The Physiological Role of Inulin in Wild Cardoon (Cynara cardunculus L. var. sylvestris Lam.)
Abstract
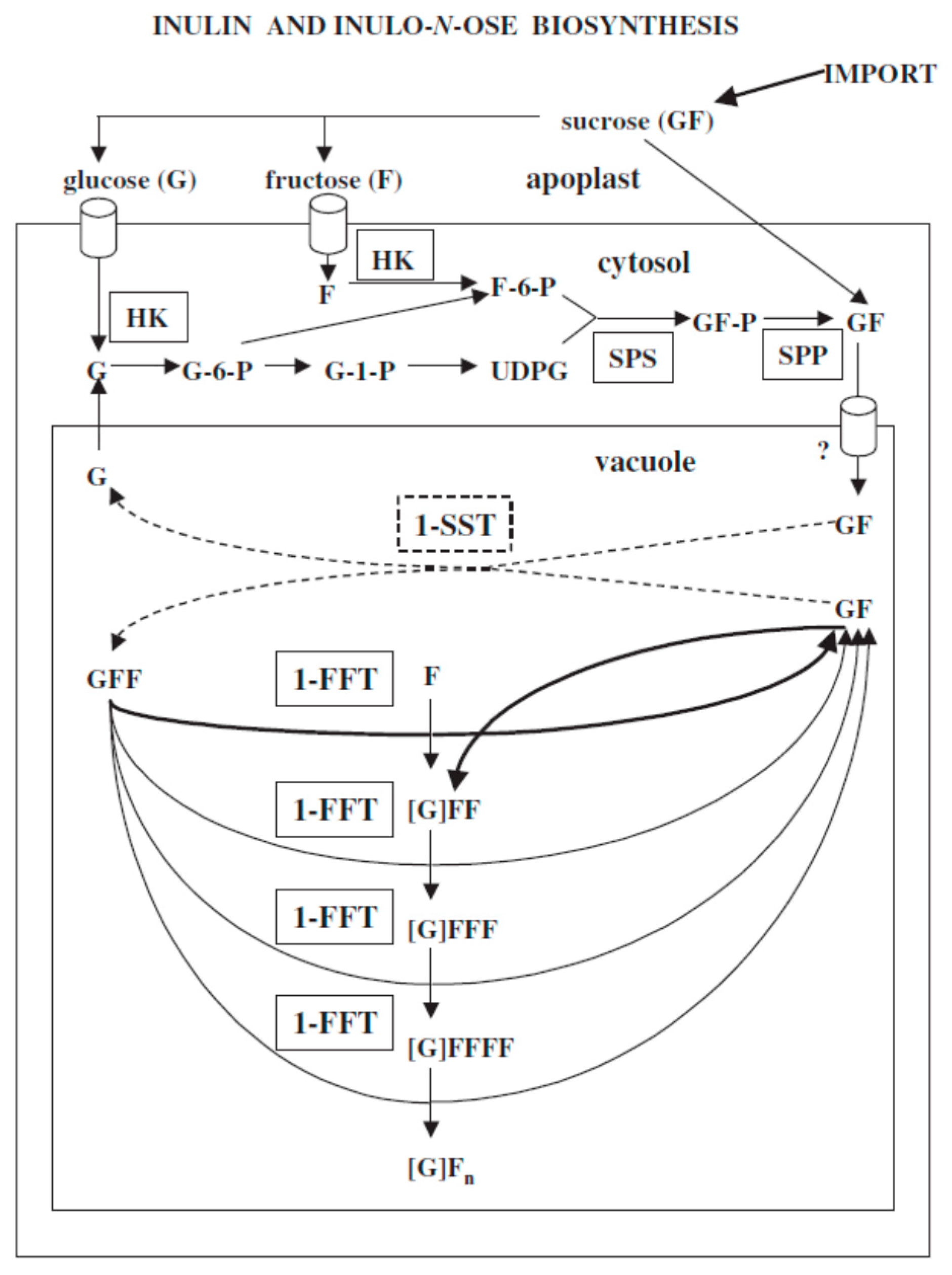
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Crop Management
2.2. Harvesting Procedures and Data Collection
2.3. Protein Extraction and Enzyme Assay
2.4. Carbohydrate Extraction and Analysis
2.5. Data Analysis
3. Results
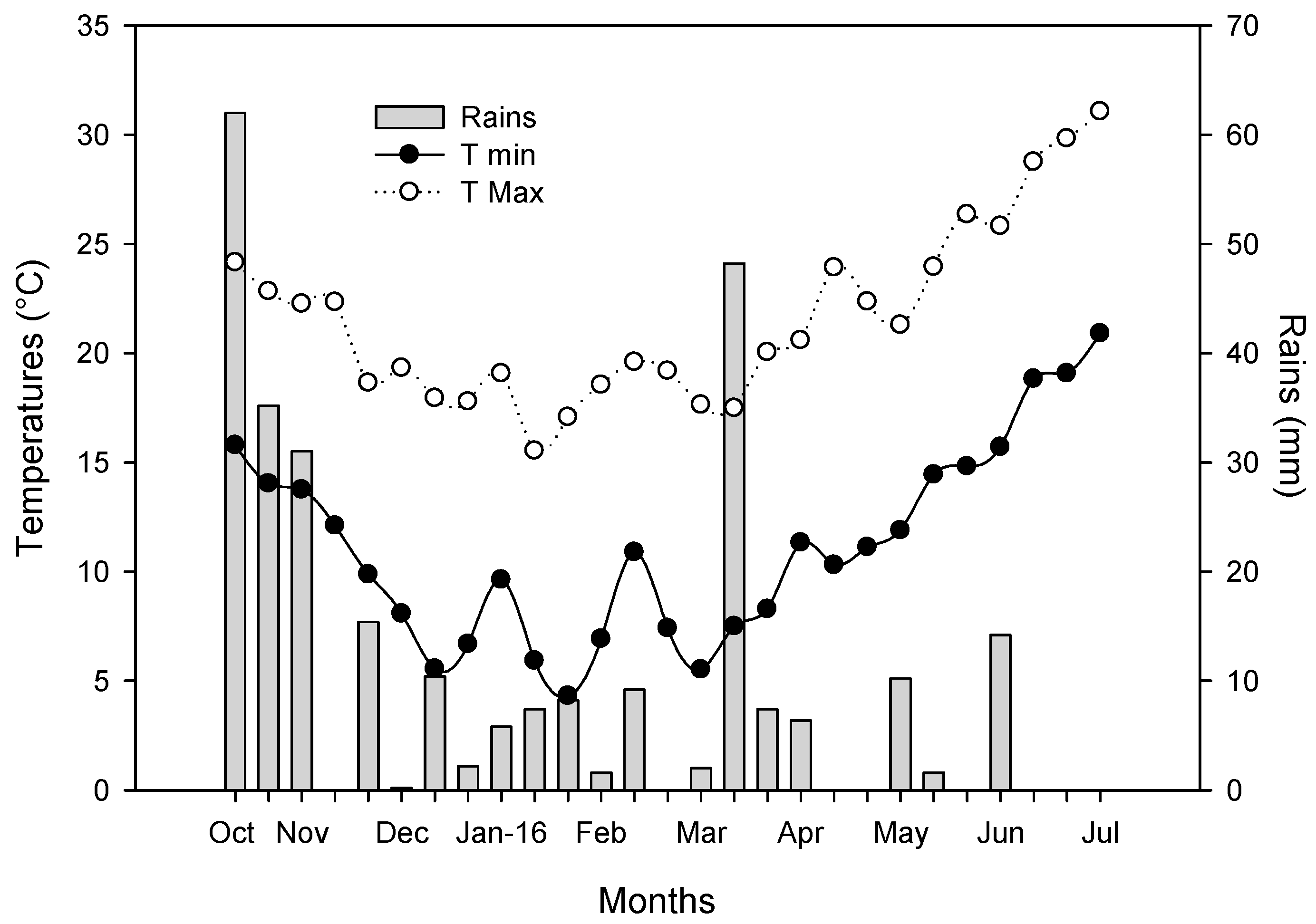
3.1. Meteorological Data
3.2. Plant Growth
3.3. Changes in Carbohydrates Content in Roots
3.4. Enzyme Activities in Roots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendry, G.A.; Wallace, R.K. The origin, distribution and evolutionary significance of fructans. In Science and Technology of Fructans; CRC Press: Boca Raton, FL, USA, 1993; p. 119. [Google Scholar]
- Pollock, C.J.; Cairns, A.J. Fructan metabolism in grasses and cereal. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 77–101. [Google Scholar] [CrossRef]
- Melilli, M.; Sillitti, C.; Conte, A.; Padalino, L.; Del Nobile, M.; Bognanni, R.; Pagliaro, A. Quality characteristics of cereal based foods enriched with quinoa and inulin. In Quinoa: Cultivation, Nutritional Properties and Effects on Health; Peiretti, P.G., Gai, F., Eds.; Nova: Osaka, Japan, 2019; p. 328. [Google Scholar]
- Raccuia, S.A.; Genovese, C.; Leonardi, C.; Bognanni, R.; Platania, C.; Calderaro, P.; Melilli, M.G. Fructose production by Cynara cardunculus inulin hydrolysis. Acta Hortic. 2016, 1147, 309–314. [Google Scholar] [CrossRef]
- Padalino, L.; Costa, C.; Conte, A.; Melilli, M.G.; Sillitti, C.; Bognanni, R.; Raccuia, S.A.; Del Nobile, M.A. The quality of functional whole-meal durum wheat spaghetti as affected by inulin polymerization degree. Carbohydr. Polym. 2017, 173, 84–90. [Google Scholar] [CrossRef]
