Simultaneous Estimation of Rhein and Aloe-Emodin in Traditional and Ultrasound-Based Extracts of Rheum palmatum L. (Rhubarb) Using Sustainable Reverse-Phase and Conventional Normal-Phase HPTLC Methods
Abstract
:1. Introduction
2. Materials and Methods
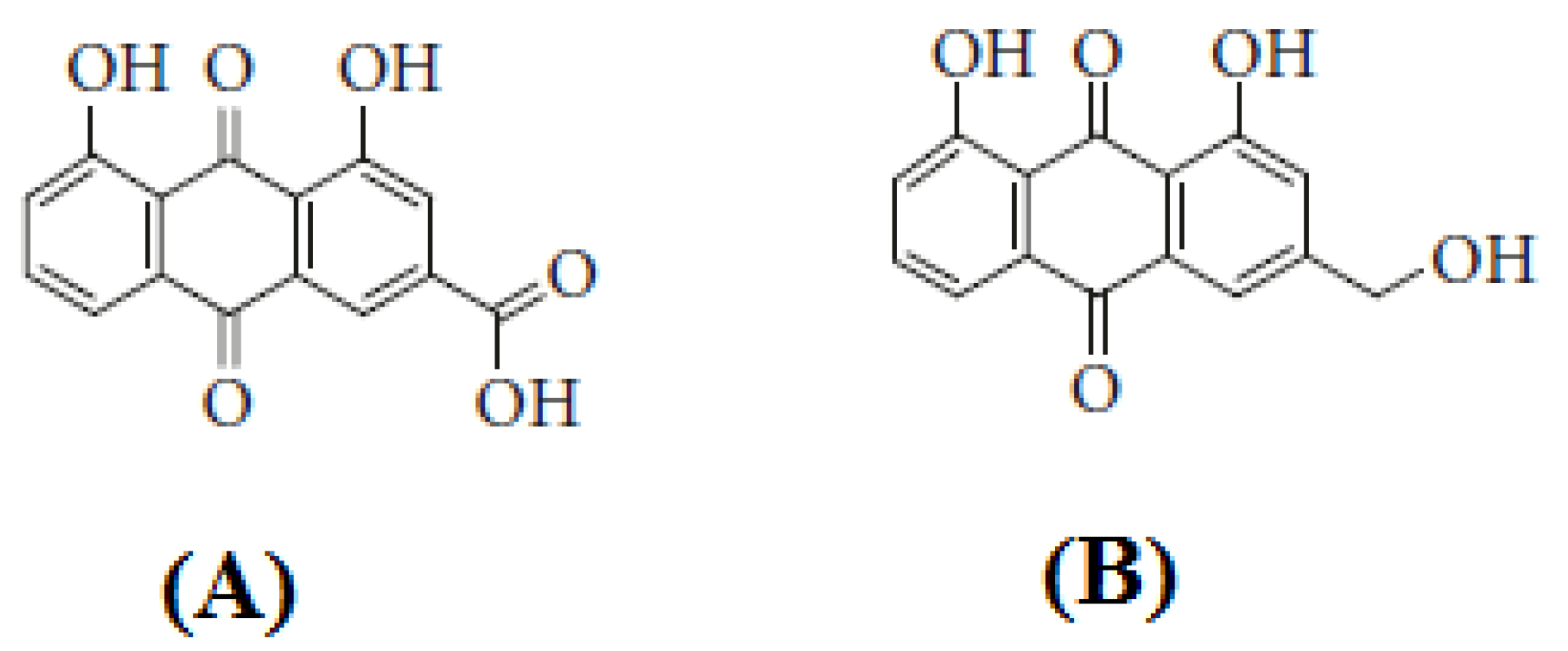
2.1. Materials
2.2. Instrumentation and Chromatography
2.3. Calibration Plots and Quality Control (QC) Solutions for Rhein and Aloe-Emodin
2.4. Sample Preparation for the Simultaneous Determination of Rhein and Aloe-Emodin in TE of Marketed Rhubarb and Rhubarb Plant Extract
2.5. Sample Preparation for the Simultaneous Quantification of Rhein and Aloe-Emodin in UBE of Commercial Rhubarb and Rhubarb Plant Extract
2.6. Validation Parameters
2.7. Application of Conventional and Sustainable Analytical Methods in the Simultaneous Estimation of Rhein and Aloe-Emodin in Commercial Rhubarb and Rhubarb Plant Extracts
2.8. Greenness Assessment by AGREE Approach
3. Results and Discussion
3.1. Method Development
3.2. Validation Parameters
3.3. Application of Conventional and Sustainable Analytical Methods in the Simultaneous Analysis of Rhein and Aloe-Emodin in TE and UBE of Marketed Rhubarb and Rhubarb Plant Extracts
3.4. Greenness Assessment Using AGREE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Wang, X.-H.; Yu, X.; Cao, F.; Cai, X.; Chen, P.; Li, M.; Feng, Y.; Li, H.; Wang, X. Pharmacokinetics of anthraquinones from medicinal plants. Front. Pharmacol. 2021, 12, E638993. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.-C.; Duh, P.-D.; Chuang, D.-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.-G. Health functions and structure-activity relationships of natural anthraquinones from plants. Food Funct. 2018, 9, 6063–6080. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nonaka, G.I.; Nishioka, I. Studies on Rhubarb (Rhei rhizoma). XV: Simultaneous determination of phenolic constituents by high-performance liquid chromatography. Chem. Pharm. Bull. 1989, 37, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Koyama, J.; Morita, I.; Kobayashi, N. Simultaneous determination of anthraquinones in rhubarb by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2007, 1145, 183–189. [Google Scholar] [CrossRef]
- Pecere, T.; Gazzola, M.V.; Mucignat, C.; Parolin, C.; Vecchia, F.D.; Cavaggioni, A.; Basso, G.; Diaspro, A.; Salvato, B.; Carli, M.; et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000, 60, 2800–2804. [Google Scholar]
- Guo, J.M.; Xiao, B.X.; Liu, Q.; Zhang, S.; Liu, D.H.; Gong, Z.H. Anticancer effect of aloe-emodin on cervical cancer cells involves G2/M arrest and induction of differentiation. Acta Pharmacol. Sin. 2007, 28, 1991–1995. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Wan, M.; Zhu, Z.; Chen, G.; Huang, X. Simultaneous determination of eight major bioactive compounds in Dachengqi Tang (DT) by high-performance liquid chromatography. Chin. Med. 2008, 3, E5. [Google Scholar] [CrossRef] [Green Version]
- Ren, P.; Qin, F.; Huang, X.; Zhu, Z. Simultaneous LC analysis of aloe-emodin, rhein, emodin, and chrysophanol in Rhizoma rhei-type preparations. Chromatographia 2009, 70, 1515–1517. [Google Scholar] [CrossRef]
