The Combined Application of Mineral Fertilizer and Organic Amendments Improved the Stability of Soil Water-Stable Aggregates and C and N Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Site Description
2.2. Experimental Layout and Sampling
2.3. Soil Aggregate Fractionation and Chemical Analysis
2.4. Cost Analysis
2.5. Statistical Analysis
3. Results
3.1. Size Distribution of Soil Aggregates
3.2. Mean Weight Diameter (MWD)
3.3. Soil Organic Carbon (SOC) and Total Nitrogen (TN) Concentrations in Aggregates
3.4. Relationship between Aggregate Size Fractions, Aggregate Stability, and Soil Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Six, J.; Elliott, E.T.; Paustian, K. Soil structure and soil organic matter. II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 2000, 64, 1042–1049. [Google Scholar] [CrossRef]
- Wu, Q.C.; Zhang, C.Z.; Yu, Z.H.; Zhang, J.B.; Zhu, C.W.; Zhao, Z.H.; Xiong, J.A.R.; Chen, J.L. Effects of elevated CO2 and nitrogen addition on organic carbon and aggregates in soil planted with different rice cultivars. Plant Soil 2018, 432, 245–258. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Miller, R.M.; Lussenhop, J. Contributions of interacting biological mechanisms to soil aggregate stabilization in restored prairie. Soil Biol. Biochem. 1998, 30, 905–916. [Google Scholar] [CrossRef]
- Miller, R.M.; Jastrow, J.D. Hierarchy of root and mycorrhizal fungal interactions with soil aggregation. Soil Biol. Biochem. 1990, 22, 579–584. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, M.K.; Zhuang, S.Y.; Chiang, P.N. Chemical and physical properties of rhizosphere and bulk soils of three tea plants cultivated in Ultisols. Geoderma 2006, 136, 378–387. [Google Scholar] [CrossRef]
