Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils
Abstract
1. Introduction
2. Halobacteria
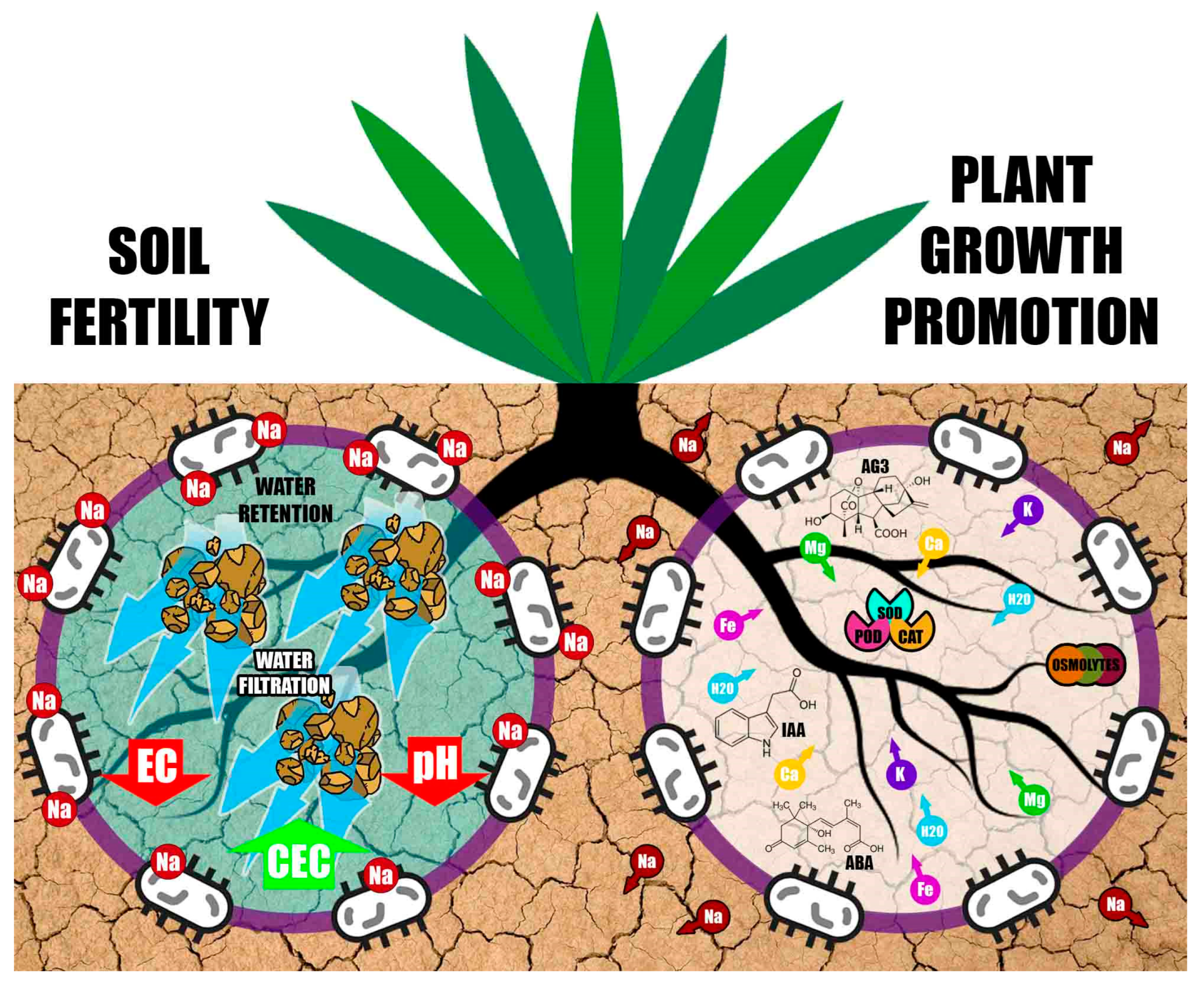
3. Plant Growth-Promoting Halobacteria and Protection against Abiotic Stress
- (1)
- Activating the antioxidant defense machinery of plants by regulating the activity of enzymes such as superoxide dismutase, peroxidase and catalase, which eliminate reactive oxygen species and protect plants from salinity
- (2)
- Improving plant nutrition by fixing atmospheric nitrogen, solubilizing nutrients such as P and K, or by producing siderophores that improve Fe uptake
- (3)
- Increasing selective ion uptake to maintain a high K+:Na+ ratio, which can reduce the accumulation of toxic ions such as Na+ and Cl−
- (4)
- Reducing the accumulation of Na+ in the plant by excreting exopolysaccharides that fix this cation in roots and prevent its translocation to leaves and the rest of the plant; the exopolysaccharides produced by halobacteria also improve soil structure by promoting soil aggregation
- (5)
- Generating ACC-deaminase activity to reduce ethylene levels in the plant
- (6)
- Modifying the structure and morphology of roots, thus facilitating the uptake of nutrients and water from soil. Szepesi [21] established that halotropism helps roots navigate and remodel their architecture through cost-effective energy supply to survive high-salinity conditions
- (7)
- Accumulating amino acids such as glutamate and proline, amines such as carnitine, glycine and betaine, and sugars such as sucrose and trehalose to reduce intracellular osmotic stress
- (8)
- Increasing stomatal conductance and photosynthetic activity
- (9)
- Inducing and regulating the expression of stress-response genes in plants
| Halobacteria | Place of Isolation | Crop Evaluated | Mechanisms That Promote Plant Growth | Effect on the Plant | Ref. |
|---|---|---|---|---|---|
| Micrococcus yunnanensis, Planococcus rifietoensis, Variovorax paradoxus | Halophyte plants from the Gurbanunggut desert, northwest China | Beta vulgaris L. (sugar beet) | ACC-deaminase activity, phosphate solubilization and production of siderophores and indole-acetic acid (IAA) | The effect of halobacteria was assessed under five NaCl concentrations (50 mM, 75 mM, 100 mM, 125 mM and 150 mM). Seed germination increased by 37% and 94% under 50 mM and 125 mM NaCl, respectively). The highest increase in growth parameters was observed at 125 mM NaCl (SL = 24%, RL = 13%, SDW = 98%, RDW = 42%) | [53] |
| Pseudomonas pseudoalcaligenes, Bacillus subtilis | Rhizosphere of rice (Oryza sativa) and sugarcane (Saccharum officinarum) in saline agricultural soils in Pakistan | Glycine max L. (soybean) | ACC-deaminase activity, production of siderophores and IAA | Increase in growth parameters: SL = 20%, SFW = 81%, SDW = 48%, RFW = 124%, RDW = 67%, LA = 174%. Increase in relative leaf water content (26%), increase in K+ level in shoots (61%) and roots (197%), as well as in the levels of antioxidant enzymes in shoots (SOD = 118%, CAT = 20%, APX = 65%, POD = 83%, PAL = 35%, PPO = 50%) and roots (SOD = 12%, CAT = 36%, APX = 87%, POD = 117%, PAL = 18%, PPO = 48%). Leaf proline content increased (118%). Lipid peroxidation decreased in shoots (MDL = 52%) and roots (MDL = 45%) | [51] |
| B. velenzensis, B. subtilis subsp. spizizenii | Sfax solar saltern in the central portion of the Eastern Tunisian coast. | Solanum lycopersicum var. Rio Grande (tomato) | Siderophore production, biofilm formation, exopolysaccharide production, phosphate solubilization and protease and lipase activity | Seed germination improved under salt stress and in the presence of heavy metals (Co, Ni, Cu and Cr) (Vigor index = 166 in inoculated plants, contrasting with 87.33 in non-inoculated plants). 50–90% reduction in the degree of infection caused by the pathogenic fungus Botrytis cinerea in fruits | [54] |
| Halomonas elongata, Bacillus sp. | Salicornia utahensis, Salicornia rubra and Allenrolfea occidentalis | Medicago sativa (alfalfa) | Biofilm formation and possible sequestration of Na+ ions during growth | An endophytic interaction with alfalfa roots developed; root length (15.9 cm) and total biomass (386 mg) increased significantly in the inoculated plants (2.6- and 4.5-fold, respectively) | [55] |
| Pseudomonas fluorescens, Enterobacter hormaechei, Pseudomonas migulae | Setaria italica L. (moha) | Setaria italica L. | ACC-deaminase activity and production of exopolysaccharides | The effect of halobacteria was evaluated under three different levels of hydric stress (drought conditions): −0.3 MPa, −0.49 MPa and −1.03 MPa. Percent germination increased within a range of 13.68–141.82% at all drought levels. Seed dry weight increased by 122%. Exopolysaccharide production facilitated colonization (the CFU ml−1 dropped in only 18.93% 30 days after inoculation). Soil moisture increased by 95.27% and the proportion of soil adhered to roots also improved (75.58%) | [56] |
