Abstract
This work examines in silico the dominant geochemical processes that control inorganic nutrients (Ca, Mg, Na, K) availability in irrigated agricultural soil amended with potassium-enriched biochar (from olive mill wastes) at mass doses of 0.5%, 1%, 2% and 10%. The geochemical modelling step was supported by analytical measurements regarding the physicochemical characteristics of the irrigation water, the agricultural soil and the biochar. Two geochemical approaches, namely equilibrium exchange (E.E.) and kinetic exchange (K.E.) models were applied and compared to assess nutrient release with an emphasis on potassium availability. Equilibrium exchange perspective assumed that nutrient release is controlled by ion-exchange reactions onto the biochar surface, whilst kinetic exchange perspective assumed the contribution of both ion-exchange and dissolution of salts. Results indicated that for the E.E. model, the soluble amount of potassium is readily available for transport within the pores of the porous media, and therefore is leached from the column within only 10 days. For the K.E. model that assumes a kinetically controlled release of potassium due to interactions occurring at the solid-solution interface, the assessed retention times were more realistic and significantly higher (up to 100 days). Concerning the applied doses of biochar, for a 2% biochar fraction mixed with soil, for example, the available K for plants doubled compared with the available K in the soil without biochar. In any case, the use of numerical modeling was proven helpful for a quick assessment of biochar performance in soil, by avoiding time-consuming and laborious experimental set-ups. Validation of the models by experimental data will further establish the proposed mechanisms.
Keywords:
biochar; olive mill wastes; potassium; column experiments; kinetics; ion-exchange; PHREEQC 1. Introduction
Carbon storage in healthy agricultural soils constitute a key factor for soil biodiversity and fertility, food security, and climate change mitigation [1,2]. Agricultural soil fertilization is also a critical factor, as it can act beneficially in soil improvement, and therefore in crops growth and yields [3]. At the same time, the excessive and irrational use of inorganic fertilizers may be responsible for land degradation [4], nutrients leaching from agricultural soils [5] and freshwater body eutrophication [6]. One of the promising strategies for achieving a better soil nutrient balance as well as reducing aquifer contamination is to use slow-release nutrient biofertilizers instead of synthetic industrial fertilizers [7,8] and environmentally friendly amendments for the remediation of metal-contaminated soil and water [9,10].
Biochars, which are the carbonaceous residues of organic biomass carbonization in the absence of oxygen, have been identified as promising and eco-friendly materials for agricultural soil amendment and soil conditioning [11,12,13,14]. Indeed, it has been clearly reported that these materials: (i) have slow release capacities for the contained nutrients, (ii) significantly improve agricultural soils’ physico-chemical, biological and hydrodynamic properties, (iii) present high carbon sequestration potential and low CO2 emissions to the atmosphere.
Many efforts have been recently made for the study of biochar valorization in agriculture as organic fertilizers, with the potential to overcome the limitations of low fertility soils, such as high acidity, low organic and available nutrients contents. At this point, the utilization of biochar as an agricultural waste composting additive was studied, indicating that the addition of the produced co-compost to soil increased the total contents of main nutrients such as K, Ca, P and S [15]. Biochars generally contain important amounts of carbon and oxygen. They could furnish significant concentrations of nutrients such as phosphorus, potassium and calcium, which are vital for plant growth [16,17]. Although biochar decomposes at a slow rate and has a recalcitrant behavior, it contains specific functional groups (hydroxyl-, aldehyde and ketone groups mainly) that could promote the adsorption of nutrients and heavy metals [18,19,20].
The availability of nutrients in biochar-amended soils depends on the application rate, the solubility of biochar compounds, the texture and physicochemical properties of biochar and the soil, as well as the soil moisture [17,21,22,23]. The mobilization of nutrients in biochar-amended soils results from various complex geochemical interactions that occur at the solid-liquid interface, including mainly dissolution and ion-exchange reactions. The mobile fraction of nutrients includes the water-leachable and the exchangeable nutrient pools. The water-leachable fraction refers to the amount of nutrients released with water due to the dissolution of soluble salts present in biochar and soil such as sylvine (KCl, pKsp = −0.85) and arcanite (K2SO4, pKsp = 1.8) [24]. However, the amount of K released from the dissolution of these salts exceeds the holding capacity of the soil. Therefore, it is suggested that most of K would be washed away from biochar-amended soils in less than a year after application, resulting in its short-term availability [23]. Leaching experiments showed that the availability of cations released from mallee biochars lasted less than 25 days [22]. Besides, sawdust biochar-based fertilizer were depleted in K after 40 days of sequential elution with water [25]. Likewise, the availability of K in sandy soils amended with manure biochars at a dose of 2% was significantly decreased after 40 days [21].
On the other hand, the exchangeable fraction refers to the amount of cations exchanged by biochar and the fine fraction of soil and is expressed in terms of the cation exchange capacity (CEC). Biochar may enhance the CEC of amended soils by providing various chemical elements such as Ca, Mg, Na, and K through electrostatic forces [26]. Exchange reactions result in a dynamic equilibrium between the different cations in the soil solution, increasing the long-term availability of critical nutrients in biochar-amended soils [17]. The exchange with the cations in the soil solution is also an important mechanism for leaching K from soils, especially when high amounts of Ca are present in the aqueous solution. Calcium is abundant in alkaline agricultural soils and irrigation water and is favourable in the exchange sites over K. The leaching of K from soils (with or without biochar) due to its displacement by Ca is a well-documented process [27,28]. Limwikran et al. [29] noted an interchange between K and Ca in soils amended with fruit-originated biochars, which was attributed to the sorption of Ca by biochar at the expense of K. Potassium fixation by the clays of amended soils may inhibit elution and aid the long-term availability of K in these soils with low Ca contents [30]. However, since most studies use distilled water as an extractant, the leachability of K in field-scale applications of agricultural lands, where irrigation water is a constant supplier of Ca, is expected to differ.
The kinetics of nutrient release decreases with time exponentially and can be well described using first-order, pseudo-second-order, diffusion and Elovich equations [8,16,22,23,26]. These equations were fitted to experimental data to estimate the leaching rates of nutrients from biochar and their availability. For instance, it has been reported that the leaching rate of cations from mallee biochars increased with decreasing particle size and followed the order Mg > Na > K > Ca [22]. The release of K, Mg, Ca and P from pig manure and sewage sludge biochar was well described by first and second-order kinetics [26]. Similar result was found for K and P release form poultry manure derived biochars [31].
