Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Treatments
2.2. Rhizobacteria Selection
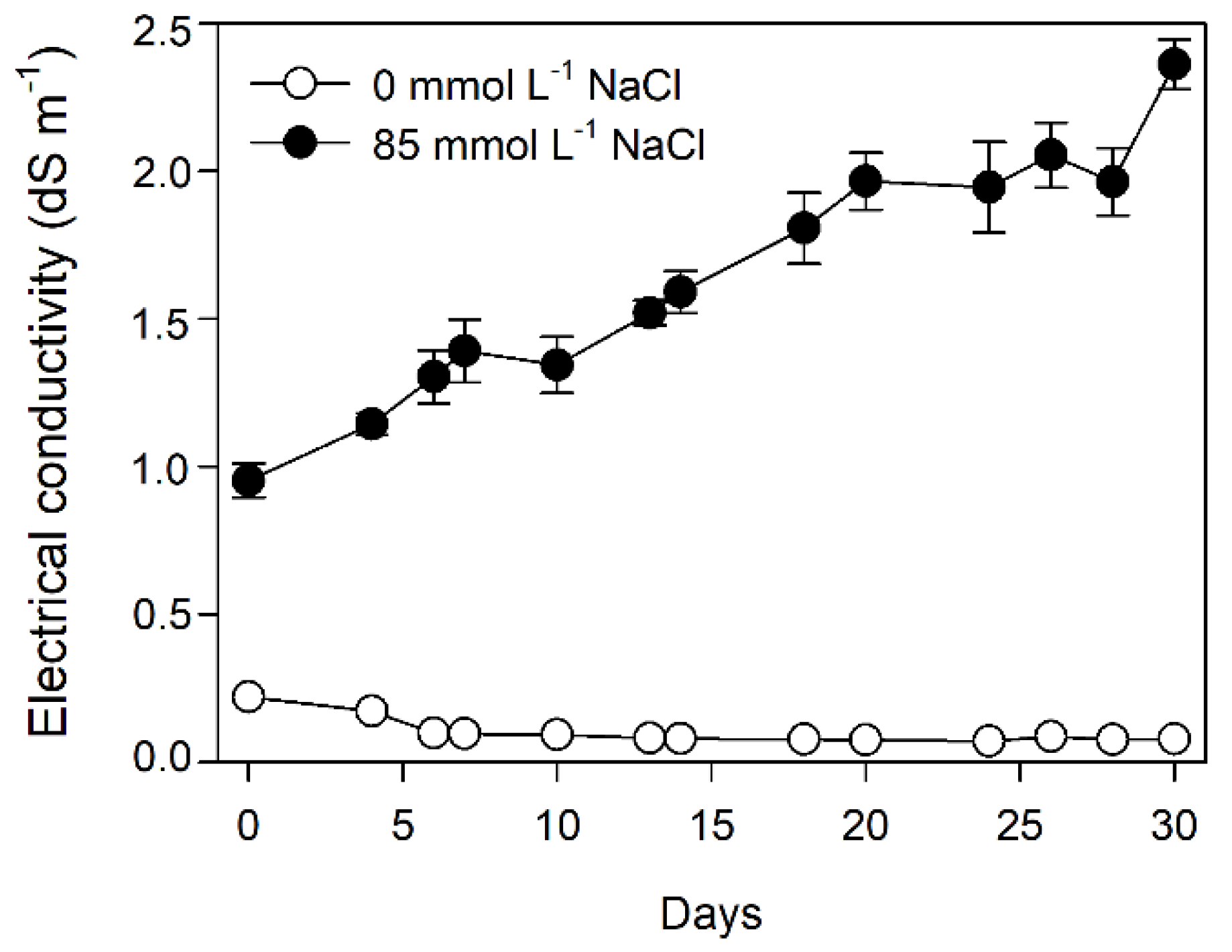
2.3. Electrical Conductivity and Growth Measurements
2.4. Leaf Relative Water Content
2.5. Gas Exchange
2.6. Chlorophyll Fluorescence
2.7. Photosynthetic Pigments
2.8. Nutrient Content
2.9. Biochemical Assays in Swiss Chard Leaves
2.10. Statistical Analysis
3. Results
3.1. Electrical Conductivity and Growth Measurements
3.2. Leaf Relative Water Content and Gas Exchange
3.3. Chlorophyll Fluorescence
3.4. Photosynthetic Pigments
3.5. Nutrient Content
3.6. Flavonoid, Phenolic, and Anthocyanin Concentrations and Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Socolow, R.H. Nitrogen management and the future of food: Lessons from the management of energy and carbon. Proc. Natl. Acad. Sci. USA 1999, 96, 6001–6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoba, P.; Ramakrishnan, S.S. Modeling the contributing factors of desertification and evaluating their relationships to the soil degradation process through geomatic techniques. Solid Earth 2016, 7, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.J.; Flowers, S.A. Why does salinity pose such a difficult problem for plant breeders? Agric. Water Manag. 2005, 78, 15–24. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Sakai, M.; Futamata, H.; Kim, J.S.; Matsuguchi, T. Effect of soil salinity on population structure of fluorescent pseudomonads in spinach rhizosphere. Soil Sci. Plant Nutr. 1998, 44, 701–705. [Google Scholar] [CrossRef]
- Zafar-Ul-Hye, M.; Mahmood, F.; Danish, S.; Hussain, S.; Gul, M.; Yaseen, R.; Shaaban, M. Evaluating efficacy of plant growth promoting rhizobacteria and potassium fertilizer on spinach growth under salt stress. Pak. J. Bot. 2020, 52, 1441–1447. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.S.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Imm. Hungar. 2009, 56, 263–284. [Google Scholar] [CrossRef]
- Mesa-Marín, J.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E.; Redondo-Gómez, S. Synergic Effect Rhizobacteria—Halophytes as a Rising Strategy to Face a Changing World. In Halophytes and Climatic Change: Adaptive mechanisms and Potential Uses; Hasanuzzaman, M., Shabala, S., Fujita, M., Eds.; CABI: Wallingford, UK, 2019; pp. 240–254. [Google Scholar]
- Seneviratne, G.; Weerasekara, M.L.M.A.W.; Seneviratne, K.A.C.N.; Zavahir, J.S.; Kecskes, M.L.; Kennedy, I.R. Importance of Biofilm Formation in Plant Growth Promoting Rhizobacterial Action. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Das, N.; Basak, L.V.G.; Salam, J.A.; Abigail, E.A. Application of biofilms on remediation of pollutants—An overview. J. Microbiol. Biotechnol. 2012, 2, 783–790. [Google Scholar]
- Welbaum, G.E. Family Amaranthaceae, Subfamily Chenopodiaceae. In Vegetable Production and Practices; Welbaum, G.E., Ed.; CPI Group Ltd.: London, UK, 2015; pp. 349–368. [Google Scholar]