| Staphylococcus sp. | Salicornia sp. (Succulent, halophyte plant) | Salicornia sp. | Production of IAA, ACC-deaminase activity and phosphate solubilization | The effect of endophytic and rhizosphere Staphylococcus sp. was evaluated at three NaCl concentrations (0 mM, 200 mM, 400 mM and 600 mM). Increases were observed in germination (33.2%), plant weight (71.77% at 0 mM, 88.9% at 200 mM, 72.2% at 400 mM and 85.7% at 600 mM NaCl) and root dry weight (69.7% at 200 mM, 35.6% at 400 mM and 12.7% at 600 mM NaCl). At all concentrations, increases were also recorded in leaf water content (161.1%), K+ content (22.8% at 200 mM, 21.9% at 400 mM and 86.6% at 600 mM NaCl) and superoxide dismutase activity | [57] |
| Enterobacter sp. | Rhizosphere of rice (Oryza sativa) in fields near the Odisha coast, India | Rice | Phosphate solubilization, production of IAA, siderophores and HCN | Germination percentages (Vigor index = 881.6 versus 57.6 in non-inoculated plants) and morphological parameters of shoots (length = 23.07%, fresh weight = 35.71%, dry weight = 90%) and roots (length = 60%, fresh weight = 40%, dry weight = 50%) increased, as well as the total chlorophyll content (114%). Ethylene concentration decreased (66.6%). The activity of antioxidant enzymes increased (SOD = 40%, CAT = 41.66%, POD = 34.42%, PPO = 50%), as well as sugar (113.5%), protein (50%) and IAA (45.83%) content | [45] |
| Klebsiella sp., Pseudomonas sp., Pseudomonas stutzeri, Agrobacterium tumefaciens, Ochrobactrum anthropi | Roots of Arthronemum indicum in coastal marshes | Arachis hypogaea L. (maní) | Nitrogen fixation, IAA production, phosphate solubilization and ACC-deaminase activity | Total nitrogen content in shoots and roots increased significantly (76%) compared to the control. Fewer reactive oxygen species accumulated. Parameters such as shoot (19–31%) and root (45–64%) biomass increased under 4–8% NaCl | [58] |
| Arthrobacter woluwensis, Microbacterium oxydans, Arthrobacter aurescens, Bacillus megaterium, Bacillus aryabhattai | Artemisia princeps, Chenochloa crusgalli, Oenothera biennis growing in sand dunes at Pohang Beach, Korea | Glycine max L. (soybean) | Phosphate solubilization and production of IAA, gibberellins and siderophores | Superoxide dismutase (108.95%) and glutathione peroxidase (40.89%) levels increased, K+ uptake improved (25.56%) and Na+ concentration decreased (31%). Chlorophyll content (63.24%) and all plant growth parameters (SL = 23.52%, RL = 31.17%, SFW = 106.39%, RFW = 114.94%, SDW = 172.72% and RDW = 118.87%) increased under 200 mM NaCl | [50] |
| Bacillus cereus, Serratia marcescens, Pseudomonas aeruginosa | Triticum aestivum (wheat) | Wheat | Production of IAA, HCN and siderophores; nitrogen fixation, phosphate solubilization | Under two levels of saline stress (150 mM and 300 mM NaCl), the uptake system of reactive oxygen species was strengthened, especially at 300 mM NaCl (CAT = 77.77%, SOD = 76.25%, POX = 69.23%) and K+ selectivity increased (19.16% at 300 mM NaCl), restricting Na+ uptake (Na+ ion content in leaves = 27.55%) | [52] |
| Zhihengliuella halotolerans, Brachybacterium sp. | Suaeda sp. in different areas of Iran | Suaeda maritima | Nitrogen fixation | Growth was favored by increasing nutrient uptake through an improved root system, showing higher root and shoot fresh weight (0.0956 g plant−1 and 0.2284 g plant−1, respectively). Salinity resistance also increased | [26] |
| Staphylococcus jettensis, S. arlettae, Bacillus marisflavi, Zhihengliuella flava, Halomonas nanhaiensis, Exiguobacterium mexicanum | Suaeda fructicosa (L.) of north-eastern Pakistan | Zea mays L. | Auxin production, ACC-deaminase activity and biofilm formation | Root (98%) and shoot length (59%) significantly increased in the presence of 200 to 400 mM NaCl. Under co-inoculation, plants showed a high accumulation of proline (140%) compared with the non-inoculated plants | [48] |
| Pseudomonas putida, Alcaligenes sp., Klebsiella sp., Pseudomonas cedrina subsp. Fulgida | Rizosphere of Medicago sativa (alfalfa) from southern Morocco | Alfalfa | Phosphate solubilization; nitrogen fixation; cellulase, pectinase and chitinase activity; production of IAA, ammonia and HCN | The dry weight of shoots (69.3%), roots (36.87%) and chlorophyll content (36.69%) increased. The accumulation of hydrogen peroxide (48.13%) in plant tissue and proline content in shoots (68.27%) decreased significantly. Colonization by arbuscular mycorrhizal fungi increased (25.1%) under saline stress. Phosphatase (49.7%) and galactosidase (91.7%) activity increased in the rhizosphere of inoculated plants | [47] |
