Abstract
In this work, 75 quality protein maize (QPM) inbred lines were evaluated for aluminum tolerance using a nutrient solution assay in a laboratory and a soil-based technique in a greenhouse tunnel. The experiment was set up in a completely randomized design with three replications in the laboratory, and a randomized complete block design was used in the greenhouse. Aluminum toxicity was generated by amending a nutrient solution with 600 µM of aluminum sulfate (Al2 [SO4]3) in the laboratory, and Al2 [SO4]3 was applied at a rate of 24 mg kg−1 of soil in the greenhouse experiment. Relative root length (RRL) and hematoxylin staining (HS) scores were used to identify tolerant genotypes in the laboratory. According to RRL, 94.7% of genotypes were tolerant and 5.3% were sensitive, while Hematoxylin (HS) classified 77.9% of the genotypes as tolerant, and 22.1% as sensitive. RRL and HS presented a very strong negative association (−0.788). In the soil-based method, the experiments were conducted twice in successive summer seasons of 2019 and 2020. Several growth traits were measured and most genotypes that exhibited tolerance in the nutrient solution also had similar tolerance in the soil-based screening technique. Genetic variability for tolerance was identified, revealing potentially useful donors of tolerance genes in breeding programs.
1. Introduction
Aluminum toxicity is a significant issue in maize production. It damages the apex of the root, and retards root hair growth. This results in nutrient deficiency and leaf disorders such as chlorosis [1]. The chemical form of Al is mainly dependent on soil pH [2]. At low soil pH (<5.0), toxic forms of Al (Al3+) are discharged into the soil at amounts that impair plant root growth [3]. Aluminum (Al) toxicity damages the plant root system, and inhibits root hair growth, resulting in nutrient deficiency, thus affecting the development of the whole plant. The most toxic form of Al regarding plants is Al3+ [4]. Thus, Al toxicity has become one of the major factors that inhibit plant growth and development in acidic soils.
Soil acidity has been identified as one of the significant abiotic constraints in South Africa. A recent study conducted at ARC-Small Grain Institute in 2015 determined that a very high percentage of soils from KwaZulu Natal, North-Western Free State, and Eastern Free State are acidic. It is believed that an average of 25 ppm of Al3+ is present in these soils. The Eastern Cape Province grows insufficient maize due to several limitations, including aluminum toxicity [5,6,7]. Similarly, Ref. [5] reported that 75–100% of the fields in the Eastern Cape tested low in pH. On the other hand, Ref. [6] reported that most of the soils in Lusikisiki were moderately acidic and that the acidity was associated almost entirely with extractable Al. These areas are inhabited mainly by resource-poor smallholder farmers who have limited capacity to deal with the mentioned stress.
Maize production is substantially constrained under acid soils [8]. Al toxicity is widely considered to be the most important growth-limiting factor for plants in acidic soils with pH less than 5. The problem of Al toxicity is critical in high-rainfall areas, where rain leaches alkaline elements such as calcium, potassium, and magnesium from the soil, leaving acidic elements such as aluminum, hydrogen, and manganese to replace the bases. Although many of the problems associated with Al toxicity can be corrected through amendments such as lime, most smallholder farmers in developing countries cannot afford to buy lime. There is need for alternative, affordable, and integrated approaches such as breeding for Al tolerance in the management of the problem of Al toxicity in order to increase maize productivity among the smallholder farmers in the marginalized areas.
Plant species, including varieties within a species, vary in their response to Al. Some are more tolerant than others [2]. Unfortunately, most maize germplasm is sensitive to aluminum [9]. However, specific cultivars of maize—such as those derived from an autochthonous race of the Atlantic coast of South America—are aluminum tolerant, albeit with low yields [10]. Hence, genetic variability for Al tolerance has been demonstrated among maize inbred lines, hybrids, and open-pollinated varieties [11]. To address the toxicity imposed by Al stress, developing tolerant genotypes is the primary aim of plant breeding [12]. For successful breeding of cultivars with desirable characteristics, information on the degree and nature of variability in a plant population has becomes one of the prerequisites. Developing a strategy to enhance QPM performance on soils with high levels of aluminum requires a prior understanding of the morphological and physiological responses of genotypes with distinct genetic backgrounds [13].