- Abdulmalek, M.; Ximba, B.J.; Lewu, F.B. Soil properties, growth, mineral content and ultra-structural leaf morphology of Swiss chard in response to landfill leachates used as irrigation water. Int. J. Agric. Biol. 2017, 19, 403–409. [Google Scholar] [CrossRef]
- Liu, L.; El-Shemy, H.A.; Saneoka, H. Effects of 5-aminolevulinic acid on water uptake, ionic toxicity, and antioxidant capacity of Swiss chard (Beta vulgaris L.) under sodic-alkaline conditions. J. Plant Nutr. Soil Sci. 2017, 180, 535–543. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mesa-Marín, J.; Pérez-Romero, J.A.; López-Jurado, J.; García-López, J.V.; Mariscal, V.; Molina-Heredia, F.P.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Flowers, T.J.; et al. Consortia of plant-growth-promoting rhizobacteria isolated from halophytes improve response of eight crops to soil salinization and climate change conditions. Agronomy 2021, 11, 1609. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. Amst. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Bernabeu-Meana, M.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Redondo-Gómez, S. Effect of Plant Growth-Promoting Rhizobacteria on Salicornia ramosissima Seed Germination under Salinity, CO2 and Temperature Stress. Agronomy 2019, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Gómez, S.; Mateos Naranjo, E.; Davy, A.J.; Fernández-Muñoz, F.; Castellanos, E.M.; Luque, T.; Figueroa, E. Growth and Photosynthetic Responses to Salinity of the Salt-marsh Shrub Atriplex portulacoides. Ann. Bot. 2007, 100, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Marín, J.; Pérez-Romero, J.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Impact of Plant Growth Promoting Bacteria on Salicornia ramosissima Ecophysiology and Heavy Metal Phytoremediation Capacity in Estuarine Soils. Front. Microbiol. 2020, 11, 553018. [Google Scholar] [CrossRef]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Duarte, B.; Santos, D.; Marques, J.C.; Caçador, I. Ecophysiological constraints of two invasive plant species under a saline Gradient: Halophytes versus Glycophytes. Estuar. Coast. Shelf Sci. 2015, 167, 154–165. [Google Scholar] [CrossRef]
- Küpper, H.; Seibert, S.; Parameswaran, A. Fast, sensitive, and inexpensive alternative to analytical pigment HPLC: Quantification of chlorophylls and carotenoids in crude extracts by fitting with Gauss peak Spectra. Anal. Chem. 2007, 79, 7611–7627. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Faizan, S.; Gulzar, B.; Hakeem, K.R. Inoculation of Rhizobium alleviates salinity stress through modulation of growth characteristics, physiological and biochemical attributes, stomatal activities and antioxidant defence in Cicer arietinum L. J. Plant Growth Regul. 2021, 40, 2148–2163. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: A meta-analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Yasin, N.A.; Akram, W.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A. Halotolerant plant-growth promoting rhizobacteria modulate gene expression and osmolyte production to improve salinity tolerance and growth of Capsicum annum L. Environ. Sci. Pollut. Res. 2018, 25, 23236–23250. [Google Scholar] [CrossRef]