| Bacillus subtilis, Oceanobacillus iheyensis, Arthrobacter crystallopoietes | Triticum turgidum subsp. Durum cv. Haurani in Dead Sea nearby areas | Triticum turgidum subsp. Durum cv. Tamaroi (salt-sensitive) and var. 5004 (salt-resistant) | Nitrogen fixation, ACC-deaminase activity and production of siderophores and IAA | The germination percentage of both plant varieties improved (83.3% in the sensitive variety and 100% in the tolerant variety under 160 mM NaCl); root length (139.12% for the sensitive variety and 59% for the tolerant variety at 160 mM NaCl) increased. The tolerance and survival of salt-sensitive plants improved (var. Tamaroi) under drought stress after 10 days of water withholding | [39] |
| Bradyrhizobium sp., Paenibacillus graminis, Actinomadura sp., Bacillus sp., Streptomyces sp. | Soil with pasture (Bradyrhizombium sp.), rhizosphere of Caatinga (Actinomadura sp.), maize (Paenibacillus graminis), sugar cane (Bacillus sp.) and rúcula (Streptomyces sp.), Brazil | Vigna unguiculata (L.) (cowpea bean) | Nitrogen fixation | The co-inoculation of Bradyrhizobium with the other PGPR genera prompted responses to salinity-induced oxidative stress. Bradyrhizobium with Bacillus sp. showed an increased activity of superoxide dismutase (52%), Bradyrhizobium inoculated with Streptomyces displayed the highest catalase activity (55%), Bradyrhizobium inoculated with P. graminis increased phenol peroxidase activity (20%) and Bradyrhizobium inoculated with Bacillus sp. showed the highest levels of redox glutathione (23.9%) in root nodules | [49] |
| Bacillus tequilensis, Bacillus aryabhattai | Rice rhizosphere in various saline fields in Malaysia | Rice | IAA production, nitrogen fixation and phosphate and potassium solubilization | Inoculation with halobacteria in three rice varieties (BBRI dhan67, Putra-1 and MR279) was evaluated. Increases were recorded in the photosynthetic rate (56.23% in BRRI dhan67, 69.95% in Putra-1 and 69.04% in MR297), transpiration (92.22% in BRRI dhan67, 89.08% in Putra-1 and 82.87% in MR297) and stomatal conductance (78.94% in BRRI dhan67, 50% in Putra-1 and 47.36% in MR297). Grain yield (in weight; 15.44%, 33.12% and 57.55% in each variety, respectively) also increased, as well as some growth parameters (SL = 14.74%, RL = 19.58%, RDW = 17.64% and RWC = 4.29%). Proline content (22.73%), electrolyte loss (20.08%), malondialdehyde content in shoots (45.13%) and antioxidant enzyme activity decreased significantly (SOD = 56.72% and POD = 39.62%) | [59] |
| Klebsiella sp. | Wheat rhizosphere | Avena sativa (oat) | IAA production and ACC-deaminase activity | Shoot (14.74%) and root (19.58%) length, root dry weight (17.64%) and relative water content (4.29%) increased. Proline content (22.73%), electrolyte loss (20.08%), malondialdehyde content in shoots (45.13%) and antioxidant enzyme activity decreased significantly (SOD = 56.72% and POD = 39.62%) | [60] |
| Aneurinibacillus aneurinilyticus and Paenibacillus sp. | Garlic (Allium sativum) rhizosphere | Phaseolus vulgaris (French bean) | ACC-deaminase activity, phosphate solubilization, production of IAA, siderophores and ammonium | Ethylene concentration decreased (42%); root (110%) and shoot (60%) length, fresh weight (45% in roots and 255% in shoots), root (220%) and shoot (425%) biomass and total chlorophyll content (57%) increased | [61] |
| Bacillus pumillus FAB10 | Wheat rhizosphere | Wheat | Biofilm formation, ACC-deaminase activity, phosphate solubilization, production of exopolysaccharides and IAA | The effect of halobacteria was evaluated at three NaCl concentrations (75 mM, 125 mM and 250 mM NaCl). Internal CO2 concentration increased (8.5%, 7.5% and 5.5% for each NaCl concentration, respectively), as well as the transpiration rate (22.7% at 250 mM NaCl). Reduced activity levels of antioxidant enzymes such as catalase (42%, 25% and 22%), superoxide dismutase (10.5%, 10.8% and 12%) and glutathione reductase (50%, 42.9% and 42.9%) with each NaCl concentration were recorded | [62] |
| Glutamicybacter halophytcola KLBMP 5180 | Rhizosphere of the coastal halophyte Limonium sinense | Tomato seeds (Jingpeng No. 1) | Production of siderophores, exopolysaccharides and IAA | Root length and root fresh weight (28.6%) increased; total chlorophyll content in leaves remained unchanged under 200 mM NaCl. Proline content increased in leaves (40.4%) and stems (39.2%) under salt stress and in leaves (110%) and stems (86.7%) of non-stressed plants. The accumulation of antioxidant enzymes (SOD = 7.6% and 9.4% and POD = 35.2% and 160.8% in 0 mM and 200 mM NaCl) and the K+/Na+ ratio (102.5% and 170.5% in stems and 10.2% and 33.3% in leafs) increased at 0 mM and 200 mM NaCl, respectively | [63] |