Several numerical codes such as PHREEQC [32], HP1 [33], CXTFIT [34], etc. are employed to study the reactive transport of organic and inorganic substances within the pores of biochar-amended soils.. Most of them simulate the sorption of nutrients such as P [35,36] and other contaminants such as heavy metals [37]. The proposed models are fitted to experimental data to estimate the fate of elements in the solution under variably saturated media. Nevertheless, the leaching of nutrients from soils are limited to simple two-component systems [27], and the respective release mechanisms have rarely been modelled. Comparative studies in more complex multi-elemental systems and biochar-amended soils are still missing, despite the numerical capabilities that the aforementioned codes provide.
Meanwhile, significant quantities of wastes and by-products derived from olive oil industry, especially in the Mediterranean region, are being generated annually, including olive mill wastewaters and solid wastes, such as olive pomace [38]. Spain, Italy, Greece, Turkey, Tunisia, Portugal, Syria, and Morocco are the major olive oil producers worldwide. Although these countries produce million tons of olive oil, disposal, treatment and recovery of the huge amounts of the generated by-products and wastes in short time, represent a crucial and challenging issue [39]. Since conventional physical, chemical, mechanical, biological and thermal methods proved to be inefficient on pollutants removal and have shown moderate efficiencies in terms of waste mineralization, being also laborious and expensive, the production of biochar has been studied as a conversion technology for olive mill wastes [40].
To overcome these shortcomings, the present study aims to fill the gap by simulating nutrient release dynamics of biochar-amended soils using PHREEQC numerical code. PHREEQC code is a versatile tool in studying various geochemical reactions through reactive transport [41,42,43]. Our study provides an insight on the qualitative and quantitative characteristics of the dominant geochemical processes that control the availability of inorganic nutrients in soils amended with olive-pomace-derived biochar, as a result of the leaching process induced by irrigation water. This is achieved through the robust capabilities of geochemical modelling and based on the specific characteristics of irrigation water, agricultural soil and biochar. For this purpose, two geochemical approaches are evaluated and compared to assess nutrient release: (a) equilibrium exchange approach and (b) kinetic exchange approach. Based on the theoretical modelling results, this research envisages performing a preliminary evaluation of the specific biochars’ efficacy as soil amendments/conditioners, in the context of the circular economy and zeroing waste approach.
2. Materials and Methods
2.1. Olive-Mill Waste Biochar
The feedstock for the biochar used in this work was exhausted olive pomace from Tunisia. The biochar, prepared at a temperature of 450 °C, was collected from an industrial company in the Mahdia region. Pyrolysis temperature is an important parameter that influences not only the biochar production yield, but also its physico-chemical characteristics [44]. For agricultural valorization purposes, pyrolysis temperatures between 400 °C and 500 °C are generally preferred in order to get respectable biochar yields (>25–30%) and sufficient bioavailable nutrient contents. According to our previous work on this biomass [38], the biochar production yield at a temperature of 450 °C was evaluated to be more than 30%. Moreover, the produced biochar’s C and N content were higher than 80%, and 0.4%, respectively. The fraction of the biochar with particles size lower than 1 mm was used during this study.
The raw olive pomace and the olive pomace biochar elemental composition are given in Table 1 [38]. It shows that the biochar presents a high carbon content. This content is within the range given for lignocellulosic biomasses [45]. Moisture content (22% dry basis) was measured gravimetrically by the oven drying method conforming to the EN 14774-1:2004 standard [46]. The volatile matter (17.4% dry basis) was examined using a thermogravimetric method at 900 °C for 7 min according to the NF EN 15148:2009 standard [47]. Ash (5.6% dry basis) was determined at 815 °C, conforming to the ISO 1171:2010 standard [48]. The higher heating value (HHV) was determined by employing an adiabatic oxygen calorimeter, according to the NF EN 14918:2009 standard [49]. Ultimate analysis was determined by SOCOR laboratory (France), as reported by the EN 15104:2011 standard [50]. The produced biochar was used in its raw form, without any modification.

Table 1.
Ultimate analysis of raw olive pomace and olive pomace biochar (adapted from [38]).
2.2. Agricultural Soil
An agricultural loam soil was collected from the upper soil layer (0–20 cm) of a cultivated field in the Sindos area (northern Greece). The grain size analysis of the soil was performed on the fraction lower than 2 mm.
The main characteristics of the soil are given in Table 2. The pH was measured by a 3520 pH-meter (Jenway, UK) in water-saturated soil paste for soil samples and in 1:2 (v/v) mixture/water slurries of soil samples mixed with biochar. Electrical conductivity was measured by a GLP 32 conductimeter (Barcelona, Spain) in water-saturated soil paste for soil samples and in 1:2 (v/v) mixture/water slurries of soil samples mixed with biochar, also. The Walkey-Black method was used for soil organic matter analysis [51]. CaCO3 was measured with Automatic Digital Soil Calcimeter (Fogl, BD Inventions P.C.,Thessaloniki, Greece). Extractable phosphorus and boron were determined colorimetrically using a Lambda 35 UV/VIS spectrophotometer (Perkin Elmer, MA, USA). Exchangeable K and Na were determined after extraction with ammonium acetate by a M410 Flame Photometer (Sherwood Scientific Ltd, Cambridge, UK). Exchangeable Ca and Mg were determined after extraction with ammonium acetate with a Perkin Elmer AAnalyst 200 Atomic Absorption Spectrometer. Fe, Cu, Mn, Zn, Cd, Pb and Ni were measured after extraction with DTPA with a Perkin Elmer AAnalyst 200 Atomic Absorption Spectrometer. Total N was determined according to ISO 11261:1995 [52]. NO3− measurement was performed after extraction with KCl and by passage through a column of copperized cadmium [53]. Soil texture analysis was performed according to the Bouyoucos method [54]. Wilting point, field capacity, bulk density, and saturation were determined using soil moisture pressure plate apparatus, according to the standard methods described in Soil Survey Laboratory Methods Manual, Version 4.0 [55]. Cation Exchange Capacity was determined according to the ammonium acetate method [56]. The exchangeable sodium percentage (ESP), which describes the level of adsorbed Na in soil (exchangeable_Na, cmol/kg) versus the total cation exchange capacity (CEC, cmol/kg), was calculated by the following equation:

Table 2.
