Alleviation of Cadmium Stress in Wheat through the Combined Application of Boron and Biochar via Regulating Morpho-Physiological and Antioxidant Defense Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Design
2.2. Preparation of Rice Straw Biochar
2.3. Morphological and Yield-Related Attributes
2.4. Determination Photosynthetic Pigments
2.5. Extraction of Enzymes
2.5.1. Determination of Primary Metabolites
2.5.2. Determination of Enzymatic Antioxidant Activities
2.6. MDA Determination
2.7. Determination of Electrolytic Leakage
2.8. Data Analysis
3. Results
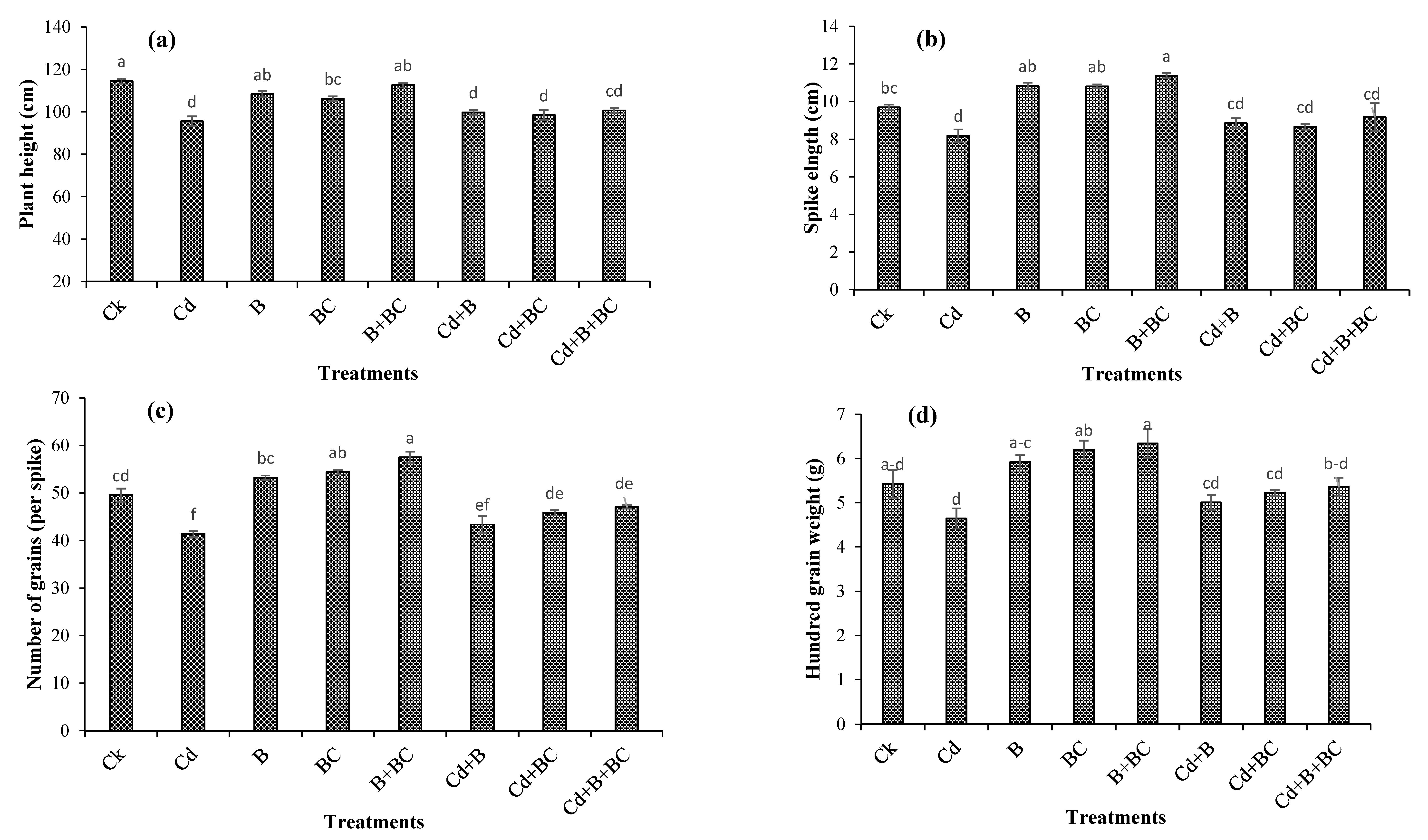
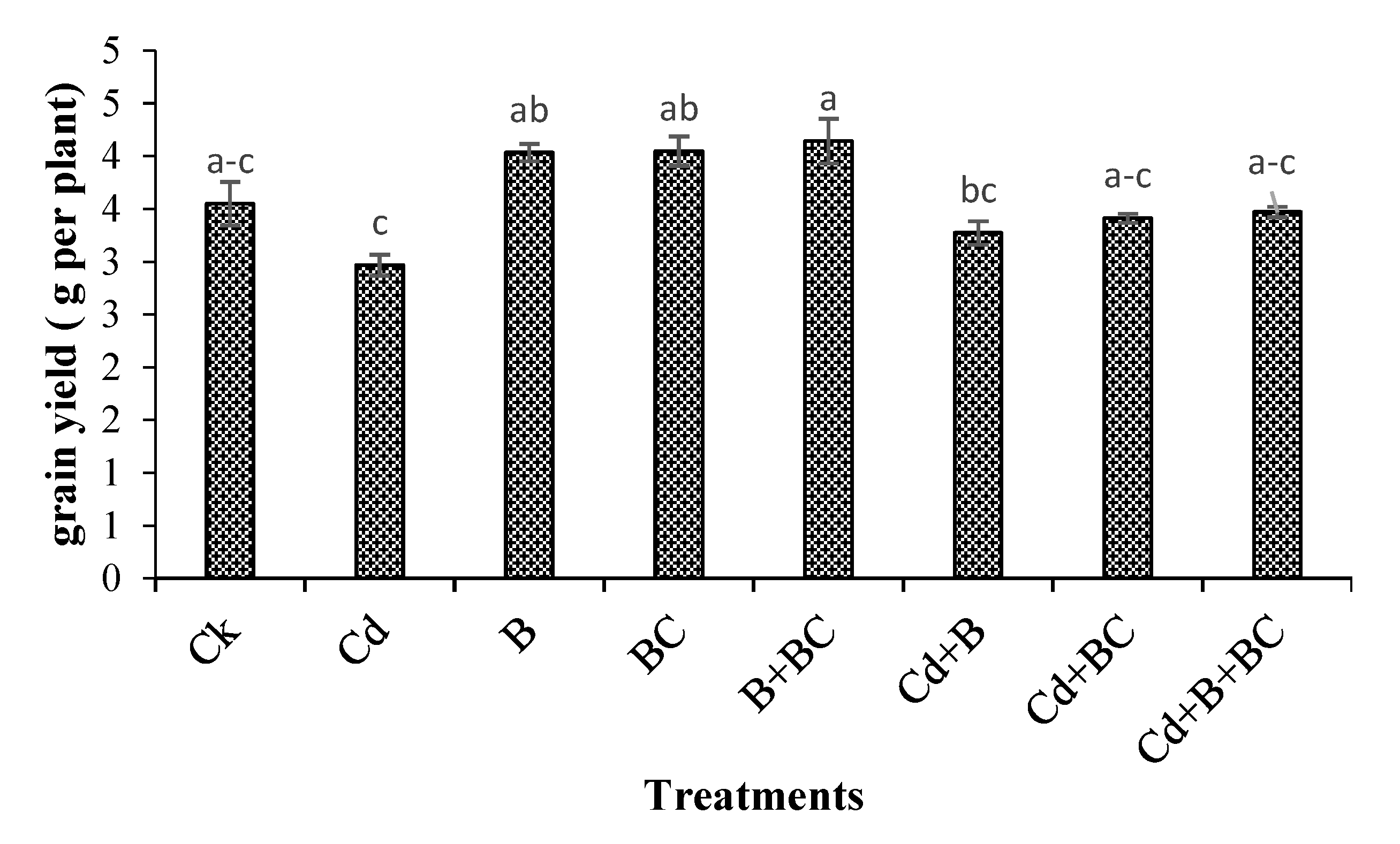
3.1. Plant Height, Yield, and Yield Components
3.2. Photosynthetic Pigments
3.3. Enzymatic Antioxidants Activities
3.4. Lipid Peroxidation
3.5. Osmo-Protectance
4. Discussion
4.1. Morphological and Yield Traits
4.2. Photosynthetic Traits
4.3. Antioxidant Enzyme Activities
4.4. Biochemical Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimumbasilicum L.). Ind. Crops Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q. Physiological responses of maize to elemental sulphur and cadmium stress. Plant Soil Environ. 2018, 52, 523–529. [Google Scholar] [CrossRef]
- Yang, Y. Assessing cadmium exposure risks of vegetables with plant uptake factor and soil property. Environ. Pollut. 2018, 238, 263–269. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Yizhu, L.; Imtiaz, M.; Ditta, A.; Rizwan, M.S.; Ashraf, M.; Mehmood, S.; Aziz, O.; Mubeen, F.; Ali, M.; Elahi, N.N.; et al. Response of growth, antioxidant enzymes and root exudates production towards as stress in Pteris vittata and in Astragalus sinicus colonized by arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2020, 27, 2340–2352. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Rehman, M.Z.U.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968. [Google Scholar] [CrossRef]
- Thind, S.; Hussain, I.; Ali, S.; Hussain, S.; Rasheed, R.; Ali, B.; Hussain, H.A. Physiological and biochemical bases of foliar silicon-induced alleviation of cadmium toxicity in wheat. J. Soil Sci. Plant Nutr. 2020, 20, 2714–2730. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37. [Google Scholar] [CrossRef]
- Bianucci, E.; Peralta, J.M.; Furlan, A.; Hernández, L.E.; Castro, S. Arsenic in wheat, maize, and other crops. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; pp. 279–306. [Google Scholar]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- Hansch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Bie, Z. Boron: Functions and Approaches to Enhance Its Availability in Plants for Sustainable Agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [Green Version]
