Comparative Study of Phosphorous-Acid-Containing Products for Managing Phytophthora Blight of Bell Pepper
Abstract
:1. Introduction
2. Materials and Methods
2.1. P. capsici Isolates and Chemicals
2.2. In vitro Tests
2.2.1. Mycelial Growth
2.2.2. Zoospore Germination
2.2.3. Sporangium Formation
2.3. Greenhouse Tests
2.3.1. Control of Phytophthora Blight of Bell Pepper by Phosphorous-Acid-Containing Products
2.3.2. Induction of Systemic Resistance in Bell Pepper by Phosphorous-Acid-Containing Products
2.4. Statistical Analysis
3. Results
3.1. Effect of Phosphorous-Acid-Containing Products on Mycelium Growth, Zoospore Germination, and Sporangium Formation of P. capsici
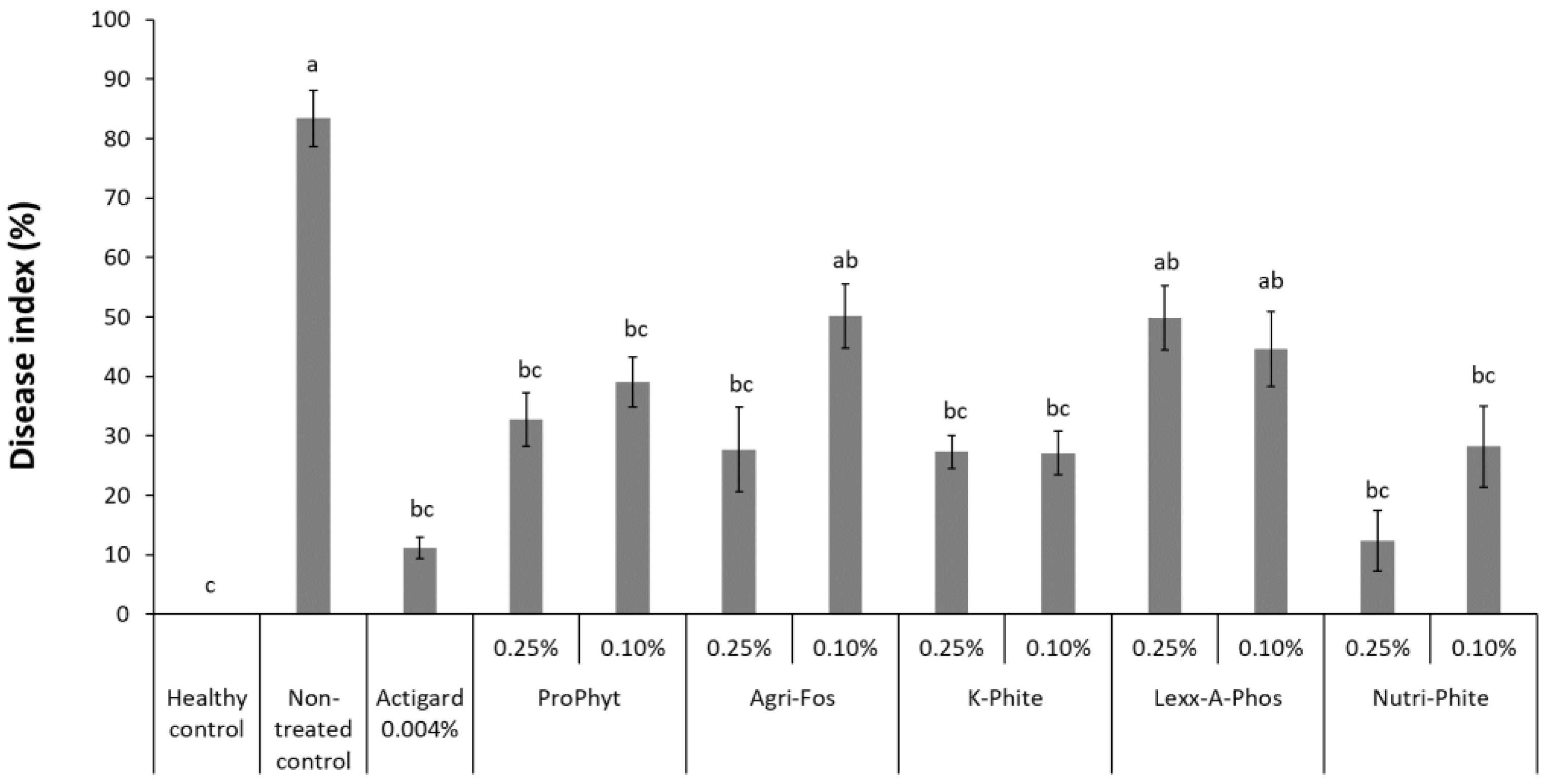
3.2. Application of Phosphorous-Acid-Containing Products as Drench in Artificially Infested Growing Medium to Suppress Phytophthora Blight
3.3. Induction of Systemic Resistance in Bell Pepper against Phytophthora Blight by Phosphorous-Acid-Containing Products
3.4. Effect of Phosphorous-Acid-Containing Products on the Growth of Bell Pepper Seedlings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Förster, H.; Adaskaveg, J.E.; Kim, D.H.; Stanghellini, M.E. Effect of phosphite on tomato and pepper plants and on susceptibility of pepper to Phytophthora root and crown rot in hydroponic culture. Plant Dis. 1998, 82, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristaino, J.B.; Larkin, R.P.; Campbell, C.L. Spatial and temporal dynamics of Phytophthora epidemics in commercial bell pepper fields. Phytopathology 1993, 83, 1312–1320. [Google Scholar] [CrossRef]
- Silvar, C.; Díaz, J.; Merino, F. Real-Time polymerase chain reaction quantification of Phytophthora capsici in different pepper genotypes. Phytopathology 2005, 95, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Babadoost, M. Host range of Phytophthora capsici from pumpkin and pathogenicity of isolates. Plant Dis. 2004, 88, 485–489. [Google Scholar] [CrossRef] [Green Version]
- Zitter, T.A.; Hopkins, D.L.; Thomas, C.E. Compendium of Cucurbit Diseases; American Phytopathological Society Press: St. Paul, MN, USA, 1996; p. 87. [Google Scholar]
- Babadoost, M.; Islam, S.Z. Fungicide seed treatment effects on seedling damping-off of pumpkin caused by Phytophthora capsici. Plant Dis. 2003, 87, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Critopoulos, P.D. Foot rot of tomato incited by Phytophthora capsici. Bull. Torrey Bot. Club 1955, 82, 168–182. [Google Scholar] [CrossRef]