Breeding for aluminum tolerance is sustainable in that it is inexpensive, permanent, and environmentally friendly. However, little is known about available quality protein maize (QPM) tolerance to Al toxicity in South Africa, which necessitates screening available germplasm for tolerance. In the current study, Al tolerance was conducted at the seedling stage because tolerance to soil acidity during seedling development has been associated with higher grain yields. Selection at early growth stages is even more helpful when there is much germplasm, which can be a resource and save time [14]. The seedling stage had not been dealt with much in maize crop improvements until recently when [15] reported that young seedlings are more susceptible to Al toxicity than older plants. Field screening is essentially the most critical and robust strategy to evaluate soil acidity tolerance. Still, the response of plants is generally much more complicated due to uneven distribution of the metal concentration in soil, and other interactive environmental factors can influence the nature of the response [1]. Selection for tolerant genotypes can be made under controlled environments in the laboratory and greenhouse to eliminate environmental variation in the field. Laboratory and greenhouse-based techniques are becoming more widely adopted because they are rapid, accurate, and non-destructive [16]. The most preferred technique is the nutrient solution technique for most research because root systems are easily accessed. There is strict control over nutrient availability and pH, and tolerance measurement is non-destructive [17].
This study used a laboratory-based hematoxylin assay and greenhouse-based soil bioassays to identify Al-tolerant QPM genotypes. The nutrient solution and soil bio-assay methods are the most commonly used techniques for screening genotypes at the seedling stage [18]. These methods provide adequate Al stress, allowing preliminary screening of many genotypes in a small area and consequently reducing the number of promising genotypes to be analyzed later in the field. Evaluating genotypes at different crop growth stages is essential as other genes might be controlling tolerance at varying stages of growth, such that screening at both growth stages might be necessary. Various selection techniques can also estimate genetic variability at the various crop growth stages [19]. The current experiment was conducted to proffer an affordable and sustainable solution to Al toxicity challenges faced by farmers in maize production by screening for Al tolerance. This strategy could facilitate development of high yielding genotypes with stable performance in acidic soils in future maize breeding programs. The study’s objectives were as follows: (i) to determine the growth of selected QPM genotypes when subjected to a toxic concentration of exchangeable aluminum, and (ii) to identify genotypes that are tolerant to Al toxicity.
2. Materials and Methods
2.1. Experimental Study Site
The study was carried out on the premises of the University of Fort Hare (UFH) in Alice, once in the laboratory in July 2019 and twice in the greenhouse at the University Research Farm during the 2019 and 2020 summer seasons. The greenhouse is a plastic tunnel made up of clear polyethylene plastic that allows transmission of sunlight. Fluorescent lights (T5) that emit 400–700 nanometers (nm) wavelength provided light for the plants in the laboratory. The average temperature in the laboratory was 25 °C; the average temperatures in the greenhouse for the two seasons were 22 °C and 25 °C, respectively.
2.2. Germplasm
Quality protein maize inbred lines used in this study were obtained from the germplasm collection of CIMMYT-Zimbabwe and Quality Seeds Company in KwaZulu-Natal, South Africa. Uniformly sized seeds were used to reduce variation within genotypes. CIMMYT Mexico provided four reference genotypes or tolerant checks (Table 1). The CIMMYT maize lines (CML) are internationally renowned and are widely used in Eastern and Southern Africa for QPM development [20]. A total of 75 QPM genotypes were used, consisting of 71 inbred lines and 4 resistant checks (Table 1).

Table 1.
Sources of germplasm used in the study.
2.3. Nutrient Solution Technique
2.3.1. Exposure of Seedlings to Aluminum Toxicity
Adequate amounts of viable QPM seeds were collected and surface sterilized with 0.1% sodium hypochlorite (house-hold bleach) solution for 5 min by shaking continuously. This was followed by thoroughly washing the seeds in tap water for 5 min and rinsing with distilled water three times. Seeds were appropriately placed in Petri dishes with moistened filter paper and germinated at 27 °C for 7 days in an incubator. After incubating the seeds for 7 days, five healthy germinated seeds per genotype, with almost the same root and shoot length, were moved into plastic trays containing Hoagland solution for 72 h at room temperature [21]. (Table 2).

Table 2.
List of nutrition elements for the Hoagland solution.
A thin perforated polystyrene sheet was placed on top of the nutrient solution to facilitate immersion of only the roots into the nutrient solution since the polystyrene floats on water (Figure 1).
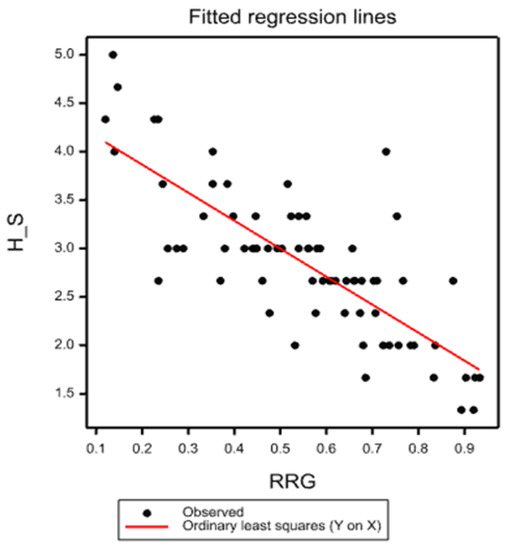
Figure 1.