- Camacho-Cristóbal, J.J.; González-Fontes, A. Boron deficiency decreases plasmalemma H+-ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta 2007, 226, 443–451. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Yang, G.; Rizwan, M.; Ali, S.; Zhou, Y.; Wang, Q.; Deng, L.; Wang, Y.; et al. Boron supply alleviates cadmium toxicity in rice (Oryza sativa L.) by enhancing cadmium adsorption on cell wall and triggering antioxidant defense system in roots. Chemosphere 2021, 266, 128938. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Tan, Q.; Xu, S.; Sun, X. Nitric oxide mediates molybdenum-induced antioxidant defense in wheat under drought stress. Front. Plant Sci. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Srivastava, H.M.; Günerhan, H.; Ghanbari, B. Exact traveling wave solutions for resonance nonlinear Schrödinger equation with intermodal dispersions and the Kerr law nonlinearity. Math. Methods Appl. Sci. 2019, 42, 7210–7221. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T. Biochar: A sustainable solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Naeem, M.A.; Shabbir, A.; Amjad, M.; Abbas, G.; Imran, M.; Murtaza, B.; Tahir, M.; Ahmad, A. Acid treated biochar enhances cadmium tolerance by restricting its uptake and improving physio-chemical attributes in quinoa (Chenopodium quinoa Willd). Ecotoxicol. Environ. Saf. 2020, 191, 110–218. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Emara, H.A.; Nower, A.A.; Abodiab, L. In vitro cultivation of potato plants. Int. J. Curr. Microbiol. Appl. 2016, 5, 858–868. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Abid, M.; Danish, S.; Saeed, M.K.; Ali, M.A. Effects of various biochars on seed germination and carbon mineralization in an alkaline soil. Pak. J. Agric. Sci. 2015, 51, 977–982. [Google Scholar]
- Aron, D. Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Van-Slyke, D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943, 150, 231–233. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A.J. The estimation of carbohydrate in plant extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Julkunen-Titto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1980, 32, 93–101. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Reis, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic peroxidases and lignification in needles of Norway Spruce Piceaabies L. Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Khalid, H.; Akmal, F.; Ali, S.; Rizwan, M.; Qayyum, M.F.; Iqbal, M.; Khalid, M.U.; Azhar, M. Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field. Environ. Pollut. 2017, 227, 560–568. [Google Scholar] [CrossRef]
- Bashir, S.; Rehman, M.; Yousaf, M.; Salam, A.; Gulshan, A.B.; Iqbal, J.; Aziz, I.; Azeem, M.; Rukh, S.; Asghar, R.M.A. Comparative efficiency of wheat straw and sugarcane bagasse biochar reduces the cadmium bioavailability to spinach and enhances the microbial activity in contaminated soil. Int. J. Phytoremediation 2019, 21, 1098–1103. [Google Scholar] [CrossRef]
- Ali, I.; Ullah, S.; He, L.; Zhao, Q.; Iqbal, A.; Wei, S.; Shah, T.; Ali, N.; Bo, Y.; Adnan, M.; et al. Combined application of biochar and nitrogen fertilizer improves rice yield, microbial activity and N-metabolism in a pot experiment. Peer J. 2020, 8, e10311. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Akmal, M.; Riaz, M.; Hu, H.Q.; Ijaz, S.S.; Iqbal, M.; Abro, S.; Mehmood, S.; Ahmad, M. Sugarcane bagasse-derived biochar reduces the cadmium and chromium bioavailability to mash bean and enhances the microbial activity in contaminated soil. J. Soils Sediments 2018, 18, 874–886. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Ziaeyan, A.H.; Rajaie, M. Combined effect of zinc and boron on yield and nutrients accumulation in corn. Int. J. Plant Prod. 2009, 3, 33–45. [Google Scholar]
- Ahmed, W.; Niaz, A.; Kanwal, S.; Rahmatullah, A. Role of boron in plant growth. J. Agric. Res. 2009, 47, 329–338. [Google Scholar]
- Xu, C.Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Li, L.; Ai, S.; Li, Y.; Wang, Y.H.; Tang, M.D. Exogenous silicon mediates alleviation of cadmium stress by promoting photosynthetic activity and activities of antioxidative enzymes in rice. J. Plant Growth Regul. 2018, 37, 602–611. [Google Scholar] [CrossRef]
- Deng, X.P.; Cheng, Y.J.; Wu, X.B.; Kwak, S.S.; Chen, W.; Egrinya, A. Exogenous hydrogen peroxide positively influences root growth and metabolism in leaves of sweet potato seedlings. Aust. J. Crop Sci. 2012, 6, 1572–1578. [Google Scholar]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Parrotta, L.; Guerriero, G.; Sergeant, K.; Cai, G.; Hausman, J.F. Target or barrier? The cell walls of early-and later-diverging plants vs. cadmium toxicity: Differences