| Alcaligenes faecalis SBN01 and SBN02 | Rhizosphere of native plants (Sesbania aculeata and Atriplex lentiformis) from the Kewra salt mine in Pakistan | Wheat | Nitrogen fixation, IAA production, phosphate solubilization and catalase and protease activity | Under two levels of salt stress (450 mM and 600 mM NaCl), plant biomass increased (LA = 29.47%, PH = 6.5%, SpL = 11.8%, VT = 90% at 450 mM NaCl and LA = 36.78%, PH = 19.15%, SpL = 21.31%, VT = 114.28% at 600 mM NaCl); accumulation of photosynthetic pigments (Chla = 83.82%, Total Chl = 22.41%, Carotenoid = 39.94% at 600 mM NaCl) and the efficiency of the photosystem II (PIABS = 63.64% and Fv/Fm = 4% at 450 mM and PIABS = 45.22% and Fv/Fm = 14% at 600 mM) increased versus non-inoculated plants | [41] |
4. Effect of Halobacteria on Soil Fertility
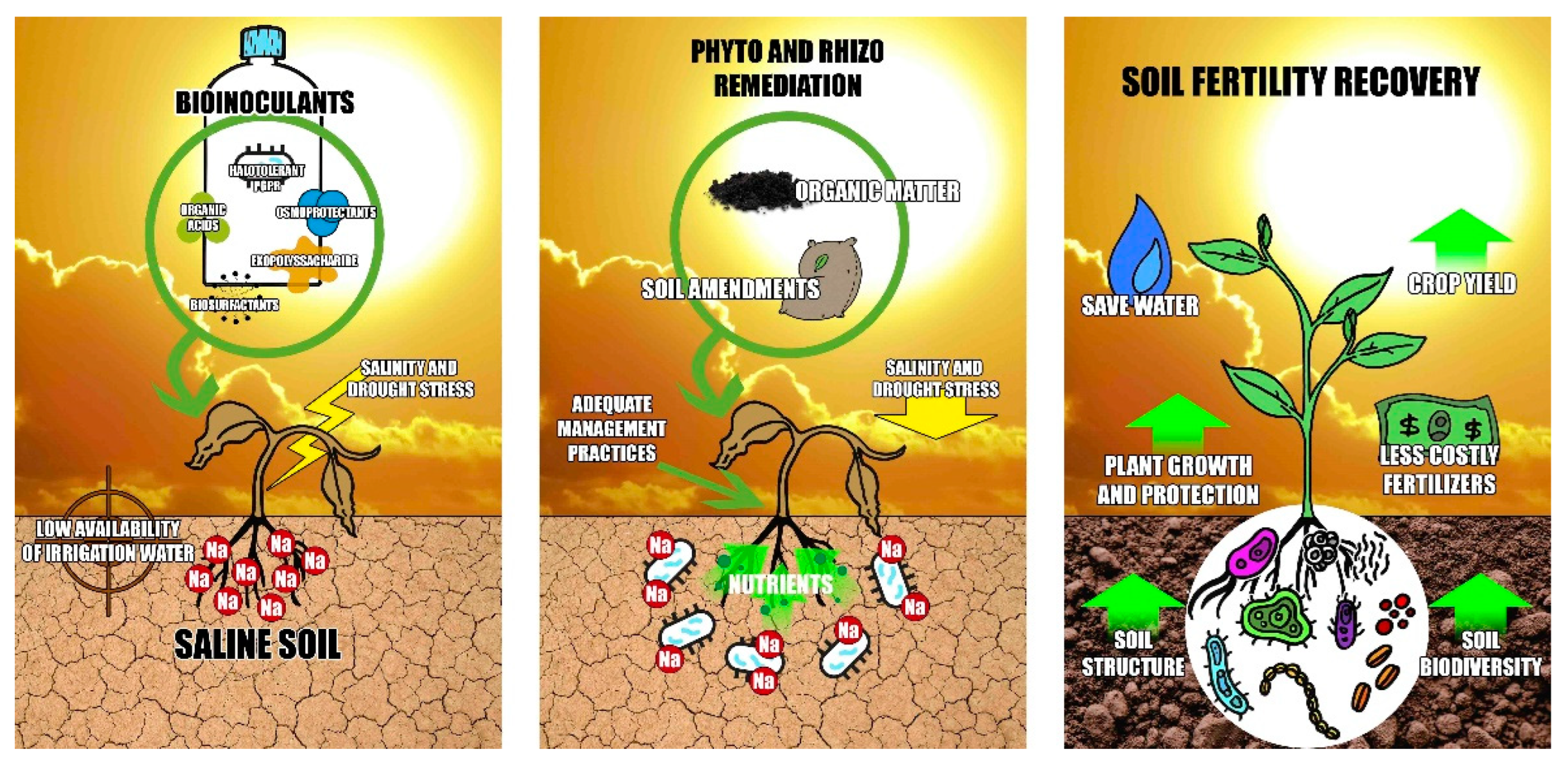
5. Abiotic Characteristics of Arid and Semi-Arid Zones
6. Halobacteria Potential in the Recovery of Saline Soils. A Key Characteristic
7. Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kulkarni, J.; Sharma, S.; Srivastava, A.K.; Penna, S. Halotolerant Microbes and Their Applications in Sustainable Agriculture. In Physiological and Biotechnological Aspects of Extremophiles; Salwan, R., Sharma, V., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2020; pp. 39–49. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing Climate Change: Application of Microbial Biostimulants to Mitigate Stress in Horticultural Crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A Hope for Cultivation of Saline Soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant Plant Growth–Promoting Bacteria: Prospects for Alleviating Salinity Stress in Plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing Climate-Resilient Crops: Improving Plant Tolerance to Stress Combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Mining Halophytes for Plant Growth-Promoting Halotolerant Bacteria to Enhance the Salinity Tolerance of Non-Halophytic Crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, S. Microbe-Based Inoculants: Role in Next Green Revolution. In Environmental Concerns and Sustainable Development; Shukla, V., Kumar, N., Eds.; Springer: Singapore, 2019; pp. 191–246. [Google Scholar] [CrossRef]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of Plant Growth-Promoting Bacteria in Sustainable Agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Arora, K.N.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P.; et al. Halo-Tolerant Plant Growth Promoting Rhizobacteria for Improving Productivity and Remediation of Saline Soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to Mitigate the Adverse Effect of Drought Stress on Crop Plants—Influences of Soil Bacteria: A Review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Potential of Halotolerant and Halophilic Microorganisms for Biotechnology. Extremophiles 2001, 5, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Babalola, O.O. Reclamation of Arid and Semi-Arid Soils: The Role of Plant Growth-Promoting Archaea and Bacteria. Curr. Plant Biol. 2020, 25, 100173. [Google Scholar] [CrossRef]
- Roohi, A.; Ahmed, I.; Iqbal, M.; Jamil, M. Preliminary Isolation and Characterization of Halotolerant and Halophilic Bacteria from Salt Mines of Karak, Pakistan. Pakistan J. Bot. 2012, 44, 365–370. [Google Scholar]
- Ruginescu, R.; Gomoiu, I.; Popescu, O.; Cojoc, R.; Neagu, S.; Lucaci, I.; Batrinescu-Moteau, C.; Enache, M. Bioprospecting for Novel Halophilic and Halotolerant Sources of Hydrolytic Enzymes in Brackish, Saline and Hypersaline Lakes of Romania. Microorganisms 2020, 8, 1903. [Google Scholar] [CrossRef]
- Sharma, R.; Manda, R.; Gupta, S.; Kumar, S.; Kumar, V. Isolation and Characterization of Osmotolerant Bacteria from Thar Desert of Western Rajasthan (India). Rev. Biol. Trop. 2013, 61, 1551–1562. [Google Scholar] [CrossRef][Green Version]
- Agu, K.C.; Nmecha, C.O.; Ikedinma, J.C.; Awah, N.S.; Eneite, H.C.; Victor-Aduloju, A.T.; Umeoduagu, N.; Onwuatuegwu, J.T.C. Isolation and Characterization of Halotolerant Bacteria from Ezzu River Amansea, Awka, Anambra State. Bioeng. Biosci. 2017, 5, 86–90. [Google Scholar] [CrossRef]
- Szepesi, Á. Halotropism: Phytohormonal Aspects and Potential Applications. Front. Plant Sci. 2020, 11, 1448. [Google Scholar] [CrossRef]