- Whalley, W.R.; Riseley, B.; Harrison, P.B.L.; Bird, N.R.A.; Leech, P.K.; Adderley, W.P. Structural differences between bulk and rhizosphere soil. Europ. J. Soil Sci. 2005, 56, 353–360. [Google Scholar] [CrossRef]
- Tripathi, R.; Nayak, A.K.; Bhattacharyya, P.; Shukla, A.K.; Shahid, M.; Raja, R.; Panda, B.B.; Mohanty, S.; Kumar, A.; Thilagam, V.K. Soil aggregation and distribution of carbon and nitrogen in different fractions after 41 years long-term fertilizer experiment in tropical rice-rice system. Geoderma 2014, 213, 280–286. [Google Scholar] [CrossRef]
- Barbosa, G.M.C.; Oliveira, J.F.; Miyazawa, M.; Ruiz, D.B.; Filho, J.T. Aggregation and clay dispersion of an oxisol treated with swine and poultry manures. Soil Till. Res. 2015, 146, 279–285. [Google Scholar] [CrossRef]
- Soon, Y.K.; Lupwayi, N.Z. Straw management in a cold semi-arid region: Impact on soil quality and crop productivity. Field Crops Res. 2012, 139, 39–46. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, T.; Jia, Z.K.; Han, Q.F.; Ren, X.L. Soil aggregate and crop yield changes with different rates of straw incorporation in semiarid areas of northwest China. Geoderma 2014, 230–231, 41–49. [Google Scholar] [CrossRef]
- Xin, X.L.; Zhang, J.B.; Zhu, A.N.; Zhang, C.Z. Effects of long-term (23 years) mineral fertilizer and compost application on physical properties of fluvo-aquic soil in the North China Plain. Soil Till. Res. 2016, 156, 166–172. [Google Scholar] [CrossRef]