in the response mechanisms. Front. Plant Sci. 2015, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Voxeur, A.; Fry, S.C. Glycosylinositolphosphorylceramides (GIPCs) from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. Plant J. 2014, 79, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Koszelnik-Leszek, A. Structural, physiological and genetic diversification of Silene vulgaris ecotypes from heavy metal-contaminated areas and their synchronous in vitro cultivation. Planta 2019, 249, 1761–1778. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Ippolito, J.A.; Watts, D.W.; Sigua, G.C.; Ducey, T.F.; Johnson, M.G. Biochar compost blends facilitate switchgrass growth in mine soils by reducing Cd and Zn bioavailability. Biochar 2019, 1, 97–114. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. Springerplus 2016, 5, 1290. [Google Scholar] [CrossRef] [Green Version]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Wang, Y.; Imran, M.; Jiang, C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J. Environ. Manag. 2018, 208, 149–158. [Google Scholar] [CrossRef]
- Gutsch, A.; Keunen, E.; Guerriero, G.; Renaut, J.; Cuypers, A.; Hausman, J.F.; Sergeant, K.; Luo, Z.B. Long-term cadmium exposure influences the abundance of proteins that impact the cell wall structure in Medicago sativa stems. Plant Biol. 2018, 20, 1023–1035. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; He, Y.; Ye, L.; Shen, T.; Liu, F.; Kong, W. Moisture influence reducing method for heavy metals detection in plant materials using laser-induced breakdown spectroscopy: A case study for chromium content detection in rice leaves. Anal. Chem. 2017, 89, 7593–7600. [Google Scholar] [CrossRef]
- Noreen, S.; Faiz, S.; Akhter, M.S.; Shah, K.H. Influence of Foliar Application of Osmoprotectants to Ameliorate Salt Stress in Sunflower (Helianthus annuus L.). Sarhad J. Agric. 2019, 35, 1316–1325. [Google Scholar] [CrossRef]
- Wang, Y.M.; Gao, Q.; Xue, L.H.; Yang, L.Z.; Li, H.X.; Feng, Y.F. Effects of different biochar application patterns on rice growth and yield. J. Agric. Resour. Environ. 2018, 1, 58–65. [Google Scholar]
- Hasanuzzaman, M.; Banerjee, A.; BorhannuddinBhuyan, M.H.M.; Roychoudhury, A.; Al Mahmud, J.; Fujita, M. Targeting glycinebetaine for abiotic stress tolerance in crop plants: Physiological mechanism, molecular interaction and signaling. Phyton 2019, 88, 185–221. [Google Scholar] [CrossRef] [Green Version]