- Lamour, K.H.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant Pathol. 2012, 13, 329–337. [Google Scholar] [CrossRef]
- De Cara, M.; Ayala-Doñas, A.; Aguilera-Lirola, A.; Campoadra, S.C.A.; Badillo-López, E.; Gómez-Vázquez, J. First report of Phytophthora capsici causing wilting and crown and root rot on eggplant (Solanum melongena) in Southeastern Spain. Plant Dis. 2018, 102, 2044. [Google Scholar] [CrossRef]
- Hwang, B.K.; Kim, C.H. Phytophthora blight of pepper and its control in Korea. Plant Dis. 1995, 79, 221–227. [Google Scholar] [CrossRef]
- Hausbeck, M.K.; Lamour, K.H. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 2004, 88, 1292–1303. [Google Scholar] [CrossRef] [Green Version]
- Schwinn, F.; Staub, T. Oomycete fungicides. In Modern Selective Fungicides, Properties, Applications, Mechanisms of Action; Lyr, H., Ed.; Gustav Fischer Verlag: New York, NY, USA, 1995; pp. 323–346. [Google Scholar]
- Parra, G.; Ristaino, J. Insensitivity to Ridomil Gold (mefenoxam) found among field isolates of Phytophthora capsici causing Phytophthora blight on bell pepper in North Carolina and New Jersey. Plant Dis. 1998, 82, 711. [Google Scholar] [CrossRef] [PubMed]
- Lamour, K.H.; Hausbeck, M.K. Mefenoxam insensitivity and the sexual stage of Phytophthora capsici in Michigan cucurbit fields. Phytopathology 2000, 90, 396–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agosteo, G.E.; Raudino, F.; Cacciola, S.O. Resistance of Phytophthora capsici to metalaxyl in plastic-house capsicum crops in Southern Italy. EPPO Bull. 2000, 30, 257–261. [Google Scholar] [CrossRef]
- French-Monar, R.D.; Jones, J.B.; Ozores-Hampton, M.; Roberts, P.D. Survival of inoculum of Phytophthora capsici in soil through time under different soil treatments. Plant Dis. 2007, 91, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, L.R.; Little, G. The effect of foliar application of phosphonate formulations on the susceptibility of potato tubers to late blight. Pest Manag. Sci. 2002, 58, 17–25. [Google Scholar] [CrossRef]
- Johnson, D.A.; Inglis, D.A.; Miller, J.S. Control of potato tuber rots caused by oomycetes with foliar applications of phosphorous acid. Plant Dis. 2004, 88, 1153–1159. [Google Scholar] [CrossRef] [Green Version]