Regression analysis of HS on RRG. HS—Hematoxylin stain, RRG—relative root growth.
Seedlings were grown for 3 days in the nutrient solution after transference to the stress solution amended with aluminum concentration levels of 0 (control) and 600 µM of aluminum sulfate (Al2 [SO4]3). Five seedlings per genotype were used for measurements, and each treatment was replicated three times in a Completely Randomized Design (CRD). The Al concentration used in this study was adopted from [21], whose research showed that with 600 µM ionic strength in the nutrient solution, the aluminum concentration would sufficiently discriminate aluminum-tolerant and aluminum-sensitive maize genotypes. The pH of the stress solution was adjusted to 4.0 using 1M HCl solution by the dropwise method and remained stable during the entire experiment without any need for further corrections. A pH of 4 is necessary to activate Al toxicity [22], since the availability of most toxic forms of Al (Al3+) depends on the pH of the solution [23]. The control solution had a pH of 5.8, which is high enough to cause some form of acidity stress.
2.3.2. Measurements
Three root traits, namely initial root length, final root length, and relative root length, were evaluated (as shown in Table 3).

Table 3.
Maize root characteristics assessed.
2.3.3. Classification of Genotypes According to Relative Root Length and Hematoxylin Staining Techniques
In maize, seminal root length is a biological trait that represents the Al tolerance rate with great precision and assurance [24]. In this study, seminal root measurements were used as a criterion to discriminate genotypes for Al tolerance. Five plants per genotype were selected, and a Marlin flexible ruler measured the root length of each plant. The length of the seminal root before (initial root growth (IRL)) and after treatment (final root growth (FRL)) were determined. The difference between FRL and IRL (FRL − IRL) was the net root growth (NRL). Each genotype’s relative root length (RRL) was determined as RRL = NRL of Al-treated seedlings ÷ NRL of untreated seedlings (control). Three biological replicates were used for each genotype for both the Al-treated and non-treated (control) seedlings. Roots and shoots were dried at 80 °C for 48 h and weighed using a microgram balance (d = 0.1 mg, Sartorius CP 64, Goettingen, Germany) to determine plant biomass until constant weight was reached. Based on the RRL, all the QPM genotypes were categorized into three groups: tolerant (RRL > 30%), moderately tolerant (15% < RRL ≤ 30%), and sensitive (RRL ≤ 15%) to Al.
The localization of aluminum was detected with Hematoxylin (Merck) using the method described by [25]. Roots of intact seedlings were washed in distilled water for 15 min to remove aluminum ions on the root surface and stained with 250 mL Hematoxylin solution in beakers for 15 min, at room temperature. In this staining protocol, aluminum is a mordant that facilitates the binding of hematin (oxidized hematoxylin) to cells constituencies and forms colored complexes. The procedure described in [26] for preparing the hematoxylin solution was used, which it consisted of dissolving 2.0 g of hematoxylin in a mixture of ethylene glycol (250 mL) and H2O (750 mL). Then, 2.0 g of hematoxylin, 200 mg of sodium iodate, 17.6 g of aluminum sulfate, and 20 mL of glacial acetic acid were added with continuous stirring using a magnetic stirrer. The reagents were mixed and left for 1 h at room temperature; after that, the stain was ready for use. The plants were rewashed for about 15 min in distilled water to remove excess hematoxylin stains, and tainted root tips of the seedlings were evaluated by scoring as described by [9]. Visual scores for root staining intensity were made on a scale of 1–5 using a Dissecting Microscope AC22V (made in Japan) as follows: non-stained roots were classified as very tolerant (1), slightly stained roots as tolerant (2), moderately stained roots as relatively tolerant (3), well-stained roots as sensitive (4), and deeply stained roots as very sensitive (5).
2.4. Soil-Based Screening Procedure
Homogeneous soil collected from the UFH farm was used for the pot experiment, conducted twice in successive summer seasons in September 2019 and October 2020. Soils at UFH farm are alluvial soils, classified as Haplic Cambisol in accordance with the World Reference Base for Soil Resources (WRB) system [27]. The soil was watered to field capacity and incubated with the Al toxicity for 48 h before planting. The temperatures ranged from approximately 30 °C during the day to 19 °C during the night. The soil was air-dried and sieved through a 3 mm mesh. To optimize growth, the soil was amended with a basal compound fertilizer—hygrofert (153 g/kg N, 69 g/kg P, 183 g/kg Mg, and 14 g/kg S) at planting. Polyvinyl chloride (PVC) tubes measuring 60 cm in height and 11 cm in diameter were filled with soil, and two seeds per genotype were sown in each pot, reduced to one plant after the seedlings emerged. The soil was watered to 75% field capacity (FC) and incubated with Al2 [SO4]3 for 48 h before planting. The Al2 [SO4]3 was applied at a rate of 24 mg kg−1 of soil based on the fact that the critical concentration of exchangeable hydrogen cations in the soil (mg/kg−1) above which toxicity is observed for barley, which is a cereal similar to maize, is 23–24 mg/kg−1 [28] A pressure plate apparatus was used to determine the field capacity of the soil [29]. The soil was weighed regularly to maintain constant moisture at 75% FC. The irrigation solution was maintained at pH 4.0 using HCl. Since Al toxicity alters with soil pH, measurements of the soil pH were taken bi-weekly. The pH was always under 4.5, signifying that Al3+ in the soil was available to the plant during the course of the experiment. The experiment was laid in a randomized complete block design (RCBD) with three replications.