- Garbetta, A.; D’Antuono, I.; Melilli, M.G.; Sillitti, C.; Linsalata, V.; Scandurra, S.; Cardinali, A. Inulin enriched durum wheat spaghetti: Effect of polymerization degree on technological and nutritional characteristics. J. Funct. Foods 2020, 71, 104004. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Ritsema, T.; Smeekens, S. Fructans: Beneficial for plants and humans. Curr. Opin. Plant Biol. 2003, 6, 223–230. [Google Scholar] [CrossRef]
- Melilli, M.G.; Branca, F.; Sillitti, C.; Scandurra, S.; Calderaro, P.; Di Stefano, V. Germplasm evaluation to obtain inulin with high degree of polymerization in Mediterranean environment. Nat. Prod. Res. 2020, 34, 187–191. [Google Scholar] [CrossRef]
- Van Laere, A.; Van den Ende, W. Inulin metabolism in dicots: Chicory as a model system. Plant Cell Environ. 2002, 25, 803–813. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G.; Tringali, S. Genetic and environmental influence on inulin yield in wild cardoon (Cynara cardunculus L. var. sylvestris Lam. Acta Hortic. 2004, 660, 47–53. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Biomass and grain oil yields in Cynara cardunculus L. genotypes grown in a Mediterranean environment. Field Crops Res. 2007, 101, 187–197. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Seasonal dynamics of biomass, inulin, and water-soluble sugars in roots of Cynara cardunculus L. Field Crops Res. 2010, 116, 147–153. [Google Scholar] [CrossRef]
- Livingston, D.P.; Hincha, D.K.; Heyer, A.G. Fructan and its relationship to abiotic stress tolerance in plants. Cell. Mol. Life Sci. 2009, 66, 2007–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hincha, D.K.; Zuther, E.; Hellwege, E.M.; Heyer, A.G. Specific effects of fructo- and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology 2002, 12, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hincha, D.K.; Livingston, D.P.; Premakumar, R.; Zuther, E.; Obel, N.; Cacela, C.; Heyer, A.G. Fructans from oat and rye: Composition and effects on membrane stability during drying. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 1611–1619. [Google Scholar] [CrossRef] [Green Version]