- Galvan-Ampudia, C.S.; Julkowska, M.M.; Darwish, E.; Gandullo, J.; Korver, R.A.; Brunoud, G.; Haring, M.A.; Munnik, T.; Vernoux, T.; Testerink, C. Halotropism Is a Response of Plant Roots to Avoid a Saline Environment. Curr. Biol. 2013, 23, 2044–2050. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mehnaz, S.; Mirza, M.S.; Malik, K.A. Isolation and Characterization of Bacteria Associated with the Rhizosphere of Halophytes (Salsola stocksii and Atriplex amnicola) for Production of Hydrolytic Enzymes. Braz. J. Microbiol. 2019, 50, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, S.; Franken, P.; Witzel, K. Properties of the Halophyte Microbiome and Their Implications for Plant Salt Tolerance. Funct. Plant Biol. 2013, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Złoch, M.; Ruppel, S.; Hrynkiewicz, K. Metabolic Potential and Community Structure of Endophytic and Rhizosphere Bacteria Associated with the Roots of the Halophyte Aster tripolium L. Microbiol. Res. 2016, 182, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Alishahi, F.; Alikhani, H.A.; Khoshkholgh-Sima, N.A.; Etesami, H. Mining the Roots of Various Species of the Halophyte Suaeda for Halotolerant Nitrogen-Fixing Endophytic Bacteria with the Potential for Promoting Plant Growth. Int. Microbiol. 2020, 23, 415–427. [Google Scholar] [CrossRef]
- González-Hernández, J.C.; Peña, A. Estrategias de Adaptación de Microorganismos Halófilos y Debaryomyces hansenii (Levadura Halófila). Rev. Latinoam. Microbiol. 2002, 44, 137–156. [Google Scholar]
- Sánchez-Leal, L.C.; Arguello-Arias, H. Capacidad de Bacterias Halófilas Para Capturar Sodio in vitro y Su Posible Aplicación En Bioremediación En Suelos Salinos-Sódicos. Nova 2006, 4, 19–31. [Google Scholar] [CrossRef]
- Shivanand, P.; Mugeraya, G. Halophilic Bacteria and Their Compatible Solutes-Osmoregulation and Potential Applications. Curr. Sci. 2011, 100, 1516–1521. [Google Scholar]
- Kaushal, M.; Wani, S.P. Rhizobacterial-Plant Interactions: Strategies Ensuring Plant Growth Promotion under Drought and Salinity Stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Kaushal, M.; Wani, S.P. Plant-Growth-Promoting Rhizobacteria: Drought Stress Alleviators to Ameliorate Crop Production in Drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z. The Production of Exopolysaccharide by Pseudomonas putida GAP-P45 under Various Abiotic Stress Conditions and Its Role in Soil Aggregation. Microbiol. 2015, 84, 512–519. [Google Scholar] [CrossRef]
- Pazos-Rojas, L.A.; Marín-Cevada, V.; Elizabeth, Y.; García, M.; Baez, A. Uso de Microorganismos Benéficos Para Reducir Los Daños Causados Por La Revolución Verde. Rev. Iberoam. Ciencias 2016, 3, 72–85. [Google Scholar]
- Molina-Romero, D.; del Bustillos-Cristales, M.R.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Santiago-Saenz, Y.; Castañeda-Lucio, M.; Muñoz-Rojas, J. Mecanismos de Fitoestimulación Por Rizobacterias, Aislamientos En América y Potencial Biotecnológico. Biológicas 2015, 17, 24–34. [Google Scholar]
- Saghafi, D.; Delangiz, N.; Lajayer, B.A.; Ghorbanpour, M. An Overview on Improvement of Crop Productivity in Saline Soils by Halotolerant and Halophilic PGPRs. 3 Biotech 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R.; Białkowska, A.; Canfora, L.; Pinzari, F. When Salt Meddles Between Plant, Soil, and Microorganisms. Front. Plant Sci. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Afridi, M.S.; Sumaira, A.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; et al. Induction of Tolerance to Salinity in Wheat Genotypes by Plant Growth Promoting Endophytes: Involvement of ACC Deaminase and Antioxidant Enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y.; Alananbeh, K.M.; Al-Sayaydeh, R. Isolation and Characterization of Halotolerant Plant Growth Promoting Rhizobacteria From Durum Wheat (Triticum turgidum Subsp. Durum) Cultivated in Saline Areas of the Dead Sea Region. Front. Microbiol. 2019, 10, 1639. [Google Scholar] [CrossRef]
- Farahat, M.G.; Mahmoud, M.K.; Youseif, S.H.; Saleh, S.A.; Kamel, Z. Alleviation of Salinity Stress in Wheat by ACC Deaminase-Producing Bacillus aryabhattai EWR29 with Multifarious Plant Growth-Promoting Attributes. Plant Arch. 2020, 20, 417–429. [Google Scholar]
- Babar, M.; Saif-ur-Rehman; Rasul, S.; Aslam, K.; Abbas, R.; ur Athar, H.R.; Manzoor, I.; Kashif Hanif, M.; Naqqash, T. Mining of Halo-Tolerant Plant Growth Promoting Rhizobacteria and Their Impact on Wheat (Triticum aestivum L.) under Saline Conditions. J. King Saud Univ. Sci. 2021, 33, 101372. [Google Scholar] [CrossRef]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas maltophilia BJ01 Induces Salt Tolerance by Modulating Physiology and Biochemical Activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 2530. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Chi, X.; Wang, M.; Chen, M.; Chen, N.; Pan, L. Role of Halotolerant Phosphate-Solubilising Bacteria on Growth Promotion of Peanut (Arachis hypogaea) under Saline Soil. Ann. Appl. Biol. 2019, 174, 20–30. [Google Scholar] [CrossRef]