Main physicochemical characteristics of the agricultural soil z.
The soil was treated and mixed with the biochar in different proportions and placed in columns, with a diameter of 47.5 cm. The lower 30 cm of the columns were filled with the reference soil (0% of biochar), whilst the upper 20 cm were filled with a biochar-soil mix in four different proportions, 0.5%, 1%, 2% and 10% of biochar (Figure 1). The first three doses of biochar used were such that they could be applied in a realistic agronomic application [8,16], while the 10% dose was used for modeling and exploratory purposes. After filling the columns, they remained intact for 45 days to achieve homogenization between the soil and the biochar.
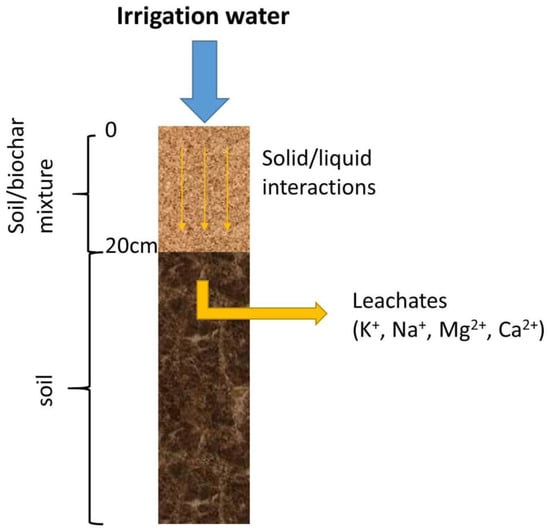
Figure 1.
Graphic representation of the theoretical leaching model of soil columns by irrigation water.
It was noted that the pH of the biochar-soil mix did not significantly change among different applications. The mean pH value of the mixtures was 7.13 ± 0.3. The concentration of the main exchangeable cations and the cation exchange capacity (CEC, cmol/kg) in the biochar-amended soils mixtures are given in Table 3.

Table 3.
Concentration of the exchangeable cations (in mg/kg) and CEC (in cmol/kg) of the biochar/soil mixtures.
Table 4 shows the main physicochemical characteristics of the irrigation water considered in this work. They were determined according to Standard Methods for the Examination of Water and Wastewater Handbook [57]. The pH was measured by a JENWAY 3520 pH Meter. Electrical conductivity was measured by a CRISON GLP 32 Conductimeter. K and Na were determined by Sherwood M410 Flame Photometer and Ca and Mg were determined with a Perkin Elmer AAnalyst 200 Atomic Absorption Spectrometer. NO3−, NH4+ and SO42− were determined using a Perkin Elmer Lambda 35 UV/VIS spectrophotometer, while HCO3− and Cl− were measured titrimetrically, according to EN ISO 9963-1:1996 [58], and Mohr method, EN ISO 9297:1989 [59], respectively.

Table 4.
Main physicochemical characteristics of the irrigation water.
2.3. Modelling K Leaching from Soil Columns Amended with Biochar
2.3.1. Data Selection and Input Parameters
The release of nutrients (Ca, Mg, K, Na) from biochar-amended soils was modelled using PHREEQC v3.3. geochemical code employed with phreeqc.dat database [32]. The conceptual model assumed that irrigation water was applied in a column of agricultural soil amended with biochar (0–20 cm) and subsequently mobilized the water-soluble and the exchangeable fraction of nutrients from the solids, which are transferred within the soil column (Figure 1).
The one-dimensional transport of each element within the soil column was modelled using the advection-reaction-dispersion equation (ARD):
where C is the soluble elemental concentration (mol/kg of water), t is the time (s), x is the distance (m), u is the linear water velocity (m/s), DL is the hydrodynamic dispersion coefficient (m2/s) and q the elemental concentration per mass of solid (mol/kg). The ARD equation was implemented using the TRANSPORT subroutine, assuming 5 cells each having 0.04 m length, a constant flow velocity of 0.1 m/d and a total simulation time of 365 days (Figure S1).
The biochar/soil column that was divided into five (5) cells was saturated with irrigation water (SOLUTION 1–5, Table 4). The column is assumed to be in equilibrium with PCO2 = 10−1.5 atm, a value that characterizes the vadose zone of most agricultural fields [60] and with 0.05 moles of calcite, as estimated by the equivalent amounts of CaCO3 in the agricultural soil (Table 2). These values were fixed in all simulations. The biochar/soil column was leached with irrigation water (SOLUTION 0, Table 4). The concentration of the cations at the end of the column (cell 5) were plotted against simulation time. The cumulative mass of K eluted from the columns (Mel, mg) with time (t, days) was calculated as follows [61]:
where Q is the flow rate (L/d), C is the soluble elemental concentration (mol/kg of water) of K at the outlet at time t (days). The flow rate is calculated from the water velocity (v = 0.1 m/d) and the porosity of the column (ε = 0.45) having a cross-sectional area of A = 1 m2 as Q = 45 L/d.
The analytical approaches in Table 2 were used to simulate the leaching of cations from the columns: (a) the equilibrium exchange model (EE), and (b) the kinetic exchange model (K.E.), which are described in detail below.
2.3.2. Equilibrium Exchange Model (E.E. Model)
The equilibrium exchange model was used to test nutrients release from the exchange sites during irrigation of the biochar-amended soil. This approach assumes that all the exchangeable cations (Table 3) are in equilibrium with the solution of the saturated column (SOLUTION 1–5). Equilibrium exchange within the column is introduced using the EXCHANGE 1–5 subroutine and occurs during the whole simulation. The concentrations of the exchangeable cations (in moles) for transport within the pore space (Table S1) were computed from Table 3, assuming that 1 kg of solids came in contact with 1 L of water. These cations are in dynamic equilibrium within the soil solution and readily available to interact with the exchange sites. The ion exchange coefficients between the different pairs of ions were determined as half-reactions (X− + 1/z Iz+ = I − X1/z, KI) relative to Na reference half-reaction (Na+ + X− = NaX, KNa = 1) according to Gaines-Thomas convention (Table S2) [60]. An example of the script for the E.E. model is given in Figure S2.