- Edelman, J.; Jefford, T.G. The mechanisim of fructosan metabolism in higher plants as exemplified in Helianthus tuberosus. New Phytol. 1968, 67, 517–531. [Google Scholar] [CrossRef]
- Valluru, R.; Van Den Ende, W. Plant fructans in stress environments: Emerging concepts and future prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.G.; Cangussu, L.M.B.; Paula, S.L.A.; Melo, G.A.; Silva, E.A. Seasonal changes in fructan accumulation in the underground organs of Gomphrena marginata Seub. (Amaranthaceae) under rock-field conditions. Theor. Exp. Plant Physiol. 2013, 25, 46–55. [Google Scholar] [CrossRef]
- Asega, A.F.; Machado De Carvalho, M.A. Fructan metabolising enzymes in rhizophores of Vernonia herbacea upon excision of aerial organs. Plant Physiol. Biochem. 2004, 42, 313–319. [Google Scholar] [CrossRef]
- De Sadeleer, E.; Vergauwen, R.; Struyf, T.; Le Roy, K.; Van den Ende, W. 1-FFT amino acids involved in high DP inulin accumulation in Viguiera discolor. Front. Plant Sci. 2015, 6, 616. [Google Scholar] [CrossRef] [Green Version]
- Rottenberg, A.; Zohary, D. The wild ancestry of the cultivated artichoke. Genet. Resour. Crop Evol. 1996, 43, 53–58. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Mainolfi, A.; Mandolino, G.; Melilli, M.G. Genetic diversity in Cynara cardunculus revealed by AFLP markers: Comparison between cultivars and wild types from Sicily. Plant Breed. 2004, 123, 280–284. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Cavallaro, V.; Melilli, M.G. Intraspecific variability in Cynara cardunculus L. var. sylvestris Lam. Sicilian populations: Seed germination under salt and moisture stresses. J. Arid Environ. 2004, 56, 107–116. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Patanè, C.; Melilli, M.G. Multiple utilisation of the plant in Cynara cardunculus L. var. sylvestris Lam.: Inulin yield. Acta Hortic. 2005, 681, 475–482. [Google Scholar] [CrossRef]