- Khan, A.; Sirajuddin; Zhao, X.Q.; Javed, M.T.; Khan, K.S.; Bano, A.; Shen, R.F.; Masood, S. Bacillus pumilus Enhances Tolerance in Rice (Oryza sativa L.) to Combined Stresses of NaCl and High Boron Due to Limited Uptake of Na+. Environ. Exp. Bot. 2016, 124, 120–129. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, P.K.; Pramanik, K.; Mitra, S.; Soren, T.; Pandey, S.; Mondal, M.H.; Maiti, T.K. A Halotolerant Enterobacter sp. Displaying ACC Deaminase Activity Promotes Rice Seedling Growth under Salt Stress. Res. Microbiol. 2018, 169, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC Deaminase and Antioxidant Enzymes Producing Halophilic Enterobacter sp. PR14 Promotes the Growth of Rice and Millets under Salinity Stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Tirry, N.; Kouchou, A.; Laghmari, G.; Lemjereb, M.; Hnadi, H.; Amrani, K.; Bahafid, W.; El Ghachtouli, N. Improved Salinity Tolerance of Medicago sativa and Soil Enzyme Activities by PGPR. Biocatal. Agric. Biotechnol. 2021, 31, 101914. [Google Scholar] [CrossRef]
- Aslam, F.; Ali, B. Halotolerant Bacterial Diversity Associated with Suaeda fruticosa (L.) Forssk. Improved Growth of Maize under Salinity Stress. Agronomy 2018, 8, 131. [Google Scholar] [CrossRef]
- de Santos, A.A.; da Silveira, J.A.G.; Bonifacio, A.; Rodrigues, A.C.; do Figueiredo, M.V.B. Antioxidant Response of Cowpea Co-Inoculated with Plant Growth-Promoting Bacteria under Salt Stress. Braz. J. Microbiol. 2018, 49, 513–521. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Halotolerant Rhizobacterial Strains Mitigate the Adverse Effects of NaCl Stress in Soybean Seedlings. Biomed Res. Int. 2019, 2019, 15. [Google Scholar] [CrossRef]
- Yasmin, H.; Naeem, S.; Bakhtawar, M.; Jabeen, Z.; Nosheen, A.; Naz, R.; Keyani, R.; Mumtaz, S.; Hassan, M.N. Halotolerant Rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis Mediate Systemic Tolerance in Hydroponically Grown Soybean (Glycine max L.) against Salinity Stress. PLoS ONE 2020, 15, e0231348. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Saad, A.M.; El-Saadony, M.T.; Merwad, A.R.M.; Rady, M.M. Plant Growth-Promoting Rhizobacteria: Potential Improvement in Antioxidant Defense System and Suppression of Oxidative Stress for Alleviating Salinity Stress in Triticum aestivum (L.) Plants. Biocatal. Agric. Biotechnol. 2020, 30, 101878. [Google Scholar] [CrossRef]
- Zhou, N.; Zhao, S.; Tian, C.Y. Effect of Halotolerant Rhizobacteria Isolated from Halophytes on the Growth of Sugar Beet (Beta vulgaris L.) under Salt Stress. FEMS Microbiol. Lett. 2017, 364, fnx091. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, F.; Abdelmalek, N.; Tounsi, S.; Dunlap, C.A.; Trigui, M. Abiotic Stress Resistance, Plant Growth Promotion and Antifungal Potential of Halotolerant Bacteria from a Tunisian Solar Saltern. Microbiol. Res. 2019, 229, 126331. [Google Scholar] [CrossRef] [PubMed]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-Tolerant Halophyte Rhizosphere Bacteria Stimulate Growth of Alfalfa in Salty Soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Associated with Foxtail Millet in a Semi-Arid and Their Potential in Alleviating Drought Stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef] [PubMed]
- Komaresofla, R.B.; Alikhani, H.A.; Etesami, H.; Khoshkholgh-Sima, N.A. Improved Growth and Salinity Tolerance of the Halophyte Salicornia Sp. by Co–Inoculation with Endophytic and Rhizosphere Bacteria. Appl. Soil Ecol. 2019, 138, 160–170. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant Rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [PubMed]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of Salt-Tolerant Plant Growth-Promoting Rhizobacteria and the Effect on Growth and Yield of Saline-Affected Rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella Sp. Confers Enhanced Tolerance to Salinity and Plant Growth Promotion in Oat Seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC Deaminase Producing Bacteria with Multifarious Plant Growth Promoting Traits Alleviates Salinity Stress in French Bean (Phaseolus vulgaris) Plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Growth Stimulation and Alleviation of Salinity Stress to Wheat by the Biofilm Forming Bacillus pumilus Strain FAB10. Appl. Soil Ecol. 2019, 143, 45–54. [Google Scholar] [CrossRef]
- Xiong, Y.W.; Gong, Y.; Li, X.W.; Chen, P.; Ju, X.Y.; Zhang, C.M.; Yuan, B.; Lv, Z.P.; Xing, K.; Qin, S. Enhancement of Growth and Salt Tolerance of Tomato Seedlings by a Natural Halotolerant Actinobacterium Glutamicibacter halophytocola KLBMP 5180 Isolated from a Coastal Halophyte. Plant Soil 2019, 445, 307–322. [Google Scholar] [CrossRef]
- Mapelli, F.; Marasco, R.; Rolli, E.; Barbato, M.; Cherif, H.; Guesmi, A.; Ouzari, I.; Daffonchio, D.; Borin, S. Potential for Plant Growth Promotion of Rhizobacteria Associated with Salicornia Growing in Tunisian Hypersaline Soils. Biomed Res. Int. 2013, 2013, 248078. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; De, T.K.; Maiti, T.K. Role of ACC Deaminase as a Stress Ameliorating Enzyme of Plant Growth-Promoting Rhizobacteria Useful in Stress Agriculture: A Review. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer Nature Singapore: Singapore, 2018; p. 398. [Google Scholar] [CrossRef]