- Aramrak, S.; Chittamart, N.; Wisawapipat, W.; Rattanapichai, W.; Phun-lam, M.; Aramrak, A. Dynamics of soil aggregate stability as induced by potassium in a soil-plant system. Soil Sci. Plant Nutr. 2021, 67, 371–379. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.Q.; Sun, J.W.; Wang, X.B.; He, P.; Zhou, W. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol. Biochem. 2013, 57, 30–42. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis, Part1. Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar] [CrossRef]
- Wang, X.J.; Jia, Z.K.; Liang, L.Y.; Yang, B.P.; Ding, R.X.; Nie, J.F.; Wang, J.P. Maize straw effects on soil aggregation and other properties in arid land. Soil Till. Res. 2015, 153, 131–136. [Google Scholar] [CrossRef]
- Pinheiro, E.F.M.; Pereira, M.G.; Anjos, L.H.C. Aggregate distribution and soil organic matter under different tillage systems for vegetable crops in a Red Latosol from Brazil. Soil Till. Res. 2004, 77, 79–84. [Google Scholar] [CrossRef]
- Tian, J.; Fan, M.S.; Guo, J.H.; Marschner, P.; Li, X.L.; Kuzyakov, Y. Effects of land use intensity on dissolved organic carbon properties and microbial community structure. Eur. J. Soil Biol. 2012, 52, 67–72. [Google Scholar] [CrossRef]
- Gao, Q.; Mi, W.H.; Xia, S.Q.; Liu, M.Y.; Mao, W.; Ju, J.; Zhao, H.T. Aggregate composition, stability and nutrient distribution characteristics in yellow clayey paddy soil under long-term different fertilization measures. J. Henan Agric. Sci. 2021, 50, 70–81. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.P.; Lair, G.J.; Kram, P.; Nikoladidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N.J. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247–248, 24–37. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Bendall, J.S.; Jara, A.A.; Welland, M.E.; Theng, B.K.G.; Rumpel, C.; de la Luz Mora, M. Nanoclays from an Andisol: Extraction, properties and carbon stabilization. Geoderma 2011, 161, 159–167. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S.; Stephen, R.C. Influence of mixed cropping rotations (pasture-arable) on organic matter content, water stable aggregation and clod porosity in a group of soils. Soil Till. Res. 1991, 19, 77–87. [Google Scholar] [CrossRef]
- Jastrow, J.D. Soil aggregate formation and the accural of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- Zhang, R.F.; Vivanco, J.M.; Shen, Q.R. The unseen rhizosphere root-soil-microbe interactions for crop production. Cur. Opin. Microbio. 2017, 37, 8–14. [Google Scholar] [CrossRef]
- Xie, M.M.; Zou, Y.N.; Wu, Q.S.; Zhang, Z.Z.; Kuča, K. Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ. 2020, 66, 287–294. [Google Scholar] [CrossRef]
- Akinola, S.A.; Babalola, O.O. The importance of adverse soil microbiomes in the light of omics: Implications for food safety. Plant Soil Environ. 2020, 66, 421–430. [Google Scholar] [CrossRef]
- Beauregrad, P.B.; Chai, Y.R.; Vlamakis, H.; Losick, R.; Kolter, R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc. Natl. Acad. Sci. USA 2013, 110, E1621–E1630. [Google Scholar] [CrossRef] [Green Version]