- Raccuia, S.A.; Melilli, M.G. Cynara cardunculus L., a potential source of inulin in the Mediterranean environment: Screening of genetic variability. Aust. J. Agric. Res. 2004, 55, 693. [Google Scholar] [CrossRef]
- Melilli, M.G.; Raccuia, S.A. Influence of shading on flowering induction and inulin metabolism in roots of Cynara cardunculus L. Acta Hortic. 2013, 983, 415–420. [Google Scholar] [CrossRef]
- Melilli, M.G.; Raccuia, S.A. Inulin and inulin metabolizing enzyme activities during the growth cycle of wild cardoon. Acta Hortic. 2012, 942, 419–426. [Google Scholar] [CrossRef]
- Official Methods of Analysis of AOAC International. Method; AOAC International: Gaithersburg, MD, USA, 2001; p. 12. [Google Scholar]
- Sillitti, C.; Melilli, M.G.; Padalino, L.; Bognanni, R.; Tringali, S.; Conte, A.; Raccuia, S.A.; Del Nobile, M.A. Healthy pasta production using inulin from cardoon: First results of sensory evaluation. Acta Hortic. 2016, 1147, 407–412. [Google Scholar] [CrossRef]
- Baert, J.R.A. The effect of sowing and harvest date and cultivar on inulin yield and composition of chicory (Cicorium intybus L.) roots. Ind. Crops Prod. 1997, 195–199. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1989; ISBN 0 8138 1561 6. [Google Scholar]
- Raccuia, S.A.; Melilli, M.G. Genetic variation for assimilate accumulation and translocation in Cynara spp. Acta Hortic. 2004, 660, 241–248. [Google Scholar] [CrossRef]