2.5. Data Collection
Plants were harvested 4 weeks after planting, and roots and shoots were separated. The roots were washed with a stream flow of tap water, and together with shoots, were oven-dried at 65 °C until a constant weight was reached. Dry matter yield was measured using a microgram balance (d = 0.1 mg, Sartorius AG, Gottingen, Germany).
2.6. Data Analysis
Analysis of variance (ANOVA) was conducted to compare the 75 genotypes’ growth traits using GenStat software version 17. Means were separated according to Turkey’s test at 0.05 level of probability, with regression analysis for relative root growth and hematoxylin staining. The levels of significance presented are p < 0.05, p < 0.01, and p < 0.001
3. Results
3.1. Classification of QPM Genotypes According to RRL and HS
Analysis of variance indicated highly significant differences (p < 0.0001) between the genotypes for tolerance to Al stress for HS and RRL (Table 4).

Table 4.
Mean square values for hematoxylin stains and relative root growth of maize plants exposed to Al toxicity.
Al toxicity treatment on QPM seedlings led to plant morphological changes and growth inhibition of the roots (Table 5). According to the RRL of the longest root, 64 genotypes were categorized as Al-tolerant, 7 genotypes as moderately tolerant, and 4 genotypes as Al sensitive. The 10 most tolerant and sensitive genotypes based on RRG and HS techniques are presented in Table 5. The hematoxylin stains detected Al accumulation in plant root tissue, which showed more Al accumulation than untreated plants. The quantitative measurement of the hematoxylin staining showed significant variations among the genotypes. Based on visual scores for root staining intensity, 2 genotypes were considered very tolerant, 18 as tolerant, 43 as moderately tolerant, 10 as sensitive, and 2 as very sensitive. The results showed a lower tolerance of Al in sensitive lines by exhibiting a deep blue-purplish color, indicating the highest sensitivity to Al toxicity. In contrast, the most tolerant lines showed no apparent intense coloration by the hematoxylin stains. Similarly, these tolerant genotypes were previously selected by RRL as the most sensitive and most tolerant genotypes.

Table 5.
Mean square values for relative root growth and hematoxylin staining scores of the 10 most Al-tolerant -sensitive genotypes under 600 µM Al concentration in nutrient solution.
3.2. Regression Analysis for RRL ans HS
Regression analysis for RRL and HS yielded a statistically significant correlation coefficient (−0.788); thus, a strong negative phenotypic correlation was observed between the two variables (Figure 1).
3.3. Dry Matter Partitioning of 75 QPM Genotypes under Soil-Based Screening Method
There was a considerable variation in acid tolerance among the 75 genotypes tested. Growth characteristics were significantly (p < 0.001) influenced by the main effects of aluminum and genotype (Table 6). Various traits were measured: leaf number, shoot height, shoot dry weight, root dry weight, total dry weight. The growth traits showed a wide range of variability among genotypes. Similarly, the mean squares of genotype, environment, and the genotype × environment (G × E) interaction were highly significant (p < 0.001), highlighting the genotypic differences with respect to the Al concentration under the stress and non-stress environments (Table 6).

Table 6.
Analysis of variance for growth traits under greenhouse conditions.
Significant differences were observed for dry matter partitioned to the shoot and root parts of the plant. The growth of genotypes as shown by the number of leaves, shoot height, shoot, root, and total dry weight were significantly different among genotypes (Table 7). The 10 best-performing genotypes were comparable for each of the studied growth traits, but were significantly different from the 10 worst-performing genotypes. After plant exposure to Al stress, the dry weight of shoots, roots, and total dry weight significantly decreased to different levels. Shoot height was significantly greater for the best-performing genotype (IBL 21) by 33.8% compared to the worst-performing genotype (QSW 16). The best-performing genotype based on leaf number IBL 4 had significantly more leaves (30%) compared to the worst-performing genotype (QSY 9). The proportion of dry matter partitioned to shoot of the best-performing genotype (IBL 8) increased by 75.5% compared to the worst-performing genotype (QSY 18). Furthermore, the most Al-tolerant genotype (IBL 13) based on root dry weight proliferated more roots (56.8%) than did the most Al-sensitive cultivar (QSW 13). The best-performing genotype for total dry weight was IBL 8, which accumulated significantly higher biomass (50.4%) compared to the worst-performing genotype (QSW 27).