- Öztaş, T.; Sönmez, K.; Canbolat, M.Y. Strength of individual soil aggregates against crushing forces I. Influence of aggregate characteristics. Turk. J. Agric. For. 1999, 23, 567–572. [Google Scholar]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability-A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A. Economic considerations on nutrient utilization in wastewater management. Energies 2021, 14, 3468. [Google Scholar] [CrossRef]
- Maroušek, J.; Marouškov, A.; Zoubek, T.; Bartoš, P. Economic impacts of soil fertility degradation by traces of iron from drinking water treatment. Environ. Develop. Sustain. 2021, 1–10. [Google Scholar] [CrossRef]
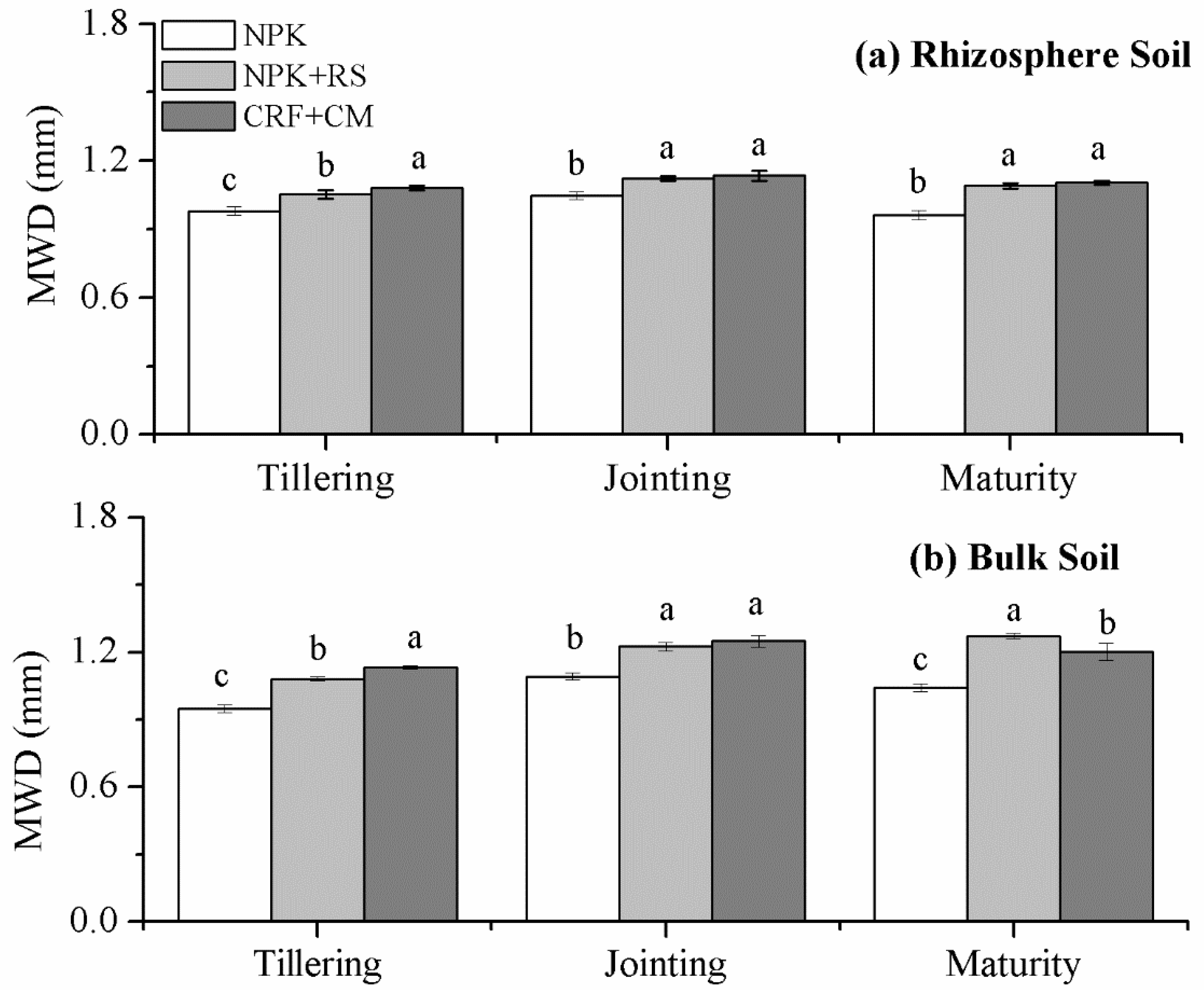
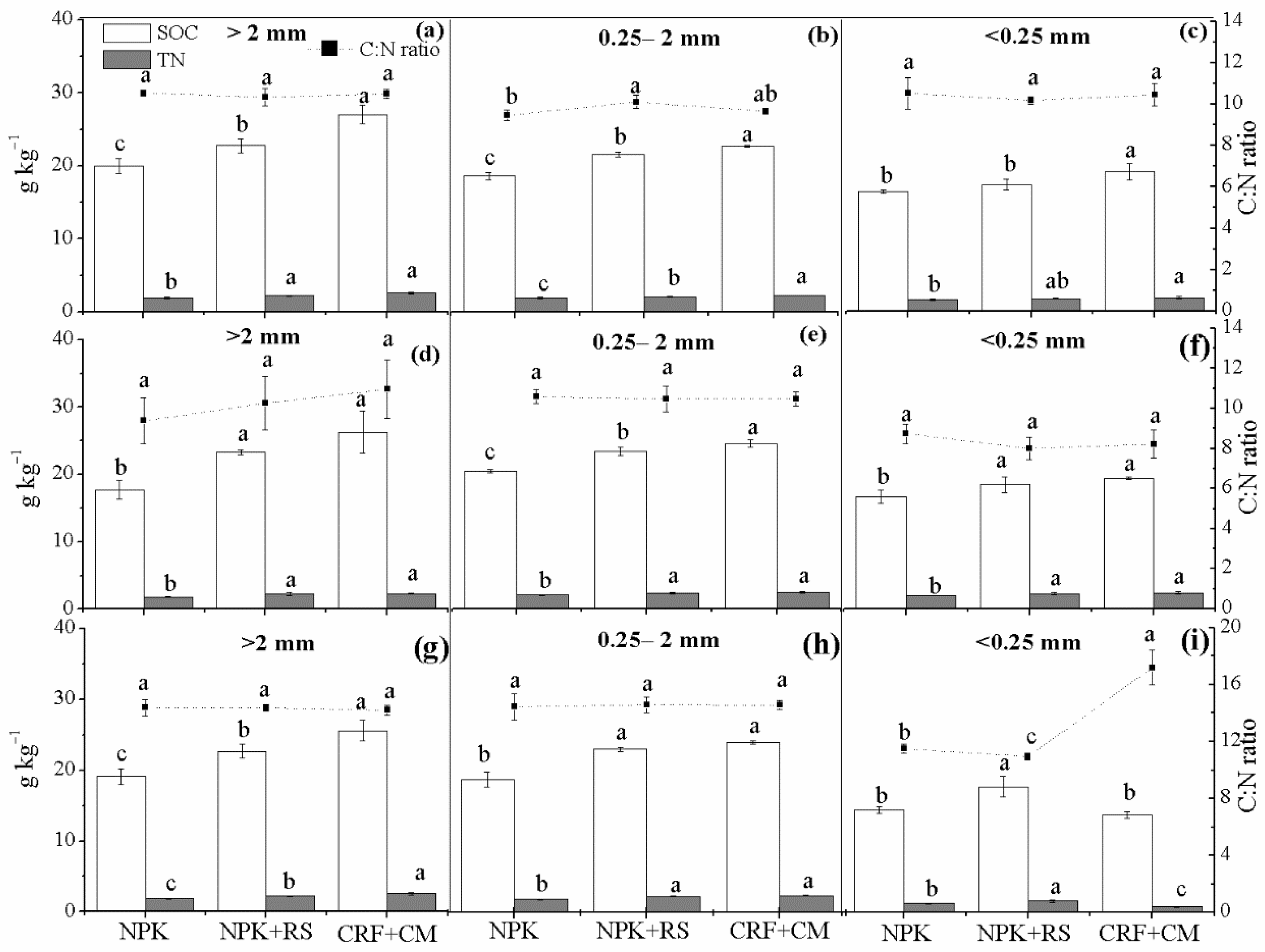
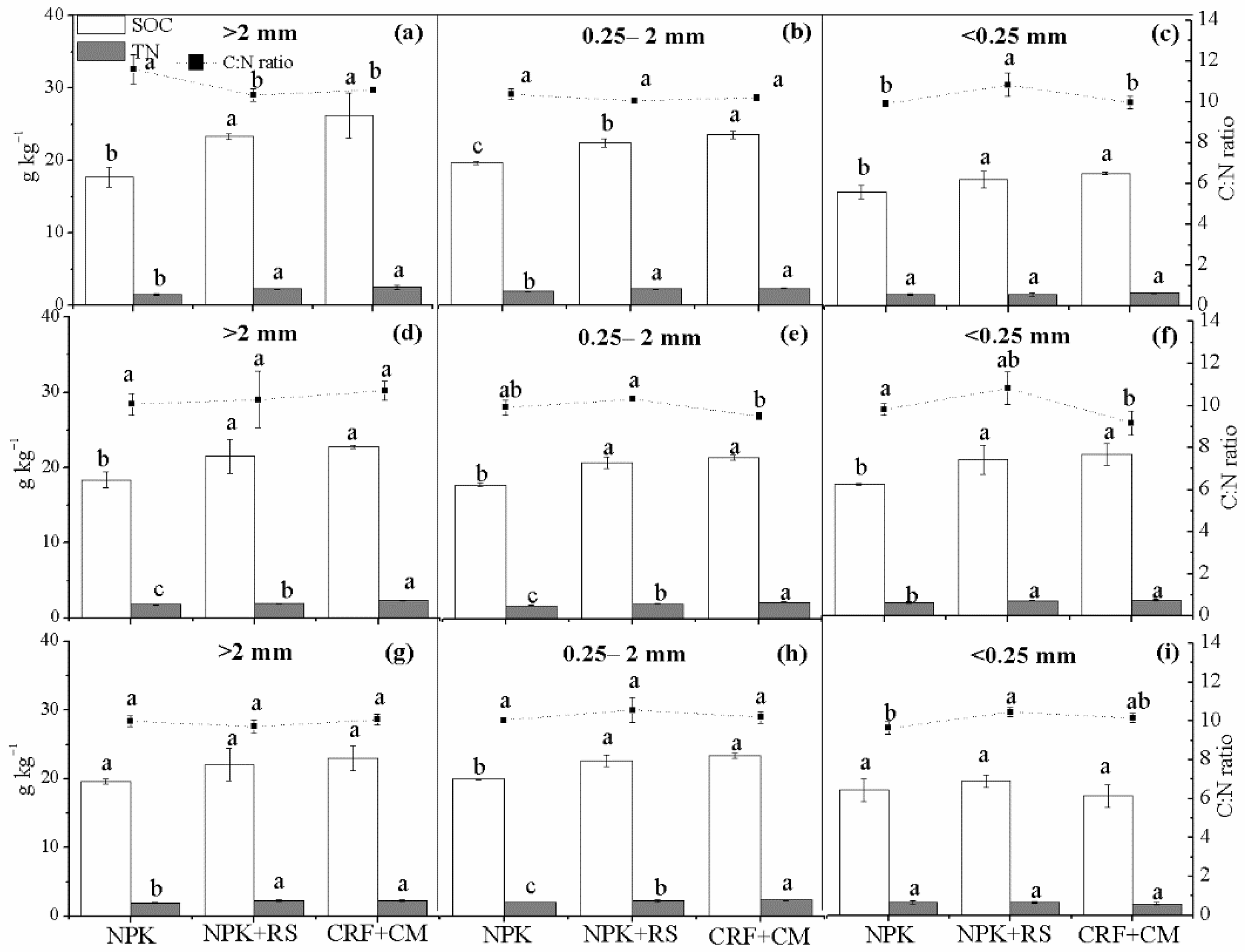
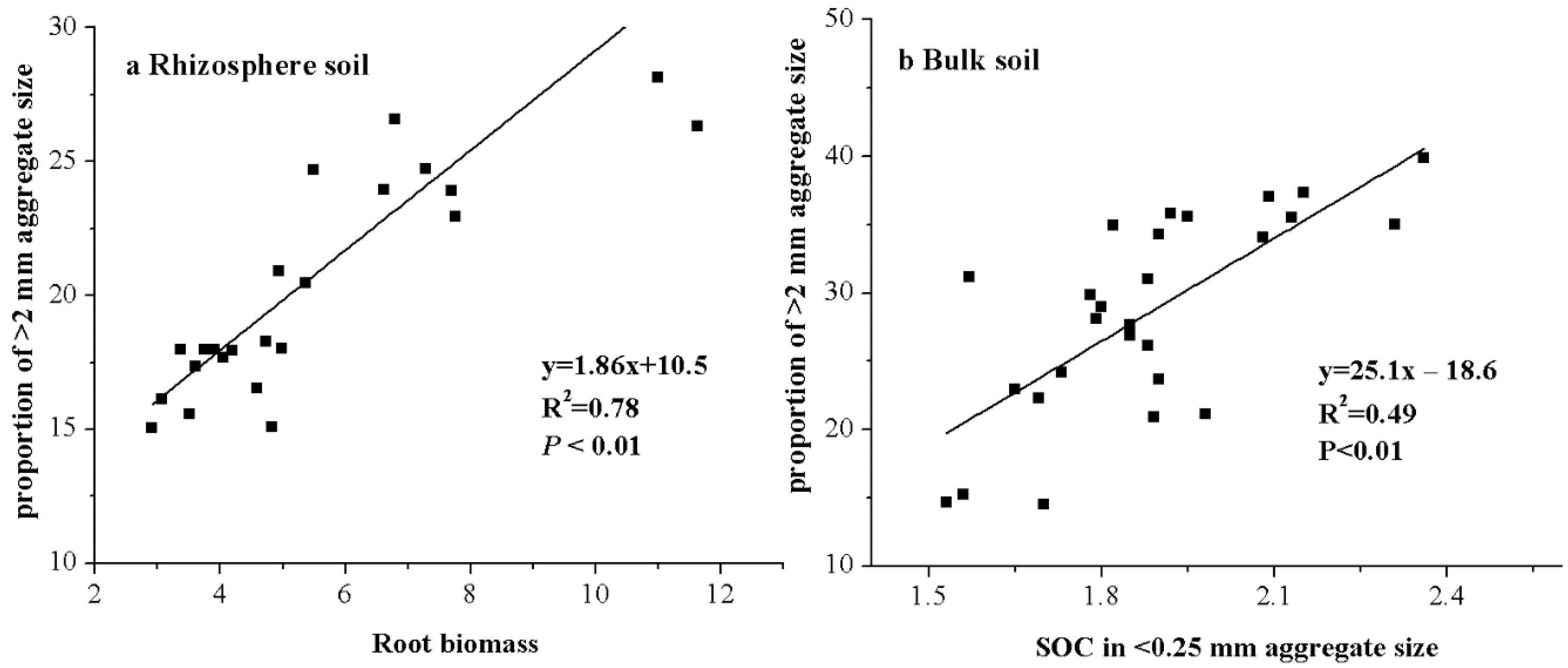
| Sampling Time | Treatment | Rhizosphere Soil | Bulk Soil | ||||
|---|---|---|---|---|---|---|---|
| >2 mm | 0.25–2 mm | <0.25 mm | >2 mm | 0.25–2 mm | <0.25 mm | ||
| Tillering | NPK | 15.6 ± 0.5 b | 52.2 ± 1.3 c | 32.2 ± 1.7 a | 14.8 ± 0.4 c | 50.3 ± 2.0 a | 34.9 ± 2.0 a |
| NPK + RS | 17.8 ± 0.4 a | 56.3 ± 1.4 b | 26.0 ± 1.7 b | 23.4 ± 1.0 b | 48.3 ± 1.7 a | 28.3 ± 1.0 b | |
| CRF + CM | 17.9 ± 0.2 a | 59.3 ± 0.9 a | 22.8 ± 1.1 c | 26.9 ± 0.8 a | 47.0 ± 2.1 a | 26.0 ± 1.4 b | |
| Jointing | NPK | 25.1 ± 1.4 b | 41.1 ± 0.9 b | 33.8 ± 0.5 a | 29.0 ± 0.9 b | 38.2 ± 1.1 a | 32.8 ± 1.1 a |
| NPK + RS | 31.1 ± 0.4 a | 37.6 ± 0.4 c | 31.3 ± 0.7 b | 36.1 ± 1.2 a | 39.3 ± 0.5 a | 24.6 ± 1.1 b | |
| CRF + CM | 28.3 ± 2.1 a | 44.5 ± 2.1 a | 27.2 ± 1.1 c | 37.5 ± 2.2 a | 39.2 ± 1.4 a | 23.3 ± 0.8 b | |
| Maturity | NPK | 16.6 ± 1.6 c | 47.8 ± 1.1 b | 36.2 ± 0.6 a | 21.7 ± 1.1 b | 47.3 ± 1.4 a | 31.0 ± 1.3 a |