2.3.3. Kinetic Exchange Model (K.E. Model)
A kinetic exchange model was used to test the leaching of nutrients with time. This model assumes a time-dependent release of nutrients from the solid surface (e.g., dissolution reactions as well as exchange reactions). Kinetic exchange can be expressed as a first-rate order reaction [60]:
where k is the leaching rate constant (s−1), q0 is the initial elemental concentration in the column per mass of solid (mol/kg), and q is the elemental concentration retained in the exchange sites of solid (mol/kg). Therefore, the term (q0 − q) refers to the mobile elemental concentration that moves within the column at each time step.
The kinetic exchange rate constant, k, is determined experimentally and depends on the various retention mechanisms that occur within the solid particles of the column (exchange, sorption, precipitation of mineral phase, etc.) In this approach, k was assumed the same for all the cations and was determined by the flow rate (Q, 45 L/d) and the volume of voids (Vw, L) (Tiruta-Barna [62]) as:
where V is the volume of the column (L), L the length of the column (m), while the other parameters have already been presented. The estimated k was 5.8 × 10−6 s−1.
Integration of Equation (4) is done by the RATES and KINETICS subroutines, where the kinetic exchange rate expression and input parameters are defined. The initial cation concentration q0 was calculated from the exchangeable cations present in the column (Table 4) and inserted in the model through the KINETICS data block. The retained cation concentration through exchange q0, was calculated from the CEC of the solids (Table 3) and entered using EXCHANGE data block. The conversion of CEC (cmol/kg) to X− total exchange sites (mol) was achieved using the porosity (ε = 0.45) and bulk density (pb = 1.6 g/cm3) of the solids (Table 2) as 0.68 mol.
In the kinetic exchange model (K.E. model), the column (SOLUTION 1–5) is firstly saturated with irrigation water and equilibrated with calcite and atmospheric CO2(g) (EQUILIBRIUM_PHASES 1–5). The column is then equilibrated with the exchanger as defined by the CEC of the solids (Table 2), and the exchanger composition is calculated (EXCHANGE 1–5) to maintain a mass balance between the different ions. It is noted that biochar did not affect significantly the soil CEC (18 cmol/kg) (Table 3); hence this value was kept constant throughout the simulations. The TRANSPORT parameters are the same as in the E.E. model. An example of the script of K.E. model is presented in Figure S3.
3. Results & Discussion
3.1. Application of the Equilibrium Exchange Model (E.E. Model)
The equilibrium exchange model (E.E. model) assumes that only ion exchange reactions control the mobility of the cations within the biochar/soil column. The effluent concentration of each cation at the end of the column (cell 5) with respect to time is presented in Figure 2. Based on the simulations, the porewater pressure in the column (SOLUTION 1–5) is equilibrated with PCO2 = 10−1.5 atm and 0.05 mole calcite, resulting in dissolution of 0.0002 moles of CaCO3. The Ca ions released from the dissolution of calcite have higher affinity towards the exchange sites (Table S2, Figure S4), and displace K, Na and Mg. The amount of cation released depends on the initial elemental concentration in the exchanger (i.e., CaX2, MgX2, KX and NaX). The exchanger composition versus simulation time is presented in Figure S4. The constant supply of Ca from both the inlet (SOLUTION 0) and the dissolution of calcite increases the Ca gradient in the porewater and prompts the sorption of Ca by the biochar (Figure 2a). Therefore, Ca is retained by the columns during the first 10 days of the simulation. The excess of Ca contents is flushed from the column increasing the outlet concentrations higher than those in the irrigation water (Figure 2a).
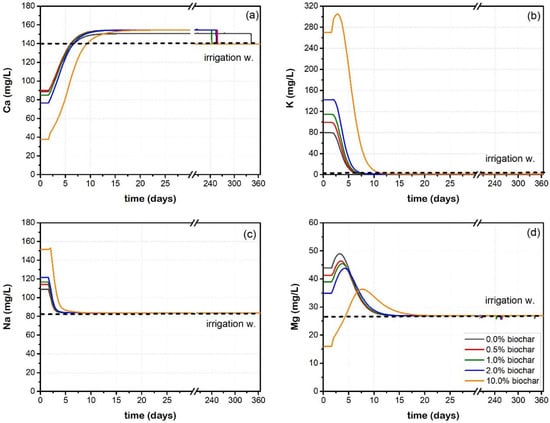
Figure 2.
Elution curves of (a) Ca, (b) K, (c) Na and (d) Mg versus time as obtained from E.E. Model.
The retention of Ca by biochars and biochar-amended soils has been related to the subsequent release of K and Na [29], and has been attributed to the higher affinity of Ca for the exchange site as well as its abundance in irrigation water, soils and biochars [28]. The higher affinity of Ca for the exchange sites (Table S2, Figure S4) is responsible for the retention of Ca and elution of the other cations from the columns, [28]. The released K concentrations range between 80 and 300 mg/L (Figure 2a), whereas Na concentrations range between 80 and 150 mg/L (Figure 2c). Both elements have completely exited the columns within the first 10 days of the simulation (i.e., the final elemental concentrations equal those of the irrigation water). After the first 10 days, the columns have become depleted in K and Na. This is expected, since the biochar used contains significant amounts of sylvite [3], which is readily soluble in water. Water soluble K was readily removed from plant-based biochars containing sylvite (1 week) compared to those containing KHCO3 or KH2PO4 compounds (8 weeks) [29]. Short depletion times (<50 days) were also reported by Kong et al. [22] and Gwenzi et al. [25]. Therefore, higher proportions of biochar into the soil would contain higher amounts of sylvite, and release more K. This also finds application for Na and Ca as noted by the increase in their exchangeable concentrations with increasing biochar proportions in soil, whilst the CEC remains the same (Table 3). It is recommended that soluble salts, may be dissolved by the ammonium acetate method, overestimating the exchangeable elemental concentrations in soils [63].
Dissolved Mg concentration peaks at day four (4) for the 0–2% biochar doses, and then decreases (Figure 2d). At a biochar dose of 10%, the maximum elution occurred at 10 days. Magnesium exhibits relative retardation to exit the columns, following the behaviour of Ca and K, as it was retained into the exchange sites (Figure S4). Complete elution occurs after ten (10) days for the columns with <2% biochar. Retardation is more evident in the 10% biochar column, where Mg is significantly retained by the exchange sites; the released amount is lower and exhaustion occurs after twenty (20) days of elution (Figure 2d).
3.2. Application of the Kinetic Exchange Model (K.E. Model)
The kinetic exchange model assumes that the leaching rate of cations from the solids depends on the reactions occurring in the solid-liquid interface, involving both dissolution and exchange. In this approach the exchanger composition (i.e., CaX2, MgX2, KX, and NaX) represents the initial elemental amount present in the column, (retained through sorption and/or precipitation of salts), whereas the exchanged elemental amount is calculated by the total CEC of the solids.