Table 7.
Mean values for various growth parameters of 75 QPM genotypes under Al stress and non-stress conditions.
4. Discussion
4.1. Classification of 75 QPM Genotypes According to RRL and HS
In the current study, 75 QPM genotypes were classified into groups according to the RRL and HS after exposure to 600 µM Al for 72 h. The two techniques measured aluminum tolerance in QPM seedlings. An essential aspect of the HS technique is that the reaction between Hematoxylin and Al was specific, such that other stressing factors would exert a minimal effect, if any, on the evaluation processes of the Al effects [4]. On the other hand, the RRL, a broadly used phenotypic index for rapid assessment of Al tolerance in cereals, was used to estimate Al tolerance among the QPM inbred lines in the current study. Both the techniques showed statistical differences among the genotypes. These results confirmed the efficiency of using the two methods for screening stress-tolerant genotypes [11].
Several studies [2,3,16] showed that Al toxicity reduces RRL, as reported for maize in hydroponic systems [30,31], and barley [32]. These findings could be attributed to Al-induced inhibition of root elongation, and thus, it becomes inefficient in absorbing nutrients. The results of this study confirmed the findings of [15], which revealed that root growth retardation is a result of Al-induced inhibition of the root. The decreased root growth might also be the leading cause for stem growth reduction as it brings about physiological stresses such as nutrient deficiency and water deficit [33]. The variations could also be attributed to the differences in genetic potency of each genotype, as different genotypes vary in tolerance to specific stress environments. The germplasm used in this study was collected from various sources and possessed different genetic backgrounds. Corroborating this result is [34], who asserted that the formation of the total root system is more controlled by genetics rather than environmental mechanisms. These results confirm the effectiveness of using RRL as a selection criterion for Al tolerance. This is supported by [4,16,35], who used the RRL index and successfully screened maize genotypes for Al tolerance. Roots of the plants exhibited different symptoms of aluminum toxicity relative to root growth for short-term exposure. Although the RRL has been broadly used as a suitable phenotypic index for Al tolerance assessment in cereal seedlings grown in nutrient solution [16], it has been argued that this significance should not be used as the sole assessment criterion. This may be misrepresentative in genotypes that accumulate more significant amounts of Al in the above-ground part of the plant or those plants that manifest Al tolerance at the adult stage [36]. Thus, against that background, HS was conducted to compliment the RRL conclusions. The HS is a rapid and straightforward technique used to evaluate the Al-stress phenotypes of QPM seedlings in the present study. The measurement of hematoxylin staining showed significant variations among the genotypes. Similar results have been testified by [1], who asserted that the roots of tolerant sorghum inbred lines were not stained with any Al ions, highlighting that they were tolerant.
4.2. Regression Analysis for RRL and HS Techniques
The HS classification was in harmony with the results for the RRL. These results were corroborated by the findings of [3,4,11] who reported that HS was highly negatively correlated with RRL. Additionally, Anas et al [1] also reported that sorghum genotypes from ICRI-SAT confirmed tolerance with the scores of HS. Overall results agreed with other previous reports, such as [3], who reported a negative correlation between RRL and HS (−0.816) using S3 inbreed lines. This could be attributed to the fact that sensitive seedlings exhibit poor RRL due to high amounts of aluminum ions accumulated in the root cap. Therefore, these particular genotypes showed higher HS. On the contrary, tolerant seedlings have some avoidance mechanism to aluminum toxicity; thus, they present higher RRL and lower HS. Other researchers such as [31,37] found consistent results with these findings. They reported that the hematoxylin screening method had considerable potential for large-scale evaluations of inbred lines and progenies of maize, especially for the first round of screening. Wenzl et al [38] used hematoxylin staining to screen Brachiaria grass genotypes for Al tolerance. They concluded that hematoxylin staining was a good criterion for discriminating between tolerant and sensitive maize seedlings. Similar results have also been reported for maize cultivars by [16]. These results showed a possible relationship between hematoxylin scores and root growth measurements in cereals. The two techniques RRL and HS proved ideal in identifying tolerant and sensitive genotypes after a short exposure time of seedlings to Al; thus, they can be used without restraint as a simple informative phenotypic index in Al tolerance studies. In agreement with these results is [38], who reported that these screening methods were suitable for separating genotypes with varying levels of acid tolerance and thus can be used for preliminary assessment of tolerance.