| NPK + RS | 19.8 ± 1.6 b | 56.4 ± 2.0 a | 23.8 ± 0.8 c | 34.6 ± 0.8 a | 47.7 ± 0.5 a | 17.6 ± 0.5 c | |
| CRF + CM | 23.9 ± 0.9 a | 49.9 ± 1.9 b | 26.2 ± 1.2 b | 32.4 ± 2.2 a | 44.1 ± 2.0 b | 23.5 ± 2.5 b | |
| Sampling Time | Treatment | Rhizosphere Soil | Bulk Soil | ||||
|---|---|---|---|---|---|---|---|
| >2 mm | 0.25–2 mm | <0.25 mm | >2 mm | 0.25–2 mm | <0.25 mm | ||
| Tillering | NPK | 16.9 ± 0.8 c | 54.1 ± 1.4 b | 29.0 ± 2.0 a | 14.7 ± 1.2 c | 54.0 ± 0.4 a | 31.3 ± 1.3 a |
| NPK + RS | 19.3 ± 0.5 b | 59.2 ± 0.9 a | 21.5 ± 0.5 b | 25.9 ± 0.9 b | 50.3 ± 2.1 b | 23.8 ± 1.2 b | |
| CRF + CM | 20.9 ± 0.5 a | 60.1 ± 1.6 a | 19.0 ± 1.4 b | 31.1 ± 1.8 a | 47.6 ± 1.5 b | 21.4 ± 1.9 b | |
| Jointing | NPK | 25.0 ± 0.3 b | 44.5 ± 0.9 a | 30.5 ± 1.2 a | 29.2 ± 0.3 b | 38.6 ± 1.4 a | 32.2 ± 1.2 a |
| NPK + RS | 34.4 ± 0.1 a | 39.2 ± 1.1 b | 26.4 ± 1.3 b | 36.2 ± 2.5 a | 39.4 ± 2.5 a | 24.4 ± 1.6 b | |
| CRF + CM | 32.7 ± 4.6 a | 45.0 ± 3.6 a | 22.3 ± 1.7 c | 38.2 ± 1.7 a | 38.9 ± 2.1 a | 22.9 ± 0.9 b | |
| Maturity | NPK | 18.5 ± 1.4 b | 51.1 ± 2.1 b | 30.3 ± 0.7 a | 22.2 ± 1.7 b | 48.0 ± 0.7 a | 29.8 ± 2.3 a |
| NPK + RS | 21.0 ± 0.8 b | 59.4 ± 2.2 a | 19.7 ± 2.0 b | 35.2 ± 2.6 a | 48.7 ± 1.5 a | 16.1 ± 1.1 b | |
| CRF + CM | 28.5 ± 1.6 a | 54.7 ± 2.1 b | 16.8 ± 0.6 c | 34.4 ± 3.7 a | 46.5 ± 2.3 a | 19.0 ± 1.5 b | |
| Aggregate Size | MWD | |||||
|---|---|---|---|---|---|---|
| >2 mm | 0.25–2 mm | <0.25 mm | ||||
| Rhizosphere soil | ||||||
| SOC | >2 mm | 0.238 | 0.274 | −0.731 *** | 0.662 *** | |
| 0.25–2 mm | 0.320 | 0.290 | −0.865 *** | 0.808 *** | ||