| Treatments | Chlorophyll a (mg g−1) | Chlorophyll b (mg g−1) | Chlorophyll a+b (mg g−1) | Chlorophyll a/b | Total Carotenoid (mg g−1) |
|---|---|---|---|---|---|
| Control (ck) | 2.43 ± 0.02 e | 0.81 ± 0.017 d | 3.24 ± 0.037 e | 3.01 ± 0.039 cd | 0.17 ± 0.003 c |
| Cadmium (Cd) | 2.32 ± 0.01 f | 0.60 ± 0.020 g | 2.92 ± 0.015 f | 3.85 ± 0.013 a | 0.13 ± 0.004 e |
| Boron (B) | 2.79 ± 0.01 b | 0.96 ± 0.011 b | 3.75 ± 0.025 b | 2.89 ± 0.027 d | 0.20 ± 0.002 b |
| Biochar (Bc) | 2.75 ± 0.02 b | 0.86 ± 0.006 c | 3.61 ± 0.017 c | 3.21 ± 0.050 c | 0.18 ± 0.003 c |
| B+Bc | 2.97 ± 0.03 a | 1.03 ± 0.008 a | 4.00 ± 0.023 a | 2.87 ± 0.055 d | 0.22 ± 0.002 a |
| Cd+B | 2.54 ± 0.02 d | 0.72 ± 0.006 ef | 3.26 ± 0.026 e | 3.53 ± 0.015 b | 0.15 ± 0.001 d |
| Cd+Bc | 2.53 ± 0.02 d | 0.70 ± 0.008 f | 3.23 ± 0.027 e | 3.63 ± 0.027 b | 0.15 ± 0.001 d |
| Cd+B+Bc | 2.66 ± 0.01 c | 0.74 ± 0.004 e | 3.39 ± 0.011 d | 3.61 ± 0.030 b | 0.16 ± 0.002 d |
| LSD ≤ 0.01 | 0.079 | 0.038 | 0.090 | 0.203 | 0.010 |
| Treatments | Superoxide Dismutase (Unit mg−1 Protein) | Catalase (Unit mg−1 Protein) | Peroxidase (Unit mg−1 Protein) | Ascorbate Peroxidase (Unit mg−1 Protein) |
|---|---|---|---|---|
| Control (ck) | 76.50 ± 1.28 g | 9.38 ± 0.17 d | 6.50 ± 0.117 d | 0.94 ± 0.051 d |
| Cadmium (Cd) | 123.60 ± 2.73 d | 15.02 ± 0.14 b | 9.30 ± 0.098 c | 1.79 ± 0.048 c |
| Boron (B) | 89.53 ± 2.45 f | 11.31 ± 0.44 c | 7.38 ± 0.061 d | 1.02 ± 0.024 d |
| Biochar (Bc) | 94.55 ± 1.57 f | 10.28 ± 0.57 cd | 7.03 ± 0.070 d | 1.04 ± 0.027 d |
| B+Bc | 105.27 ± 2.58 e | 10.67 ± 0.14 cd | 6.97 ± 0.052 d | 1.12 ± 0.046 d |
| Cd+B | 178.01 ± 2.29 b | 16.34 ± 0.31 b | 11.67 ± 0.149 a | 2.20 ± 0.069 b |
| Cd+Bc | 163.47 ± 3.03 c | 15.33 ± 0.72 b | 10.67 ± 0.167 b | 1.93 ± 0.023 c |
| Cd+B+Bc | 187.35 ± 1.23 a | 18.65 ± 0.21 a | 12.33 ± 0.147 a | 2.53 ± 0.028 a |
| LSD ≤ 0.01 | 7.73 | 1.44 | 0.891 | 0.199 |
| Treatments | Hydrogen Peroxide (µmol g−1) | Malondialdehyde Content (µmol g−1) | Electrolyte Leakage (%) | Soluble Protein (mgg−1fw) |
|---|---|---|---|---|
| Control (ck) | 21.34 ± 0.88 g | 12.69 ± 0.30 e | 35.77 ± 0.75 d | 8.89 ± 0.42 a |
| Cadmium (Cd) | 49.77 ± 0.83 a | 26.38 ± 0.68 a | 78.93 ± 0.89 a | 2.05 ± 0.05 c |
| Boron (B) | 27.90 ± 0.54 ef | 10.65 ± 0.65 f | 28.86 ± 1.97 e | 9.22 ± 0.65 a |
| Biochar (Bc) | 25.66 ± 0.29 f | 11.63 ± 0.27 ef | 30.62 ± 0.59 de | 10.37 ± 0.10 a |
| B+Bc | 29.29 ± 1.15 df | 12.15 ± 0.19 ef | 27.26 ± 1.10 e | 10.30 ± 0.68 a |
| Cd+B | 40.47 ± 0.79 b | 18.50 ± 0.41 c | 57.63 ± 2.02 b | 3.90 ± 0.27 b |