The results of the kinetic exchange model are presented in Figure 3. The column was again equilibrated with PCO2 = 10−1.5 atm and 0.05 moles of calcite. When irrigation water (SOLUTION 0) enters the column, the exchanger composition is defined based on X-. A number of cations present in SOLUTION 0 are instantly adsorbed by the solids to fill the X sites (i.e., 0.68 mol), and the initial exchanger composition consists of 0.275 mol CaX2, 0.056 mol MgX2, 0.017 mol NaX and 0.0007 mol KX. During the elution process, a fraction of elements is released or retained based on the first-order kinetics (expressed as qCa, qMg, qK and qNa, respectively) (see Equation (4)). Another fraction is then reacting with the exchange sites based on the affinity of the cations for the exchange sites (Table S2, Figure S5). The difference between the elemental amount released through kinetics (q) and exchange (X), is the solute concentration that exited the column at a specific time.
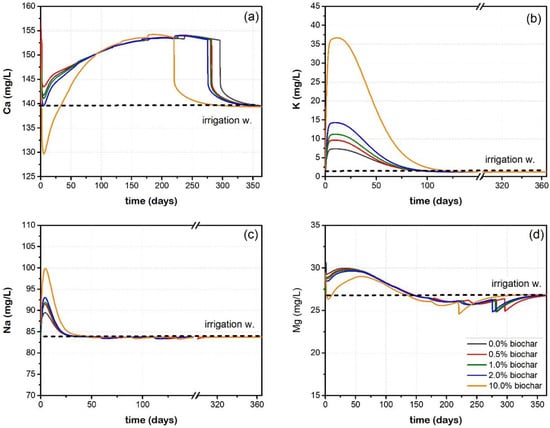
Figure 3.
Elution curves of (a) Ca, (b) K, (c) Na and (d) Mg versus time as obtained from K.E. Model.
The dissolution of calcite during equilibrium releases a high amount of Ca (~166 mg/L) regardless the amount of biochar present, because it depends solely on the PCO2 and the Ca concentration in SOLUTION 0. As the reactions proceeded there was a dynamic relation between Ca released from calcite dissolution and the solid phase, resulting in the retention of Ca. When calcite was exhausted, (and therefore there was no more supply of Ca ions), Ca ions were leached from the columns. The columns with 10% biochar dose contained relatively higher initial Ca concentrations (Table 3), resulting in higher retention of Ca in the first days of elution (<50). Consequently, the exchanger sites remained saturated with Ca for longer times compared to the other columns. As a result exhaustion occurred earlier for the 10% biochar contents (at 250 days) than control soil (at 300 days). It can be seen that the behaviour of Ca in terms of mobility, retention and leachability is primarily controlled by the equilibrium of the column with calcite and CO2(g) in both models therefore the leaching trends are the same (Figure 2a and Figure 3a).
The leaching of K from the column increases with increasing the biochar dose in soil (Figure 3b), ranging from 7.5 to 36.7 mg/L. These values are much lower than those calculated by the E.E. model (80–300 mg/L, Figure 2b) and K is slowly released from the columns until 100 days. A kinetic study of K release from pots containing amended soils with 2% biochar, showed a decline in K availability after 125 days of incubation period [30]. Similarly, in our modelling study, the column is depleted of Na within the first 25 days (Figure 3c).
Finally, Mg shows the respective retardation pattern as in Figure 3d. On day 100, the effluent Mg concentration equals the irrigation water (27 mg/L). Magnesium is released from the exchange sites (MgX2 decreases) and is retained or released from the solids through kinetics (qMg increases or decreases). When the columns become depleted in calcite, Mg is resorbed onto the exchange sites (MgX2 increases) and therefore, the effluent concentrations fall below the irrigation water (<27 mg/L).
3.3. Preliminary Assessment of Biochars’ Potential and Benefits Uses
Olive mill wastes represent a critical environmental problem in Mediterranean areas where they are generated in huge quantities in short periods [64]. Their high phenol, lipid and organic acid concentrations make them phytotoxic materials. Nevertheless, these wastes also contain valuable resources such as a large proportion of organic matter and a wide range of inorganic nutrients that could be recycled [65,66,67,68]. To this aim, the transformation of olive-mill waste and its valorization as a useful material per case in agriculture directly benefits the environment and conserves natural resources. That integrated approach is in line with the zero-waste and circular economy perspectives of the European Commission and the new common agricultural policy (CAP) that constitutes its implementation tool for sustainable agriculture in Europe [69]. However, the transformation of olive-mill wastes into biochars is not a cost-free process. It requires pre-processing and processing treatments as well as efficient and expensive industrial-scale equipment for valorizing the vast amounts of wastes produced. Therefore, the whole process should first be financially viable, especially for the farmers who are considered the main end-users of this product.
Based on the theoretical modelling results of this work, the olive-mill biochar could be potentially used as a soil amendment/conditioner, as it can provide an amount of inorganic nutrients for a considerable period in most cases. Specifically, biochar amended soils could provide K for a short period of 9 to 12 days (based on E.E. Model—Figure 2b) and for 100 days (based on K.E. Model—Figure 3b). At the same time, the elevated amount of Ca could be highly beneficial to acidic soils as a pH buffer [29], owing to the longer elution times of Ca (250 to 300 days, Figure 3a). On the contrary, Na is released in relatively smaller amounts and for a shorter period (Figure 2c); thus, minimizing any potential threat for soil sodification and alkalization caused by a long-term Na elution in more significant amounts. Therefore, the application of olive-pomace-derived biochar as amendment in agricultural soils, not only enhances its nutrient content, but can also improve its physicochemical properties. The potential effect of other cations present in biochars is rarely studied, since focused in given in its nutritious potential (K, N and P) [22].
