181. https://doi.org/10.3390/agronomy12123181
Huang S, Jia Z, Li H, Zhang S, Shen J, Gai Y, Jiao C, Sun X, Duan S, Wang M, et al. ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species. Agronomy. 2022; 12(12):3181. https://doi.org/10.3390/agronomy12123181
Chicago/Turabian StyleHuang, Suya, Zhaohui Jia, Hangfei Li, Shuting Zhang, Junying Shen, Yunpeng Gai, Chen Jiao, Xuepeng Sun, Shuo Duan, Min Wang, and et al. 2022. "ACT-Toxin, the Key Effector for the Virulence of Alternaria alternata Tangerine Pathotype to Specific Citrus Species" Agronomy 12, no. 12: 3181. https://doi.org/10.3390/agronomy12123181