- Yandoc-Ables, C.B.; Rosskopf, E.N.; Lamb, E.M. Management of Phytophthora crown rot in pumpkin and zucchini seedlings with phosphonates. Plant Dis. 2007, 91, 1651–1656. [Google Scholar] [CrossRef]
- Ouimette, D.; Colley, M. Comparative antifungal activity of four phosphonate compounds against isolates of nine Phytophthora species. Phytopathology 1989, 79, 761–767. [Google Scholar] [CrossRef]
- Smillie, R.; Grant, B.R.; Guest, D. The mode of action of phosphite: Evidence for both direct and indirect modes of action on three Phytophthora spp. in plants. Phytopathology 1989, 79, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Guest, D.; Grant, B. The complex action of phosphonates as antifungal agents. Biol. Rev. 1991, 66, 159–187. [Google Scholar] [CrossRef]
- Dobrowolski, M.P.; Shearer, B.L.; Colquhoun, I.J.; O’Brien, P.A.; Hardy, G.E.S.J. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathol. 2008, 57, 928–936. [Google Scholar] [CrossRef]
- Yin, J.; Jackson, K.L.; Candole, B.L.; Csinos, A.S.; Langston, D.B.; Ji, P. Aggressiveness and diversity of Phytophthora capsici on vegetable crops in Georgia. Ann. Appl. Biol. 2012, 160, 191–200. [Google Scholar] [CrossRef]
- Yuan, N.N.; Chen, S.N.; Zhai, L.X.; Schnabel, G.; Yin, L.F.; Luo, C.X. Baseline sensitivity of Monilia yunnanensis to the DMI fungicides tebuconazole and triadimefon. Eur. J. Plant Pathol. 2013, 136, 651–655. [Google Scholar] [CrossRef]
- Sujkowski, L.S.; Parra, G.R.; Gumpertz, M.L.; Ristaino, J.B. Temporal dynamics of Phytophthora blight on bell pepper in relation to the mechanisms of dispersal of primary inoculum of Phytophthora capsici in soil. Phytopathology 2000, 90, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Candole, B.L.; Conner, P.J.; Ji, P. Evaluation of Phytophthora root rot-resistant Capsicum annuum accessions for resistance to Phytophthora foliar blight and Phytophthora stem blight. Agric. Sci. 2012, 3, 732–737. [Google Scholar] [CrossRef]
- Fenn, M.E.; Coffey, M.D. Studies on the in vitro and in vivo antifungal activity of Fosetyl-Al and phosphorous acid. Phytopathology 1984, 74, 606–611. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; The American Phytopathological Society: St Paul, MN, USA, 1996; p. 562. [Google Scholar]