- del Orozco-Mosqueda, M.C.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Al-Mailem, D.M.; Eliyas, M.; Radwan, S.S. Ferric Sulfate and Proline Enhance Heavy-Metal Tolerance of Halophilic/Halotolerant Soil Microorganisms and Their Bioremediation Potential for Spilled-Oil under Multiple Stresses. Front. Microbiol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Hassan, H.A.; Dawah, S.E.; El-Sheekh, M.M. Monitoring the Degradation Capability of Novel Haloalkaliphilic Tributyltin Chloride (TBTCl) Resistant Bacteria from Butyltin-Polluted Site. Rev. Argent. Microbiol. 2019, 51, 39–46. [Google Scholar] [CrossRef]
- Nabti, E.; Schmid, M.; Hartmann, A. Application of Halotolerant Bacteria to Restore Plant Growth Under Salt Stress. In Halophiles: Biodiversity and Sustainable Exploitation; Maheshwari, D.K., Saraf, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–449. [Google Scholar] [CrossRef]
- Fatima, T.; Arora, N.K. Plant Growth-Promoting Rhizospheric Microbes for Remediation of Saline Soils. In Phyto and Rhizo Remediation; Arora, K.N., Kumar, N., Eds.; Springer Nature: Singapore, 2019; pp. 121–146. [Google Scholar] [CrossRef]
- Saravanan, A.; Jeevanantham, S.; Narayanan, V.A.; Kumar, P.S.; Yaashikaa, P.R.; Muthu, C.M.M. Rhizoremediation-A Promising Tool for the Removal of Soil Contaminants: A Review. J. Environ. Chem. Eng. 2020, 8, 103543. [Google Scholar] [CrossRef]
- Liu, W.; Hou, J.; Wang, Q.; Ding, L.; Luo, Y. Isolation and Characterization of Plant Growth-Promoting Rhizobacteria and Their Effects on Phytoremediation of Petroleum-Contaminated Saline-Alkali Soil. Chemosphere 2014, 117, 303–308. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Rathore, A.P.; Sharma, S. Effect of Halotolerant Plant Growth Promoting Rhizobacteria Inoculation on Soil Microbial Community Structure and Nutrients. Appl. Soil Ecol. 2020, 150, 103461. [Google Scholar] [CrossRef]
- Pankaj, U.; Singh, D.N.; Mishra, P.; Gaur, P.; Babu, C.S.V.; Shanker, K.; Verma, R.K. Autochthonous Halotolerant Plant Growth-Promoting Rhizobacteria Promote Bacoside A Yield of Bacopa monnieri (L.) Nash and Phytoextraction of Salt-Affected Soil. Pedosphere 2020, 30, 671–683. [Google Scholar] [CrossRef]
- Al-Enazy, A.A.; Al-Barakah, F.; Al-Oud, S.; Usman, A. Effect of Phosphogypsum Application and Bacteria Co-Inoculation on Biochemical Properties and Nutrient Availability to Maize Plants in a Saline Soil. Arch. Agron. Soil Sci. 2018, 64, 1394–1406. [Google Scholar] [CrossRef]
- Hassan, T.U.; Bano, A.; Naz, I. Halophyte Root Powder: An Alternative Biofertilizer and Carrier for Saline Land. Soil Sci. Plant Nutr. 2018, 64, 653–661. [Google Scholar] [CrossRef]
- Anees, M.; Qayyum, A.; Jamil, M.; ur Rehman, F.; Abid, M.; Malik, M.S.; Yunas, M.; Ullah, K. Role of Halotolerant and Chitinolytic Bacteria in Phytoremediation of Saline Soil Using Spinach Plant. Int. J. Phytoremediation 2020, 22, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Arora, S. Halotolerant Microbes for Amelioration of Salt-Affected Soils for Sustainable Agriculture. In Phyto-Microbiome in Stress Regulation; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer Nature: Singapore, 2020; pp. 323–343. [Google Scholar] [CrossRef]
- Fernández, J.G. El Recurso Suelo-Agua En Medios Áridos y Semiáridos. In C4 y CAM. Características Generales y Uso en Programas de Desarrollo de Tierras Áridas y Semiáridas; González, J., Chueca, S., Eds.; CSIC, Fundación Ramón Areces: Madrid, España, 2010; pp. 143–149. [Google Scholar]
- López-a, R.; Ramírez-barajas, J.L. Macronutrients in Desert Soils with Agricultural Potential. Terra Latinoam. 2003, 21, 333–340. [Google Scholar]
- O’Geen, A. Consejos Sobre La Sequía: Recuperar Los Suelos Salinos, Sódicos y Salino-Sódicos. ANR 2018, 8629, 1–8. [Google Scholar] [CrossRef]
- Rengasamy, P.; North, S.; Smith, A. Diagnosis and Management of Sodicity and Salinity in Soil and Water in the Murray Irrigation Region, 1st ed.; The University of Adelaide: Adelaide, SA, Australia, 2010. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of Salt-Induced Land Degradation and Restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Rengasamy, P. World Salinization with Emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Rasool, S.; Hameed, A.; Azooz, M.M.; Siddiqi, T.O.; Ahmad, P. Salt Stress: Causes, Types and Responses of Plants. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 9781461447, pp. 1–510. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas Rhizobacteria of Avicennia Marina of Indian Sundarbans Promote Rice Growth under Saline and Heavy Metal Stresses through Exopolysaccharide Production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Fatima, T.; Mishra, I.; Verma, R.; Arora, N.K. Mechanisms of Halotolerant Plant Growth Promoting Alcaligenes sp. Involved in Salt Tolerance and Enhancement of the Growth of Rice under Salinity Stress. 3 Biotech 2020, 10, 361. [Google Scholar] [CrossRef]