4.3. Dry matter Partitioning of 75 QPM Genotypes Using the Soil-Based Screening Method
Genotypic variation was observed on genotypes grown, as the technique provided a realistic rooting environment that allowed separation of the tolerant and sensitive genotypes [39]. Dry matter partitioned to the shoot and roots of the QPM genotypes differed and decreased significantly in response to aluminum toxicity. This suggested that these growth traits could be useful in germplasm screening for aluminum tolerance. These results were supported by [40], who reported that toxic levels of Al inhibited cell division and elongation, resulting in a shallow and reduced root system that limits water and nutrient uptake. The results showed that aluminum toxicity had a detrimental effect on plant growth. This was manifested by the considerable decreases observed in all the parameters assessed in response to the aluminum applied in the soil. Similarly, Bhalerao et al [41] stated that Al toxicity was expressed by the direct injury of roots and root length reduction. Most genotypes that exhibited Al tolerance in the nutrient solution technique also showed similar tolerance in the soil bioassay experiment. Conclusively, the overall effect of Al toxicity is significantly expressed through the biomass and, ultimately, grain yield of crops. Even though the objective of this study did not evaluate up to grain yield, it is envisaged that the quality of grain yield would be reduced since the abnormal root morphology directly impacts nutrient uptake as well as water absorption. The Al toxicity-induced suppression of photosynthetic capacity of shoots might also ultimately reduce the quality of grain. This aspect certainly requires further investigation as it is important to establish the extent to which the QPM genotypes will be affected.
5. Conclusions
Two different techniques, namely nutrient solution and soil-based screening assays, were used to determine the response of QPM genotypes to aluminum toxicity. Genotypic variation for tolerance was observed among the genotypes. RRL classified 94.7% of genotypes as tolerant and 5.3% as sensitive, while HS classified 77.9% of the genotypes as tolerant and 22.1% as sensitive. A strong negative correlation was observed between the RRL and HS. Interestingly, most genotypes that displayed tolerance in the nutrient solution also showed similar tolerance in the soil-based screening technique. The tolerant genotypes could be useful in future breeding programs. Thus, it can be recommended to use either the nutrient solution screening method or the soil-based screening method, as both techniques allow selection of the tolerant and sensitive genotypes.
Author Contributions
R.M.Z. designed and established the experiment, collected and analyzed experimental data, and wrote the manuscript. C.S.M. designed the experiment, supervised the experiment setup, collected and analyzed experimental data, and wrote the manuscript. L.N.T. designed the experiment, collected and analyzed experimental data, supervised the experiment setup, and wrote the manuscript. A.M. designed and supervised the experiment setup, collected experimental data, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation (NRF) of South Africa, Grant number 116256 (Ref: CSRU 180504326029).
Institutional Review Board Statement
On behalf of the University of Fort Hare’s Animal Research Ethics Committee (AREC) I hereby give ethical approval in respect of the undertakings contained in the above-mentioned project and research instrument(s). Should any other instruments be used, these require separate authorization. The Researcher may therefore commence with the research as from the date of this certificate, using the reference number: Ethical clearance certificate arec-150311-008, Certificate Reference Number: MUT011SNYA01/19/E.
Informed Consent Statement
The study did not involve human subjects so does not require informed consent.
Data Availability Statement
The data presented in this study will be made available upon request.
Acknowledgments