- Hellwege, E.M.; Raap, M.; Gritscher, D.; Willmitzer, L.; Heyer, A.G. Differences in chain length distribution of inulin from Cynara scolymus and Helianthus tuberosus are reflected in a transient plant expression system using the respective 1-FFT cDNAs. FEBS Lett. 1998, 427, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Van Den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Ende, W.; Coopman, M.; Vergauwen, R.; Van Laere, A. Presence of Inulin-Type Fructo-Oligosaccharides and Shift from Raffinose Family Oligosaccharide to Fructan Metabolism in Leaves of Boxtree (Buxus sempervirens). Front. Plant Sci. 2016, 7, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Ende, W.; De Roover, J.; Van Laere, A. Effect of nitrogen concentration on fructan and fructan metabolizing enzymes in young chicory plants (Cichorium intybus). Physiol. Plant. 1999, 105, 2–8. [Google Scholar] [CrossRef]
- Van den Ende, W.; El-Esawe, S.K. Sucrose signaling pathways leading to fructan and anthocyanin accumulation: A dual function in abiotic and biotic stress responses? Environ. Exp. Bot. 2014, 108, 4–13. [Google Scholar] [CrossRef]
- Van den Ende, W.; Van Laere, A.; Le Roy, K.; Vergauwen, R.; Boogaerts, D.; Figueiredo-Ribeiro, R.C.L.; Machado de Carvalho, M.A. Molecular cloning and characterization of a high DP fructan: Fructan 1-fructosyl transferase from Viguiera discolor (Asteraceae) and its heterologous expression in Pichia pastoris. Physiol. Plant. 2005, 125, 419–429. [Google Scholar] [CrossRef]
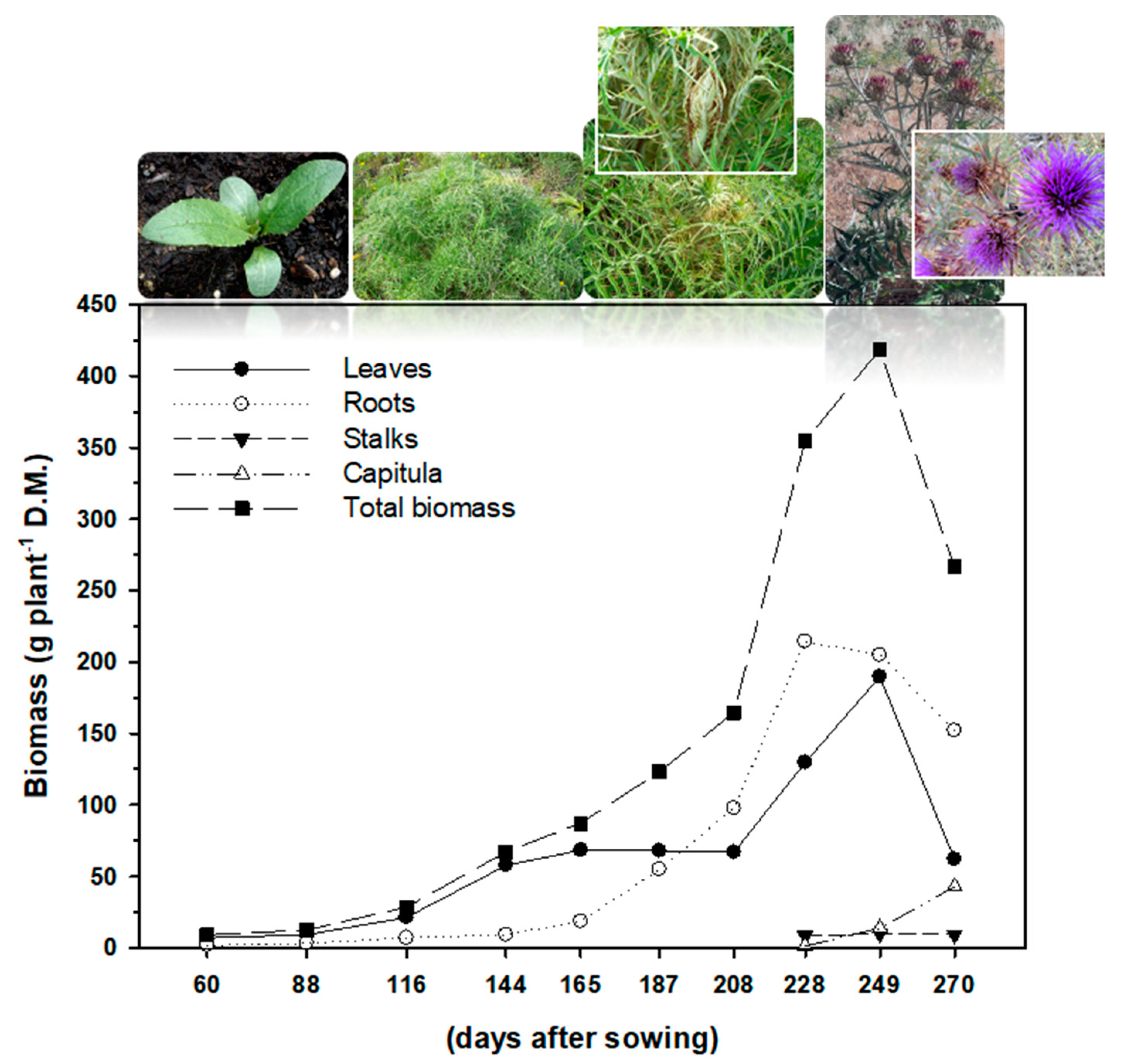

| Days after Sowing | Plant Growth Rate | Leaves | Roots | Stalks | Heads |
|---|---|---|---|---|---|
| (d) | (g plant−1 day−1 D.M.) | (% of Total Biomass) | |||
| 60 | 0.15 g | 79.7 | 20.3 | ||
| 88 | 0.14 g | 74.5 | 25.5 | ||
| 116 | 0.25 f | 74.7 | 25.3 | ||
| 144 | 0.47 e | 86.2 | 13.8 | ||
| 165 | 0.53 d | 78.5 | 21.5 | ||
| 187 | 0.66 cd | 55.3 | 44.7 | ||
| 208 | 0.79 c | 40.7 | 59.3 | ||
| 228 | 1.55 a | 36.6 | 60.6 | 2.43 | 0.39 |
| 249 | 1.47 a | 45.3 | 49.0 | 2.41 | 3.29 |
| 270 | 1.18 b | 23.3 | 57.1 | 3.52 | 16.1 |
| Days after Sowing | Glucose | Fructose | Sucrose |
|---|---|---|---|