Considering that the biochar used in this work is physically enriched in potassium, the total amount of K (g) released into the soil columns is compared in Table 5 for the two models, E.E. and K.E. The higher the initial K concentration in the solids, the higher the amount released into the columns. For example, for a 2% biochar fraction mixed with soil the available K for plants is doubled compared with the available K in the soil without biochar. When comparing the two models, the cations need more time to exit the column in the K.E. model (Figure 3), compared to the E.E. model (Figure 2). Consequently, the elemental concentrations in the column outlet were higher in model E.E. compared to model K.E, at each time step. This is expected since the K.E. model assumes more complex dynamics in the column system compared to the E.E model. Whilst in E.E. model the movement of cations within the column is controlled by the water velocity, in the K.E. model the movement of cations is also controlled by their release rate from the solid surface. This resulted in higher elemental amounts and short elution times in the E.E. model compared to the K.E model. Nevertheless, the total amount of K (g) eluted from the columns was almost the same between the two models, as calculated by Equation (3) (Table 5). It is evident that higher K amounts are released from the soils containing 10% biochar (80.3 g), compared to 2% biochar (27.3 g).

Table 5.
Total mass of K eluted from the columns (Mel, g) according to E.E. and K.E. models until exhaustion time (tex, days).
4. Conclusions
The release of cations from soil columns amended with olive-pomace-derived biochar was simulated using two modelling perspectives: (a) equilibrium exchange and (b) kinetic exchange. Equilibrium exchange perspective assumed that nutrient release is cotrolled by ion-exchange reactions onto the biochar surface, whilst kinetic exchange perspective assumed the contribution of both ion-exchange and dissolution of salts. Regardless of the modelling approach, the total mass of K eluted from the columns is almost the same and depends on the initial cation concentrations in the column, i.e., the biochar dose. Significant released K amounts sufficient for most of the typical crops are obtained when 2% of biochar was added to the mixtures. Nevertheless, the applied amounts of biochars should be proportional to the financial viability of the entire cost to ensure its success and use by the farmers.
Overall, the olive-mill produced biochar shows potential for its use as a soil amendment/conditioner. Moreover, the theoretical modelling of nutrient leaching from soils treated with biochar could be a powerful tool for sustainable soil management. This research’s theoretical modelling sheds light on the qualitative and quantitative characteristics of the geochemical mechanisms controlling the release of nutrients from biochar (i.e., exchange and dissolution), which could be a first step towards improving biochar properties through agricultural engineering. The use of numerical modeling helps in a quick assessment of biochar performance in soil, by avoiding time-consuming and laborious experimental set-ups.
Nevertheless, this studies has some inherent uncertainties of the modelling procedure, which should be validated further by experimental results. However, this is not always possible, as the experimentation could require additional resources and time. Thus, even though the modelling results should be treated with caution, yet they can be safely regarded as representative outcomes following the proper selection of input parameters and model calibration. The significant role of theoretical modelling is enhanced when considering that it could be applied in a short time for multiple numbers of combinations and scenarios, a fact which alternatively could not be possible through experimentation.
Future research in that field may include the integration of theoretical and experimental modelling to optimize results. Research may also be expanded in variable soil types (e.g., acidic or alkaline) to explore the potential impact of soil-biochar mixtures, as well as experimental investigations regarding the release of nutrients in biochar-amended soils with different physicochemical characteristics. In addition, future research may also focus on sustainable agricultural management by taking into account the replacement rate of the soil-biochar mixture or the potential of mixture regeneration to sustain its beneficial effects for more time.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/agronomy12020480/s1. Figure S1: Example of the TRANSPORT subroutine used in the simulations; Figure S2: Script example for E.E. model (input parameters for soil with 0% biochar); Figure S3: Script example for K.E. model (input parameters for soil with 0% biochar); Figure S4: Exchanger composition (KX, CaX2, NaX, MgX2) during the simulation time of E.E. model; Figure S5: Exchanger composition (KX, CaX2, NaX, MgX2) during the simulation time of K.E. model; Table S1: Total amount of moles in the exchangeable sites used in Model E.E.; Table S2: Ion-exchange coefficients relative to Na, following the Gaines-Thomas convention and implemented in PHREEQC database (EXCHANGE_SPECIES).
Author Contributions
Conceptualization: Z.K., E.T. and C.D.; Methodology: All; Formal analysis: Z.K. and V.K.; Investigation: Z.K. and V.K.; Funding acquisition: C.D., S.J. and M.J.; Data curation: Z.K.; Writing—original draft preparation: Z.K., V.K., C.D. and E.T.; Writing—review and editing: C.D., E.T., S.J. and M.J.; Visualization: C.D., E.T., Z.K. and V.K.; Supervision: C.D. and S.J.; Project administration: C.D. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by FERTICHAR project, through the ARIMNet2 Joint Call by the following funding agencies: MHESRT (Tunisia), ANR (France), and HAO332 DEMETER (Greece). ARIMNet2 (ERA-NET) has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 618127. The authors gratefully acknowledge the funding agencies for their support.
Acknowledgments
The authors also wish to thank Olivecoal (Tunisia) for providing the Olive mill waste biochar.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations. Our Priorities—The Strategic Objectives of FAO; FAO: Rome, Italy, 2019. [Google Scholar]
- “4 per 1000” Initiative Strategic Plan. 2020. Available online: https://www.4p1000.org/sites/default/files/francais/strategic_plan.pdf (accessed on 11 December 2021).
- El-Bassi, L.; Azzaz, A.A.; Jellali, S.; Akrout, H.; Marks, E.A.N.; Ghimbeu, C.M.; Jeguirim, M. Application of olive mill waste-based biochars in agriculture: Impact on soil properties, enzymatic activities and tomato growth. Sci. Total Environ. 2021, 755, 142531. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Asif, M.; Bilal, H.M.; Rehman, B.; Adnan, M.; Ahmad, T.; Rehman, H.; Anjum, M.Z. Organic and Inorganic Fertilizer; Integral Part for Crop Production Review Article. EC Agric. 2020, 6.3, 1–7. [Google Scholar]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef] [Green Version]
- Agra CEAS Consulting. Integrated Crop Management Systems in the EU. 2002. Available online: https://ec.europa.eu/environment/agriculture/pdf/icm_finalreport.pdf (accessed on 11 December 2021).