CIMMYT Zimbabwe and Mexico and Quality Seeds (PVT) LTD of South Africa are acknowledged for supplying the germplasm that was used in this study. The National Research Foundation (NRF) of South Africa is acknowledged for providing financial support for this study, grant number 116256 (Ref-CSRU 180504326029). The Govan Mbeki Research and Development Centre at the University of Fort Hare is acknowledged for providing postdoctoral funding to L.N.T.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anas, A.; Yoshida, T.; Anas, S. Screening of Al-Tolerant Sorghum by Hematoxylin Staining and Growth Response. Plant Prod. Sci. 2000, 3, 246–253. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Regon, P.; Sahoo, S.; Chowra, U.; Pradhan, A.; Roy, A.; Panda, S.K. Morpho-physiological analysis of tolerance to aluminum toxicity in rice varieties of North East India. PLoS ONE 2017, 12, e0176357. [Google Scholar] [CrossRef] [PubMed]
- Cançado, G.M.A.; Loguercio, L.L.; Martins, P.R.; Parentoni, S.N.; Paiva, E.; Borém, A.; Lopes, M.A. Hematoxylin staining as a phenotypic index for aluminum tolerance selection in tropical maize (Zea mays L.). Theor. Appl. Genet. 1999, 99, 747–754. [Google Scholar] [CrossRef]
- Roy, B.; Bhadra, S. Hydroponic screening for selection of aluminum tolerant rice (Oryza sativa L.) genotypes at seedlings stage using different indices. Cereal Res. Commun. 2014, 42, 463–473. [Google Scholar] [CrossRef]
- Mandiringana, O.T.; Mnkeni, P.N.S.; Mkile, Z.; Van Averbeke, W.; Van Ranst, E.; Verplancke, H. Mineralogy and Fertility Status of Selected Soils of the Eastern Cape Province, South Africa. Commun. Soil Sci. Plant Anal. 2005, 36, 2431–2446. [Google Scholar] [CrossRef]
- Bühmann, C.; Beukes, D.J.; Turner, D.P. Acidity/Al aspects in soils of the Lusikisiki area, Eastern Cape Province. S. Afr. J. Plant Soil 2006, 23, 87–92. [Google Scholar] [CrossRef]
- Pfunde, C.; Mutengwa, C. Combining ability of Quality Protein Maize inbred lines for seedling tolerance to drought stress. Philipp. J. Crop Sci. 2016, 41, 1–12. [Google Scholar]
- Tandzi, L.N.; Mutengwa, C.S.; Ngonkeu, E.L.M.; Gracen, V. Breeding Maize for Tolerance to Acidic Soils: A Review. Agronomy 2018, 8, 84. [Google Scholar] [CrossRef]
- Ouma, E.; Ligeyo, D.; Matonyei, T.; Agolo, J.; Were, B.; Too, E.; Onkware, A.; Gudu, S.; Kisinyo, P.; Phillip, N. Enhancing Maize Grain Yield in Acid Soils of Western Kenya Using Aluminium Tolerant Germplasm. J. Agric. Sci. Technol. 2013, A 3, 33–46. [Google Scholar]
- Minella, E.; Sorrells, M.E. Genetic analysis of aluminum tolerance in Brazilian barleys. Pesqui. Agropecuária Bras. 2002, 37, 1099–1103. [Google Scholar] [CrossRef]
- Giaveno, C.D.; Filho, J.B.M. Rapid screening for aluminum tolerance in maize (Zea mays L.). Genet. Mol. Biol. 2000, 23, 847–850. [Google Scholar] [CrossRef]
- Pattanayak, A.; Pfukrei, K. Aluminium toxicity tolerance in crop plants: Present status of research. Afr. J. Biotechnol. 2013, 12, 3752–3757. [Google Scholar]
- Legesse, H.; Nigussie-Dechassa, R.; Gebeyehu, S.; Bultosa, G. Growth and Dry Matter Partitioning of Common Bean (Phaseolus vulgaris L.) Genotypes as Influenced by Aluminum Toxicity. J. Exp. Agric. Int. 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Merga, M.; Mohammed, H.; Assefa, K. International Journal of Current Research and Academic Review. Int. J. Curr. Res. 2019, 7, 5–21. [Google Scholar]
- Mossor-Pietraszewska, T. Effect of aluminium on plant growth and metabolism. Acta Biochim. Pol. 2001, 48, 673–686. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Cui, B.; Wang, N.; Ding, J.; Liu, C.; Gao, S.; Zhang, S. Aluminium Tolerance Assessment of 141 Maize Germplasms in a Solution Culture. Univ. J. Agric. Res. 2017, 5, 1–9. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Frei, M.; Wissuwa, M. An agar nutrient solution technique as a screening tool for tolerance to zinc deficiency and iron toxicity in rice. Soil Sci. Plant Nutr. 2008, 54, 744–750. [Google Scholar] [CrossRef]
- Ahlrichs, J.L.; Karr, M.C.; Baligar, V.C.; Wright, R.J. Rapid bioassay of aluminum toxicity in soil. Plant Soil 1990, 122, 279–285. [Google Scholar] [CrossRef]
- Mustafa, H.S.B.; Farooq, j.; Hasan, E.; Bibi, T.; Mahmood, T. Cluster and principal component analyses of maize accessions under normal and water stress conditions. J. Agric. Sci. 2015, 60, 33–48. [Google Scholar]
- Masindeni, D.R. Evaluation of South African High Quality Protein Maize (Zea mays L.) Inbred Lines under Optimum and Low Nitrogen Conditions and the Identification of Suitable Donor Parents. PhD Thesis, University of Free State, Bloemfontein, South Africa, 2013. [Google Scholar]