- Guest, D.I.; Bompeix, G. The complex mode of action of phosphonates. Australas. Plant Pathol. 1990, 19, 113–115. [Google Scholar] [CrossRef]
- Walker, G.E. Phytotoxicity in mandarins caused by phosphorous acid. Australas. Plant Pathol. 1989, 18, 57–59. [Google Scholar] [CrossRef]
- Wicks, T.; Hall, B. Evaluation of phosphonic (phosphorous) acid for the control of Phytophthora cambivora on almond and cherry in South Australia. Australas. Plant Pathol. 1990, 19, 132–133. [Google Scholar] [CrossRef]
- Seymour, N.P.; Thompson, J.P.; Fiske, M.L. Phytotoxicity of Fosetyl-Al and phosphonic acid to maize during production of vesicular-arbuscular mycorrhizal inoculum. Plant Dis. 1994, 78, 441–446. [Google Scholar] [CrossRef]

| Commercial Name | Active Ingredients |
|---|---|
| Agri-Fos | Mono- and di-potassium salt of phosphorous acids (45.8%) (Agrichem Manufacturing Industries Pty. Ltd., Loganholme, QLD, AUS) |
| K-Phite | Mono- and di-potassium salts of phosphoric acid (53%) (Plant Food Systems, Zellwood, FL, USA) |
| Lexx-A-Phos | Di-potassium phosphate (22.7%), di-potassium phosphonate (22.4%) (Foliar Nutrients, Inc., Cairo, GA, USA) |
| Nutri-Phite | Total phosphoric acid (60%), soluble potash (K2O) (5%) (Biagro Western Sales, Inc., Visalia, CA, USA) |
| ProPhyt | Potassium phosphate (54.5%) (Luxembourg-Pamol, Inc., Memphis, TN, USA) |
| Ridomil Gold 480 EC 1 | Mefenoxam (phenylamide) (480 g L−1) (Syngenta Crop Protection, Inc., Greensboro, NC, USA) |
| Actigard 2 | Acibenzolar-S-methyl (50%) (Syngenta Crop Protection, Inc., Greensboro, NC, USA) |
| Fungicides | EC50 (µg mL−1) | |
|---|---|---|
| PPC1 | PPC6 | |
| Mycelial growth | ||
| Agri-Fos | 139.4 ± 3.1 | 324.4 ± 14.6 |
| Nutri-Phite | 50.5 ± 1.9 | 94.5 ± 2.8 |
| Lexx-A-Phos | 246.4 ± 10.1 | 195.5 ± 5.9 |
| K-Phite | 112.4 ± 8.2 | 181.5 ± 12.6 |
| ProPhyt | 120.3 ± 6.1 | 164.2 ± 5.4 |
| Ridomil Gold 480 EC | 7.6 ± 0.6 | 13.8 ± 2.5 |
| Zoospore germination | ||
| Agri-Fos | 39.5 ± 2.4 | 13.9 ± 1.3 |
| Nutri-Phite | 49.1 ± 2.2 | 12.5 ± 4.5 |
| Lexx-A-Phos | 280.5 ± 8.6 | 58.9 ± 2.7 |
| K-Phite | 52.0 ± 2.9 | 27.9 ± 1.1 |
| ProPhyt | 61.1 ± 3.2 | 5.2 ± 0.6 |
| Ridomil Gold 480 EC | >100 | >240 |
| Sporangium formation | ||
| Agri-Fos | 149.4 ± 11.5 | 34.0 ± 1.2 |