| (d) | (g kg−1 D.M.) | ||
| 60 | 24.9 ± 1.10 e * | 6.11 ± 1.12 f | 0.98 ± 0.65 f |
| 88 | 26.8 ± 0.77 de | 6.30 ± 1.12 f | 1.35 ± 1.16 f |
| 116 | 21.7 ± 2.55 f | 29.3 ± 0.61 b | 4.21 ± 0.24 e |
| 144 | 44.5 ± 2.62 a | 30.8 ± 0.33 ab | 3.73 ± 0.48 e |
| 165 | 38.7 ± 1.37 b | 32.3 ± 0.92 a | 5.62 ± 1.19 d |
| 187 | 29.5 ± 1.61 d | 12.7 ± 2.00 e | 16.1 ± 1.39 a |
| 208 | 34.3 ± 1.39 c | 15.6 ± 0.79 d | 9.46 ± 1.95 c |
| 228 | 15.1 ± 1.10 g | 12.3 ± 0.70 e | 9.86 ± 1.41 c |
| 249 | 10.3 ± 2.12 h | 22.4 ± 0.85 c | 12.8 ± 2.12 b |
| 270 | 9.13 ± 2.12 h | 20.7 ± 1.27 c | 13.3 ± 0.71 b |
| Days after Sowing | 1-SST | 1-FFT | 1-FEH | Invertase |
|---|---|---|---|---|
| (d) | (nkat g−1 f.w.) | |||
| 60 | 5.48 f * | 10.4 a | 30.3 c | 2.46 e |
| 88 | 24.8 c | 8.89 c | 11.7 f | 3.43 c |
| 116 | 8.81 e | 9.46 b | 8.19 g | 3.03 d |
| 144 | 18.4 d | 9.71 b | 31.7 c | 10.5 a |
| 165 | 30.2 b | 4.94 e | 32.3 c | 3.34 c |
| 187 | 69.2 a | 3.59 f | 50.8 b | 2.33 e |
| 208 | 2.74 g | 0.90 h | 57.8 a | 3.90 b |
| 228 | 0.30 h | 5.00 e | 19.7 d | 0.57 g |
| 249 | 0 h | 7.17 d | 13.0 f | 1.61 f |
| 270 | 0 h | 2.29 g | 15.8 e | 0.60 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branca, F.; Argento, S.; Paoletti, A.M.; Melilli, M.G. The Physiological Role of Inulin in Wild Cardoon (Cynara cardunculus L. var. sylvestris Lam.). Agronomy 2022, 12, 290. https://doi.org/10.3390/agronomy12020290
Branca F, Argento S, Paoletti AM, Melilli MG. The Physiological Role of Inulin in Wild Cardoon (Cynara cardunculus L. var. sylvestris Lam.). Agronomy. 2022; 12(2):290. https://doi.org/10.3390/agronomy12020290
Chicago/Turabian StyleBranca, Ferdinando, Sergio Argento, Anna Maria Paoletti, and Maria Grazia Melilli. 2022. "The Physiological Role of Inulin in Wild Cardoon (Cynara cardunculus L. var. sylvestris Lam.)" Agronomy 12, no. 2: 290. https://doi.org/10.3390/agronomy12020290