- Chanda, R.; Munyinda, K.; Kinsese, T.; Osiru, D.S. Genotypic variation in seedling tolerance to aluminum toxicity in historical maize inbred lines of Zambia. Agronomy 2015, 5, 200–219. [Google Scholar]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef]
- Abate, E.; Hussien, S.; Laing, M.; Mengistu, F. Aluminum tolerance in cereals: A potential component of integrated acid soils management in Ethiopia. Ethiop. J. Nat. Resour. 2013, 13, 43–66. [Google Scholar]
- Hochholdinger, F.; Woll, K.; Sauer, M.; Dembinsky, D. Genetic Dissection of Root Formation in Maize (Zea mays) Reveals Root-type Specific Developmental Programmes. Ann. Bot. 2004, 93, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Polle, E.; Konzak, C.F.; Kattrick, J.A. Visual Detection of Aluminum Tolerance Levels in Wheat by Hematoxylin Staining of Seedling Roots. Crop. Sci. 1978, 18, 823–827. [Google Scholar] [CrossRef]
- Gill, G.W. Special Stains. Cytopreparation. Essentials in Cytopathology, Springer: New York, NY, USA, 2013; Volume 12. [Google Scholar] [CrossRef]
- Nciizah, A.D.; Wakindiki, I.I.C. Particulate organic matter, soil texture and mineralogy relations in some Eastern Cape ecotopes in South Africa. S. Afr. J. Plant Soil 2012, 29, 39–46. [Google Scholar] [CrossRef]
- Holland, J.E.; White, P.J.; Thauvin, J.-N.; Jordan-Meille, L.; Haefele, S.M.; Thomas, C.L.; Goulding, K.W.T.; McGrath, S.P. Liming impacts barley yield over a wide concentration range of soil exchangeable cations. Nutr. Cycl. Agroecosyst. 2021, 120, 131–144. [Google Scholar] [CrossRef]
- Nyambo, P.; Taeni, T.; Chiduza, C.; Araya, T. Effects of Maize Residue Biochar Amendments on Soil Properties and Soil Loss on Acidic Hutton Soil. Agronomy 2018, 8, 256. [Google Scholar] [CrossRef]
- Mariano, E.D.; Keltjens, W.G. Long-Term Effects of Aluminum Exposure on Nutrient Uptake by Maize Genotypes Differing in Aluminum Resistance. J. Plant Nutr. 2005, 28, 323–333. [Google Scholar] [CrossRef]
- Trachsel, S.; Stamp, P.; Hund, A. Effect of high temperatures, drought and aluminum toxicity on root growth of tropical maize (Zea mays L.) seedlings. Maydica 2010, 55, 249–260. [Google Scholar]
- Zelinová, V.; Halušková, L.; Huttová, J.; Illés, P.; Mistrík, I.; Valentovičová, K.; Tamás, L. Short-term aluminium-induced changes in barley root tips. Protoplasma 2011, 248, 523–530. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Singh, D.; Kumar, J. A comparative study of screening methods for tolerance to aluminum toxicity in pigeonpea [Cajanus cajan (L.) millspaugh]. Aust. J. Crop Sci. 2011, 5, 1419–1426. [Google Scholar]
- Suwarto; Dinuriah, I.; Pramesthi, R.; Soraya. Root growth dynamics and grain yield of ten new plant type of rice lines under aerobic and flooded condition. IOP Conf. Series Earth Environ. Sci. 2019, 250, 012082. [Google Scholar] [CrossRef]
- Camacho, R.G.; Caraballo, D.F. Evaluation of morphological characteristics in Venezuelan maize (Zea mays L.) genotypes under drought stress. Sci. Agric. 1994, 51, 453–458. [Google Scholar] [CrossRef]
- Foy, C.D.; Chaney, R.L.; White, M.C. The Physiology of Metal Toxicity in Plants. Annu. Rev. Plant Physiol. 1978, 29, 511–566. [Google Scholar] [CrossRef]
- Magnavaca, R.; Gardner, C.O.; Clark, R.B. Evaluation of inbred maize lines for aluminum tolerance in nutrient solution. In Genetic Aspects of Plant Mineral Nutrition; Springer: Berlin/Heidelberg, Germany, 1987; pp. 255–265. [Google Scholar] [CrossRef]
- Wenzl, P.; Arango, A.; Chaves, A.L.; Buitrago, M.E.; Patiño, G.M.; Miles, J.; Rao, I.M. A greenhouse method to screen brachiaria grass genotypes for aluminum resistance and root vigor. Crop. Sci. 2006, 46, 968–976. [Google Scholar] [CrossRef]
- Tang, C.; Nuruzzaman, M.; Rengel, Z. Screening wheat genotypes for tolerance of soil acidity. Aust. J. Agric. Res. 2003, 54, 445–452. [Google Scholar] [CrossRef]
- De Menezes, C.B.; Lima, G.M.; Marucci, R.C.; Bernardino, K.C.; Dos Santos, C.V.; Julio, M.P.M.; Schaffert, R.E. Evaluation of grain sorghum hybrids for aluminum tolerance in nutrient solution. Acta Sci. Agron. 2018, 40, 35304. [Google Scholar] [CrossRef]
- Bhalerao, S.A.; Prabhu, D.V. Aluminium Toxicity in Plants—A Review. J. Appl. Chem. 2015, 21, 447–474. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).