| Nutri-Phite | 58.1 ± 4.5 | 12.1 ± 2.4 |
| Lexx-A-Phos | 127.2 ± 6.3 | 42.4 ± 1.5 |
| K-Phite | 217.9 ± 5.9 | 45.9 ± 1.6 |
| ProPhyt | 6.1 ± 0.7 | 225.7 ± 5.9 |
| Ridomil Gold 480 EC | 9.3 ± 1.2 | 2.3 ± 0.2 |
| Treatments | Concentration (%) | Height (cm) | Fresh Weight (g) | Dry Weight (g) | ||
|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | |||
| Healthy control | 0 | 21.7 ± 1.2 a | 12.0 ± 0.4 a | 6.3 ± 1.5 ab | 1.7 ± 0.5 a | 0.7 ± 0.1 bcd |
| Non-treated control | 0 | 17.6 ± 1.3 a | 9.6 ± 0.1 ab | 5.6 ± 1.1 b | 1.2 ± 0.2 ab | 0.5 ± 0.1 d |
| Ridomil Gold 480 EC | 0.10 | 16.8 ± 2.2 a | 7.2 ± 0.5 b | 4.5 ± 1.3 b | 1.0 ± 0.3 ab | 0.4 ± 0.1 d |
| ProPhyt | 0.25 | 18.9 ± 1.9 a | 8.5 ± 0.6 ab | 6.7 ± 0.6 ab | 1.5 ± 0.4 ab | 0.7 ± 0.1 bcd |
| 0.10 | 17.1 ± 2.8 a | 8.1 ± 0.2 b | 5.8 ± 0.4 b | 1.1 ± 0.3 ab | 0.6 ± 0.1 abcd | |
| Agri-Fos | 0.25 | 21.6 ± 1.1 a | 8.2 ± 0.3 b | 8.2 ± 2.2 a | 1.4 ± 0.2 ab | 0.9 ± 0.2 a |
| 0.10 | 18.4 ± 0.8 a | 9.0 ± 0.5 ab | 5.9 ± 0.3 b | 1.3 ± 0.1 ab | 0.6 ± 0.1 abcd | |
| K-Phite | 0.25 | 20.3 ± 1.4 a | 8.3 ± 0.1 b | 7.0 ± 0.9 ab | 1.4 ± 0.3 ab | 0.7 ± 0.2 abc |
| 0.10 | 18.4 ± 2.5 a | 8.7 ± 0.4 ab | 5.8 ± 0.5 b | 1.3 ± 0.1 ab | 0.6 ± 0.1 bcd | |
| Lexx-A-Phos | 0.25 | 21.9 ± 1.5 a | 9.5 ± 0.3 ab | 6.4 ± 2.1 ab | 1.6 ± 0.4 ab | 0.8 ± 0.1 ab |
| 0.10 | 16.5 ± 0.8 a | 8.7 ± 0.9 ab | 4.9 ± 1.7 b | 1.1 ± 0.3 ab | 0.5 ± 0.1 d | |
| Nutri-Phite | 0.25 | 20.9 ± 2.1 a | 10.7 ± 1.1 ab | 5.4 ± 0.8 b | 1.6 ± 0.5 ab | 0.5 ± 0.1 d |
| 0.10 | 20.7 ± 0.8 a | 10.3 ± 0.7 ab | 6.1 ± 1.6 ab | 1.6 ± 0.3 ab | 0.7 ± 0.2 abcd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, G.K.H.; Ji, P.; Culbreath, A.K.; Ali, M.E. Comparative Study of Phosphorous-Acid-Containing Products for Managing Phytophthora Blight of Bell Pepper. Agronomy 2022, 12, 1293. https://doi.org/10.3390/agronomy12061293
Hua GKH, Ji P, Culbreath AK, Ali ME. Comparative Study of Phosphorous-Acid-Containing Products for Managing Phytophthora Blight of Bell Pepper. Agronomy. 2022; 12(6):1293. https://doi.org/10.3390/agronomy12061293
Chicago/Turabian StyleHua, Gia Khuong Hoang, Pingsheng Ji, Albert K. Culbreath, and Md Emran Ali. 2022. "Comparative Study of Phosphorous-Acid-Containing Products for Managing Phytophthora Blight of Bell Pepper" Agronomy 12, no. 6: 1293. https://doi.org/10.3390/agronomy12061293