- Hadroug, S.; Jellali, S.; Azzaz, A.A.; Kwapinska, M.; Hamdi, H.; Leahy, J.J.; Jeguirim, M.; Kwapinski, W. Valorization of salt post-modified poultry manure biochars for phosphorus recovery from aqueous solutions: Investigations on adsorption properties and involved mechanism. Biomass Convers. Biorefinery 2021, 1–16. [Google Scholar] [CrossRef]
- Lashen, Z.M.; Shams, M.S.; El-Sheshtawy, H.S.; Slaný, M.; Antoniadis, V.; Yang, X.; Sharma, G.; Rinklebe, J.; Shaheen, S.M.; Elmahdy, S.M. Remediation of Cd and Cu contaminated water and soil using novel nanomaterials derived from sugar beet processing- and clay brick factory-solid wastes. J. Hazard. Mater. 2022, 428, 128205. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Wang, B.; Wang, J.; Slaný, M.; Yan, H.; Li, P.; El-Naggar, A.; Shaheen, S.M.; Rinklebe, J.; Feng, X. Use of biochar to reduce mercury accumulation in Oryza sativa L: A trial for sustainable management of historically polluted farmlands. Environ. Int. 2021, 153, 106527. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Nunes Valenciano, M.; Silva, C.A. Enhancing Cation Exchange Capacity of Weathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Han, J.; Zhang, A.; Kang, Y.; Han, J.; Yang, B.; Hussain, Q.; Wang, X.; Zhang, M.; Khan, M.A. Biochar promotes soil organic carbon sequestration and reduces net global warming potential in apple orchard: A two-year study in the Loess Plateau of China. Sci. Total Environ. 2022, 803, 150035. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Calabi-Floody, M.; Aponte, H.; Santander, C.; Paneque, M.; Meier, S.; Panettieri, M.; Cornejo, P.; Borie, F.; Knicker, H.; et al. Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region. Agronomy 2021, 11, 767. [Google Scholar] [CrossRef]
- Ferjani, A.; Jeguirim, M.; Jellali, S.; Limousy, L.; Courson, C.; Akrout, H.; Thevenin, N.; Ruidavets, L.; Muller, A.; Bennici, S. The use of exhausted grape marc to produce biofuels and biofertilizers: Effect of pyrolysis temperatures on biochars properties. Renew. Sustain. Energy Rev. 2019, 107, 425–433. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Chapter One—Long-Term Aging of Biochar: A Molecular Understanding With Agricultural and Environmental Implications. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 141, pp. 1–51. ISBN 978-0-12-812423-9. [Google Scholar]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef] [Green Version]
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-derived biochars: A review on the influence of synthesis conditions on pollutants removal efficiency from wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068. [Google Scholar] [CrossRef]
- Van Poucke, R.; Meers, E.; Tack, F.M.G. Leaching behavior of Cd, Zn and nutrients (K, P, S) from a contaminated soil as affected by amendment with biochar. Chemosphere 2020, 245, 125561. [Google Scholar] [CrossRef]
- Kong, Z.; Liaw, S.B.; Gao, X.; Yu, Y.; Wu, H. Leaching characteristics of inherent inorganic nutrients in biochars from the slow and fast pyrolysis of mallee biomass. Fuel 2014, 128, 433–441. [Google Scholar] [CrossRef]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- Johnson, J.; Anderson, G.; Parkhurst, D. Database “thermo. com. V8. R6. 230,” Rev. 1-11. Lawrence Livermore Natl. Lab. Livermore California 995 2000. [Google Scholar]
- Gwenzi, W.; Nyambishi, T.J.; Chaukura, N.; Mapope, N. Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 405–414. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Mei, Y.; Li, F.; Cao, X. Release of nutrients and heavy metals from biochar-amended soil under environmentally relevant conditions. Environ. Sci. Pollut. Res. 2018, 25, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Kolahchi, Z.; Jalali, M. Simulating leaching of potassium in a sandy soil using simple and complex models. Agric. Water Manag. 2006, 85, 85–94. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Jalali, M. Effect of water quality on the leaching of potassium from sandy soil. J. Arid Environ. 2007, 68, 624–639. [Google Scholar] [CrossRef]
- Limwikran, T.; Kheoruenromne, I.; Suddhiprakarn, A.; Prakongkep, N.; Gilkes, R.J. Dissolution of K, Ca, and P from biochar grains in tropical soils. Geoderma 2018, 312, 139–150. [Google Scholar] [CrossRef]
- Piash, M.I.; Iwabuchi, K.; Itoh, T.; Uemura, K. Release of essential plant nutrients from manure- and wood-based biochars. Geoderma 2021, 397, 115100. [Google Scholar] [CrossRef]
- Hadroug, S.; Jellali, S.; Leahy, J.J.; Kwapinska, M.; Jeguirim, M.; Hamdi, H.; Kwapinski, W. Pyrolysis Process as a Sustainable Management Option of Poultry Manure: Characterization of the Derived Biochars and Assessment of their Nutrient Release Capacities. Water 2019, 11, 2271. [Google Scholar] [CrossRef] [Green Version]
- Parkhurst, B.D.L.; Appelo, C.A.J. User’s Guide To PHREEQC (version 2)—A Computer Program for Speciation, and Inverse Geochemical Calculations. In Water-Resources Investigations Report 99-4259; U.S. Geological Survey: Denver, CO, USA, 1999. [Google Scholar]
- Jacques, D.; Simunek, J. User Manual of the Multicomponent Variably- Saturated Flow and Transport Model HP1; SCK-CEN, Belgian Nuclear Research Centre: Mol, Belgium, 2005. [Google Scholar]
- Toride, N.; Leij, F.J.; van Genuchten, M.T. The CXTFIT Code for Estimating Transport Parameters from Laboratory or Field Tracer Experiments Version 2.0; U.S. Department of Agriculture: Riverside, CA, USA, 1995.