- Cheng, W.; Gao, J.; Huang, Q.; Shi, B.; Zhang, W. Simultaneous determination of five active ingredients in Huangshiangsheng pills by HPLC. Asian J. Chem. 2013, 14, 7735–7737. [Google Scholar] [CrossRef]
- Feng, S.-X.; Wang, Z.; Hao, R.; Zhang, L.; Li, X.-H.; Li, M.-M. Simultaneous determination of ten anthraquinones in Rheum palmatum L. from different habitants by HPLC. Chin. J. Pharm. Anal. 2017, 37, 783–788. [Google Scholar]
- Xiao, Y.; Li, J.; Chang, J.; Chen, W.; Liu, C.; Liu, X. Simultaneous determination of seven components in Zhizi Jinhua dispersible tablets by HPLC. Chin. Pharm. 2017, 12, 2549–2553. [Google Scholar]
- Ding, M.; Ma, S.; Liu, D. Simultaneous determination of hydroxyanthraquinones in rhubarb and experimental animal bodies by high-performance liquid chromatography. Anal. Sci. 2003, 19, 1163–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.-X.; Li, J.-S.; Wang, S.-M.; Yang, R.; Zhou, T.-Q.; Li, X.-Y.; Qu, L.-B. Simultaneous determination and pharmacokinetics of five anthraquinones in dog plasma by HPLC after orally administration the rhubarb extract. Pak. J. Pharm. Sci. 2014, 27, 847–854. [Google Scholar]
- Meier, N.; Meier, B.; Peter, S.; Wolfram, E. In-silico UHPLC method optimization for aglycones in the herbal laxatives Aloe barbadensis Mill., Cassia angustifolia Vahl Pods, Rahmnus frangula L. bark, Rhamnus purshianus DC bark, and Rheum palmatum L. roots. Molecules 2017, 22, 1838. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yu, X.; Gui, J.; Wan, Y.; Chen, J.; Tan, T.; Liu, F.; Guo, L. Ultrasonic solvent extraction followed by dispersive solid phase extraction (d-SPE) cleanup for the simultaneous determination of five anthraquinones in Polygonum multiflorum by UHPLC-PDA. Foods 2022, 11, 386. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, J.-H.; Liu, L.-Y.; Fu, Q.; Liu, C.-Z. A rapid UPLC-MS/MS method for simultaneous determination of five anthraquinones in rat plasma: Application to a pharmacokinetics study. Acta Med. Mediter. 2018, 34, 175–180. [Google Scholar]
- Zhang, X.Y.; Li, J.Y.; Peng, W.W.; Liu, X.; Yang, G.M.; Chen, L.H.; Cai, B.C. Comparative pharmacokinetics of aloe-emodin, rhein and emodin determined by liquid chromatography-mass spectrometry after oral administration of a rhubarb peony decoction and rhubarb extracts to rats. Pharmazie 2013, 68, 333–339. [Google Scholar]
- Narayanan, S.; Jadhav, A.P. Simultaneous estimation of aloe emodin and emodin from Rheum emodi, in Cassia alata and Aloes by HPTLC. Indian J. Pharm. Sci. 2015, 77, 783–787. [Google Scholar]
- Chewchinda, S.; Sithisarn, P. Simultaneous HPTLC determination of rhein and aloe-emodin in Senna alata leaves from Thailand and their commercial products. Nat. Prod. Comm. 2017, 12, 399–401. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Li, X.-P.; Xin, J.; Xue, T.; Zhang, B.; Liu, Y.-J. Development of a colloidal gold immunochromatographic strip for the one-step evaluation of the total content of rhein and aloe-emodin in rhubarb. Int. J. Anal. Chem. 2022, 2022, E7067245. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Wang, Y.; Chen, Y.; Chen, X.; Hu, Z. Application of 1-alkyl-3-methylimidazolium-based ionic liquid as background electrolyte in capillary zone electrophoresis for the simultaneous determination of five anthraquinones in rhubarb. Talanta 2007, 72, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, J. Simultaneous determination of aloe-emodin and rhein by synchronous fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2002, 29, 737–742. [Google Scholar] [CrossRef]