| <0.25 mm | 0.135 | 0.189 | −0.478 * | 0.409 * | ||
| Root biomass | 0.889 *** | −0.7 *** | 0.043 | 0.668 *** | ||
| Bulk soil | ||||||
| SOC | >2 mm | 0.368 | 0.084 | −0.576 ** | 0.482 * | |
| 0.25–2 mm | 0.499 ** | 0.071 | −0.746 *** | 0.638 *** | ||
| <0.25 mm | 0.720 *** | −0.473 * | −0.582 ** | 0.700 *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, W.; Chen, C.; Ma, Y.; Guo, S.; Liu, M.; Gao, Q.; Wu, Q.; Zhao, H. The Combined Application of Mineral Fertilizer and Organic Amendments Improved the Stability of Soil Water-Stable Aggregates and C and N Accumulation. Agronomy 2022, 12, 469. https://doi.org/10.3390/agronomy12020469
Mi W, Chen C, Ma Y, Guo S, Liu M, Gao Q, Wu Q, Zhao H. The Combined Application of Mineral Fertilizer and Organic Amendments Improved the Stability of Soil Water-Stable Aggregates and C and N Accumulation. Agronomy. 2022; 12(2):469. https://doi.org/10.3390/agronomy12020469
Chicago/Turabian StyleMi, Wenhai, Chao Chen, Yingying Ma, Shaokang Guo, Mingyue Liu, Qiang Gao, Qicong Wu, and Haitao Zhao. 2022. "The Combined Application of Mineral Fertilizer and Organic Amendments Improved the Stability of Soil Water-Stable Aggregates and C and N Accumulation" Agronomy 12, no. 2: 469. https://doi.org/10.3390/agronomy12020469
APA StyleMi, W., Chen, C., Ma, Y., Guo, S., Liu, M., Gao, Q., Wu, Q., & Zhao, H. (2022). The Combined Application of Mineral Fertilizer and Organic Amendments Improved the Stability of Soil Water-Stable Aggregates and C and N Accumulation. Agronomy, 12(2), 469. https://doi.org/10.3390/agronomy12020469