- Aamir, M.; Aslam, A.; Khan, M.Y.; Jamshaid, M.U.; Ahmad, M.; Asghar, H.N.; Zahir, Z.A. Co-inoculation with Rhizobium and plant growth promoting rhizobacteria (PGPR) for inducing salinity tolerance in mung bean under field condition of semi-arid climate. Asian J. Agric. Biol. 2013, 1, 7–12. [Google Scholar]
- Yasin, N.A.; Zaheer, M.M.; Khan, W.U.; Ahmad, S.R.; Ahmad, A.; Ali, A.; Akram, W. The beneficial role of potassium in Cd-induced stress alleviation and growth improvement in Gladiolus grandiflora L. Int. J. Phytorem. 2017, 20, 274–283. [Google Scholar] [CrossRef]
- Kapoor, K.; Srivastava, A. Assessment of salinity tolerance of Vinga mungo var. Pu-19 using ex-vitro and in vitro methods. Asian J. Biotechnol. 2010, 2, 73–85. [Google Scholar]
- Ünlükara, A.; Cemek, B.; Karaman, S.; Ersahin, S. Response of lettuce (Latuca sativa var. crispa) to salinity of irrigation water. N. Z. J. Crop Hortic. 2008, 36, 265–273. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Ruggiero, C.; Barbieri, G. Physiological response of field-grown cabbage to salinity and drought stress. Eur. J. Agron. 2005, 23, 57–67. [Google Scholar] [CrossRef]
- Ntanos, E.; Kekelis, P.; Assimakopoulou, A.; Gasparatos, D.; Denaxa, N.K.; Tsafouros, A.; Roussos, P.A. Amelioration effects against salinity stress in strawberry by bentonite-zeolite mixture, glycine betaine, and Bacillus amyloliquefaciens in terms of plant growth, nutrient content, soil properties, yield, and fruit quality characteristics. Appl. Sci. 2021, 11, 8796. [Google Scholar] [CrossRef]
- Soliman, M.S.; Doss, M. Salinity and mineral nutrition effects on growth and accumulation of organic and inorganic ions in two cultivated tomato varieties. J. Plant Nutr. 1992, 15, 2789–2799. [Google Scholar] [CrossRef]
- Panwar, M.; Tewari, R.; Gulati, A.; Nayyar, H. Indigenous salt-tolerant rhizobacterium Pantoea dispersa (PSB3) reduces sodium uptake and mitigates the effects of salt stress on growth and yield of chickpea. Acta Physiol. Plant 2016, 38, 278. [Google Scholar] [CrossRef]
- Mabasa, N.C.; Jones, C.L.W.; Laing, M. The use of treated brewery effluent for salt tolerant crop irrigation. Agric. Water Manag. 2021, 245, 106590. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Fujita, M.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; DaCosta, M.; Jiang, Y. Research advances in mechanisms of grass tolerance to abiotic stress from physiology to molecular biology. Crit. Rev. Plant Sci. 2014, 33, 141–189. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Govindarajan, R.; Lavania, M.; Pushpangadan, P. Novel mechanism of modulating natural antioxidants in functional foods: Involvement of plant growth promoting rhizobacteria NRRL B-30488. J. Agric. Food Chem. 2008, 56, 4474–4481. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- Pérez-Romero, J.A.; Barcia-Piedras, J.M.; Redondo-Gómez, S.; Mateos-Naranjo, E. Impact of short-term extreme temperature events on physiological performance of the halophyte Salicornia ramosissima J. Woods under optimal and sub-optimal saline conditions. Sci. Rep. 2019, 9, 659. [Google Scholar] [CrossRef]





| Chl a | Chl b | Phe a | β-carotene | Neoxanthin | Violaxanthin | Zeaxanthin | DES | |
|---|---|---|---|---|---|---|---|---|
| 0 mmol L−1 | 227 ± 9.9 c | 75 ± 3.8 b | 13 ± 0.7 c | 13 ± 0.4 a | 15 ± 0.5 c | 12 ± 0.3 a | 14 ± 0.4 a | 0.46 ± 0.005 a |
| 0 mmol L−1+ | 265 ± 6.5 a | 89 ± 2.6 b | 16 ± 0.7 c | 12 ± 0.2 a | 18 ± 0.5 a | 12 ± 0.1 a | 13 ± 0.3 a | 0.47 ± 0.005 a |
| 85 mmol L−1 | 236 ± 11.8 bc | 90 ± 5.2 b | 45 ± 8.4 b | 9 ± 0.4 b | 11 ± 0.7 d | 6 ± 0.3 b | 10 ± 0.4 b | 0.38 ± 0.016 b |
| 85 mmol L−1+ | 269 ± 25.1 ab | 109 ± 10 a | 65 ± 6.3 a | 10 ± 0.3 b | 16 ± 0.6 b | 6 ± 0.6 b | 11 ± 0.3 b | 0.37 ± 0.022 b |
| Ca mg g−1 | Fe mg kg−1 | K mg g−1 | Na mg g−1 | P mg g−1 | Mg mg g−1 | Mn mg kg−1 | Zn mg kg−1 | |
|---|---|---|---|---|---|---|---|---|
| 0mmol L−1 | 1.20 ± 0.004 a | 98 ± 1.6 c | 3.2 ± 0.01 b | 1.7 ± 0.00 b | 1.6 ± 0.01 b | 0.88 ± 0.004 b | 1126 ± 2 a | 212 ± 1 b |
| 0 mmol L−1+ | 1.11 ± 0.019 b | 103 ± 0.2 b | 3.5 ± 0.06 a | 1.7 ± 0.03 b | 1.7 ± 0.03 b | 0.95 ± 0.032 a | 1155 ± 58 a | 259 ± 5 a |
| 85 mmol L−1 | 0.86 ± 0.009 c | 116 ± 2.9 a | 3.2 ± 0.08 b | 5.6 ± 0.11 a | 2.1 ± 0.03 a | 0.81 ± 0.004 c | 1210 ± 24 a | 215 ± 2 b |
| 85 mmol L−1+ | 1.05 ± 0.032 d | 135 ± 5.5 d | 2.6 ± 0.08 c | 5.3 ± 0.14 c | 2.1 ± 0.07 b | 0.95 ± 0.035 b | 1565 ± 50 b | 251 ± 9 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redondo-Gómez, S.; Romano-Rodríguez, E.; Mesa-Marín, J.; Sola-Elías, C.; Mateos-Naranjo, E. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization. Agronomy 2022, 12, 468. https://doi.org/10.3390/agronomy12020468
Redondo-Gómez S, Romano-Rodríguez E, Mesa-Marín J, Sola-Elías C, Mateos-Naranjo E. Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization. Agronomy. 2022; 12(2):468. https://doi.org/10.3390/agronomy12020468
Chicago/Turabian StyleRedondo-Gómez, Susana, Elena Romano-Rodríguez, Jennifer Mesa-Marín, Cristina Sola-Elías, and Enrique Mateos-Naranjo. 2022. "Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization" Agronomy 12, no. 2: 468. https://doi.org/10.3390/agronomy12020468
APA StyleRedondo-Gómez, S., Romano-Rodríguez, E., Mesa-Marín, J., Sola-Elías, C., & Mateos-Naranjo, E. (2022). Consortia of Plant-Growth-Promoting Rhizobacteria Isolated from Halophytes Improve the Response of Swiss Chard to Soil Salinization. Agronomy, 12(2), 468. https://doi.org/10.3390/agronomy12020468