- Filipovic, V.; Cerne, M.; Simunek, J.; Filipovic, L.; Romic, M.; Ondra ek, G.; Bogunovic, I.; Mustac, I.; Krevh, V.; Ferencevic, A.; et al. Modeling water flow and phosphorus sorption in a soil amended with sewage sludge and olive pomace as compost or biochar. Agronomy 2020, 10, 1163. [Google Scholar] [CrossRef]
- Hemati Matin, N.; Jalali, M.; Antoniadis, V.; Shaheen, S.M.; Wang, J.; Zhang, T.; Wang, H.; Rinklebe, J. Almond and walnut shell-derived biochars affect sorption-desorption, fractionation, and release of phosphorus in two different soils. Chemosphere 2020, 241, 124888. [Google Scholar] [CrossRef]
- Jellali, S.; Diamantopoulos, E.; Haddad, K.; Anane, M.; Durner, W.; Mlayah, A. Lead removal from aqueous solutions by raw sawdust and magnesium pretreated biochar: Experimental investigations and numerical modelling. J. Environ. Manag. 2016, 180, 439–449. [Google Scholar] [CrossRef]
- Tamoši, A.; Chouchène, A.; Valatkevičius, P.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. [Google Scholar] [CrossRef] [Green Version]
- Kinigopoulou, V.; Hatzigiannakis, E.; Guitonas, A.; Oikonomou, E.K.; Samaras, P. Utilization of biobed for the efficient treatment of olive oil mill wastewater. Desalin. Water Treat. 2021, 223, 167–179. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jeguirim, M.; Kinigopoulou, V.; Doulgeris, C.; Goddard, M.L.; Jellali, S.; Matei Ghimbeu, C. Olive mill wastewater: From a pollutant to green fuels, agricultural and water source and bio-fertilizer—Hydrothermal carbonization. Sci. Total Environ. 2020, 733, 139314. [Google Scholar] [CrossRef] [PubMed]
- Berns, A.E.; Flath, A.; Mehmood, K.; Hofmann, D.; Jacques, D.; Sauter, M.; Vereecken, H.; Engelhardt, I.; Berns, A.; Mehmood, K.; et al. Numerical and Experimental Investigations of Cesium and Strontium Sorption and Transport in Agricultural Soils; Numerical and Experimental Investigations of Cesium and Strontium Sorption and Transport in Agricultural Soils. Vadose Zone J. 2018, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Boluda-Botella, N.; Valdes-Abellan, J.; Pedraza, R. Applying reactive models to column experiments to assess the hydrogeochemistry of seawater intrusion: Optimising ACUAINTRUSION and selecting cation exchange coefficients with PHREEQC. J. Hydrol. 2014, 510, 59–69. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, C.J.; Blowes, D.W. Mercury Complexation with Dissolved Organic Matter Released from Thirty-Six Types of Biochar. Bull. Environ. Contam. Toxicol. 2019, 103, 175–180. [Google Scholar] [CrossRef]
- Jellali, S.; El-Bassi, L.; Charabi, Y.; Uaman, M.; Khiari, B.; Al-Wardy, M.; Jeguirim, M. Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: A critical review on benefits for environment and agriculture. J. Environ. Manag. 2022, 305, 114368. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crops Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- ΕΝ 14774-1:2004; Solid biofuels—Determination of Moisture Content—Oven Dry Method—Part 1: Total Moisture—Reference Method (ISO 18134-1:2015).; ISO: Geneva, Switzerland, 2004.
- ΕΝ 15148:2009; Solid Biofuels—Determination of the Content of Volatile Matter; ISO: Geneva, Switzerland, 2009.
- ISO 1171:2010; Solid Mineral Fuels—Determination of Ash; ISO: Geneva, Switzerland, 2010.
- EN 14918:2009 Solid Biofuels—Determination of Calorific Value; ISO: Geneva, Switzerland, 2009.
- EN 15104; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen—Instrumental Methods; ISO: Geneva, Switzerland, 2011.
- Nelson, D.W.; Sommers, L. A Rapid and Accurate Procedure for Estimation of Organic Carbon in Soils. Proc. Indiana Acad. Sci. 1974, 84, 456–462. [Google Scholar]
- ISO 11261:1995 Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method; ISO: Geneva, Switzerland, 1995.
- Keeney, D.R.; Nelson, D.W. Methods of Soil Analysis, Part 2-Chemical and microbiological properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy & Academic Press: Madison, WI, USA, 1982; Volume 100. [Google Scholar]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Soil Survey Laboratory Methods Manual; Version 4; Burt, R. (Ed.) United States Department of Agriculture. U.S. Govt. Print. Office: Washington, DC, USA, 2004.
- Sumner, M.E.; de Ramos, L.; Kukier, U. Compulsive exchange method for determining soil exchange capacities: Proposed time and labor saving modifications. Commun. Soil Sci. Plant Anal. 1994, 25, 567–572. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods fot the Examination of Water and Wastewater, 23rd ed.American Public Health Association, American Water Works Association, Water Environment Federation: 2017: Washington, DC, USA; ISBN 978-0-87553-287-5.
- EN ISO 9963-1:1996; Water Quality—Determination of Alkalinity—Part 1: Determination of Total and Composite Alkalinity; ISO: Geneva, Switzerland, 1996.
- EN ISO 9297:1989; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method); ISO: Geneva, Switzerland, 1989.
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 5th ed.; CRC Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Volesky, B.; Weber, J.; Park, J.M. Continuous-flow metal biosorption in a regenerable Sargassum column. Water Res. 2003, 37, 297–306. [Google Scholar] [CrossRef]
- Tiruta-Barna, L. Using PHREEQC for modelling and simulation of dynamic leaching tests and scenarios. J. Hazard. Mater. 2008, 157, 525–533. [Google Scholar] [CrossRef]
- Ciesielski, H.; Sterckeman, T.; Santerne, M.; Willery, J.P. A comparison between three methods for the determination of cation exchange capacity and exchangeable cations in soils. Agronomy 1997, 17, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Marks, E.A.N.; Kinigopoulou, V.; Akrout, H.; Azzaz, A.A.; Doulgeris, C.; Jellali, S.; Rad, C.; Zulueta, P.S.; Tziritis, E.; El-Bassi, L.; et al. Potential for production of biochar-based fertilizers from olive millwaste in mediterranean basin countries: An initial assessment for Spain, Tunisia, and Greece. Sustainability 2020, 12, 6081. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Dutournié, P.; Jeguirim, M.; Khiari, B.; Goddard, M.-L.; Jellali, S. Olive Mill Wastewater: From a Pollutant to Green Fuels, Agricultural Water Source, and Bio-Fertilizer. Part 2: Water Recovery. Water 2019, 11, 768. [Google Scholar] [CrossRef] [Green Version]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sustain. Energy Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Haddad, K.; Jeguirim, M.; Jellali, S.; Thevenin, N.; Ruidavets, L.; Limousy, L. Biochar production from Cypress sawdust and olive mill wastewater: Agronomic approach. Sci. Total Environ. 2021, 752, 141713. [Google Scholar] [CrossRef]
- European Commission. The post-2020 common agricultural policy: Environmental benefits what the future CAP will bring to the table, European Commission. Available online: https://ec.europa.eu/info/sites/default/files/food-farming-fisheries/key_policies/documents/cap-post-2020-environ-benefits-simplification_en.pdf (accessed on 1 January 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).