| Cd+Bc | 36.00 ± 0.57 c | 22.22 ± 0.33 b | 62.14 ± 2.19 b | 4.10 ± 0.08 b |
| Cd+B+Bc | 32.38 ± 0.64 d | 15.57 ± 0.40 d | 48.53 ± 0.63 c | 5.52 ± 0.12 b |
| LSD ≤ 0.01 | 3.24 | 1.85 | 5.89 | 1.72 |
| Treatments | Free Proline (mg g−1) | Soluble Sugar (mg g−1) | Total Phenolic (µmol g−1) |
|---|---|---|---|
| Control (ck) | 12.26 ± 0.052 d | 7.50 ± 0.45 cd | 4.79 ± 0.17 bc |
| Cadmium (Cd) | 19.76 ± 0.015 c | 4.80 ± 0.20 e | 2.93 ± 0.50 d |
| Boron (B) | 13.20 ± 0.258 d | 8.47 ± 0.37 bc | 5.64 ± 0.32 ab |
| Biochar (Bc) | 13.03 ± 0.619 d | 9.59 ± 0.11 ab | 6.28 ± 0.41 a |
| B+Bc | 14.21 ± 0.648 d | 9.92 ± 0.30 a | 6.22 ± 0.55 a |
| Cd+B | 23.68 ± 0.787 ab | 6.91 ± 0.05 d | 3.99 ± 0.15 cd |
| Cd+Bc | 21.59 ± 0.799 bc | 7.07 ± 0.19 d | 3.74 ± 0.06 cd |
| Cd+B+Bc | 25.04 ± 0.272 a | 7.39 ± 0.56 cd | 3.93 ± 0.06 cd |
| LSD ≤ 0.01 | 2.30 | 1.25 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Irfan, M.; Sattar, A.; Hussain, S.; Ullah, S.; Abbas, T.; Ur-Rehman, H.; Nawaz, F.; Al-Hashimi, A.; Elshikh, M.S.; et al. Alleviation of Cadmium Stress in Wheat through the Combined Application of Boron and Biochar via Regulating Morpho-Physiological and Antioxidant Defense Mechanisms. Agronomy 2022, 12, 434. https://doi.org/10.3390/agronomy12020434
Hussain S, Irfan M, Sattar A, Hussain S, Ullah S, Abbas T, Ur-Rehman H, Nawaz F, Al-Hashimi A, Elshikh MS, et al. Alleviation of Cadmium Stress in Wheat through the Combined Application of Boron and Biochar via Regulating Morpho-Physiological and Antioxidant Defense Mechanisms. Agronomy. 2022; 12(2):434. https://doi.org/10.3390/agronomy12020434
Chicago/Turabian StyleHussain, Sajjad, Muhammad Irfan, Abdul Sattar, Shabir Hussain, Sami Ullah, Tahira Abbas, Haseeb Ur-Rehman, Farukh Nawaz, Abdulrahman Al-Hashimi, Mohamed S. Elshikh, and et al. 2022. "Alleviation of Cadmium Stress in Wheat through the Combined Application of Boron and Biochar via Regulating Morpho-Physiological and Antioxidant Defense Mechanisms" Agronomy 12, no. 2: 434. https://doi.org/10.3390/agronomy12020434
APA StyleHussain, S., Irfan, M., Sattar, A., Hussain, S., Ullah, S., Abbas, T., Ur-Rehman, H., Nawaz, F., Al-Hashimi, A., Elshikh, M. S., Cheema, M., & Yang, J. (2022). Alleviation of Cadmium Stress in Wheat through the Combined Application of Boron and Biochar via Regulating Morpho-Physiological and Antioxidant Defense Mechanisms. Agronomy, 12(2), 434. https://doi.org/10.3390/agronomy12020434