- Arjumend, T.; Turan, M. Biochar and Halotolerant Bacteria in The Improvement of Saline-Sodic Soil Health and Wheat Growth. Int. J. Sci. Eng. Res. 2020, 11, 728–734. [Google Scholar]
- Lal, S.; Ratna, S.; Said, O.B.; Kumar, R. Biosurfactant and Exopolysaccharide-Assisted Rhizobacterial Technique for the Remediation of Heavy Metal Contaminated Soil: An Advancement in Metal Phytoremediation Technology. Environ. Technol. Innov. 2018, 10, 243–263. [Google Scholar] [CrossRef]
- Yaseen, R.; Hegab, R.; Kenawey, M.; Eissa, D. Effect of Super Absorbent Polymer and Bio Fertilization on Maize Productivity and Soil Fertility under Drought Stress Conditions. Egypt. J. Soil Sci. 2020, 60, 377–395. [Google Scholar] [CrossRef]
- Pourfadakari, S.; Ghafari, S.; Takdastan, A.; Jorfi, S. A Salt Resistant Biosurfactant Produced by Moderately Halotolerant Pseudomonas aeruginosa (AHV-KH10) and Its Application for Bioremediation of Diesel-Contaminated Sediment in Saline Environment. Biodegradation 2021, 32, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Ojagh, S.M.A.; Fallah, N.; Nasernejad, B. Biological Treatment of Organic Compounds in Produced Water with Use of Halotolerant Bacteria. J. Environ. Chem. Eng. 2020, 8, 104412. [Google Scholar] [CrossRef]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.H.I.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K.A. Halotolerant Marine Rhizosphere-Competent Actinobacteria Promote Salicornia bigelovii Growth and Seed Production Using Seawater Irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Tumpa, F.H.; Sultana, A.; Akter, M.A.; Chakraborty, A. Plant Microbiome–An Account of the Factors That Shape Community Composition and Diversity. Curr. Plant Biol. 2020, 23, 100161. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; del Orozco-Mosqueda, M.C.; Glick, B.R. Rhizosphere Colonization Determinants by Plant Growth-Promoting Rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patel, J.S.; Meena, V.S.; Srivastava, R. Recent Advances of PGPR Based Approaches for Stress Tolerance in Plants for Sustainable Agriculture. Biocatal. Agric. Biotechnol. 2019, 20, 101270. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Karakaş, S.; Çullu, M.A.; Dikilitaş, M. Comparison of Two Halophyte Species (Salsola soda and Portulaca oleracea) for Salt Removal Potential under Different Soil Salinity Conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Nouri, H.; Chavoshi Borujeni, S.; Nirola, R.; Hassanli, A.; Beecham, S.; Alaghmand, S.; Saint, C.; Mulcahy, D. Application of Green Remediation on Soil Salinity Treatment: A Review on Halophytoremediation. Process Saf. Environ. Prot. 2017, 107, 94–107. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.; Yao, L.; Meng, Y.; Ma, X.; Si, E.; Ren, P.; Yang, K.; Shang, X.; Wang, H. Halophyte Halogeton Glomeratus, a Promising Candidate for Phytoremediation of Heavy Metal-Contaminated Saline Soils. Plant Soil 2019, 442, 323–331. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Jiang, L.; Zhang, K.; Tanveer, M.; Tian, C.; Zhao, Z. Reclamation of Saline Soil by Planting Annual Euhalophyte Suaeda Salsa with Drip Irrigation: A Three-Year Field Experiment in Arid Northwestern China. Ecol. Eng. 2021, 159, 106090. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Canseco, J.; Bautista-Cruz, A.; Sánchez-Mendoza, S.; Aquino-Bolaños, T.; Sánchez-Medina, P.S. Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils. Agronomy 2022, 12, 804. https://doi.org/10.3390/agronomy12040804
Hernández-Canseco J, Bautista-Cruz A, Sánchez-Mendoza S, Aquino-Bolaños T, Sánchez-Medina PS. Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils. Agronomy. 2022; 12(4):804. https://doi.org/10.3390/agronomy12040804
Chicago/Turabian StyleHernández-Canseco, Jessie, Angélica Bautista-Cruz, Saúl Sánchez-Mendoza, Teodulfo Aquino-Bolaños, and Patricia S. Sánchez-Medina. 2022. "Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils" Agronomy 12, no. 4: 804. https://doi.org/10.3390/agronomy12040804
APA StyleHernández-Canseco, J., Bautista-Cruz, A., Sánchez-Mendoza, S., Aquino-Bolaños, T., & Sánchez-Medina, P. S. (2022). Plant Growth-Promoting Halobacteria and Their Ability to Protect Crops from Abiotic Stress: An Eco-Friendly Alternative for Saline Soils. Agronomy, 12(4), 804. https://doi.org/10.3390/agronomy12040804