- Jintao, X.; Yongli, S.; Liming, Y.; Quanwei, Y.; Chunyan, L.; Xingyi, C.; Yun, J. Near-infrared spectroscopy for rapid and simultaneous determination of five main active components in rhubarb of different geographical origins and processing. Spectrochim. Acta Part A 2018, 205, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, X.; Hu, S.; Tian, J.; Bai, X. Simultaneous preconcentration and analysis of anthraquinones based on ultrasound emulsification ionic liquid microextraction. J. Chromatogr. Sci. 2014, 52, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously administered madness drops (tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, E105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A green HPLC method for determination of nine sulfonamides in milk and beef, and its greenness assessment with analytical eco-scale and greenness profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating green RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem. J. 2021, 164, E105960. [Google Scholar] [CrossRef]
- Alam, P.; Salem-Bekhit, M.M.; Al-Joufi, F.A.; Alqarni, M.H.; Shakeel, F. Quantitative analysis of cabozantinib in pharmaceutical dosage forms using green RP-HPTLC and green NP-HPTLC methods: A comparative evaluation. Sus. Chem. Pharm. 2021, 21, E100413. [Google Scholar] [CrossRef]
- International Council for Harmonization (ICH). Q2 (R1): Validation of Analytical Procedures–Text and Methodology; ICH: Geneva, Switzerland, 2005. [Google Scholar]







| Parameters | Rhein | Aloe-Emodin |
|---|---|---|
| Sustainable HPTLC method | ||
| Linearity range (ng/spot) | 50–1000 | 25–1000 |
| R2 | 0.9972 | 0.9962 |
| R | 0.9985 | 0.9980 |
| Slope ± SD | 4.955 ± 0.22 | 46.34 ± 1.89 |
| Intercept ± SD | 211.46 ± 1.72 | 2913.60 ± 8.54 |
| Standard error of slope | 0.08 | 0.77 |
| Standard error of intercept | 0.70 | 3.48 |
| 95% confidence interval of slope | 4.56–5.34 | 43.02–49.66 |
| 95% confidence interval of intercept | 208.43–214.48 | 2898.59–2928.60 |
| LOD ± SD (ng/spot) | 16.81 ± 0.18 | 8.49 ± 0.12 |
| LOQ ± SD (ng/spot) | 50.53 ± 0.54 | 25.47 ± 0.36 |
| Conventional HPTLC method | ||
| Linearity range (ng/spot) | 50–600 | 100–600 |
| R2 | 0.9952 | 0.9956 |
| R | 0.9975 | 0.9977 |
| Slope ± SD | 22.93 ± 2.14 | 5.26 ± 0.34 |
| Intercept ± SD | 248.10 ± 2.61 | 238.40 ± 2.54 |
| Standard error of slope | 0.87 | 0.13 |
| Standard error of intercept | 1.06 | 1.03 |
| 95% confidence interval of slope | 19.17–26.69 | 4.66–5.85 |
| 95% confidence interval of intercept | 243.51–252.68 | 233.93–242.86 |
| LOD ± SD (ng/spot) | 18.53 ± 0.23 | 39.42 ± 0.62 |
| LOQ ± SD (ng/spot) | 55.59 ± 0.69 | 118.26 ± 1.86 |
| Parameters | Rhein | Aloe-Emodin |
|---|---|---|
| Sustainable HPTLC method | ||
| Rf | 0.20 ± 0.01 | 0.86 ± 0.02 |
| As | 1.10 ± 0.03 | 1.05 ± 0.02 |
| N/m | 3844 ± 3.98 | 4687 ± 4.87 |
| Conventional HPTLC method | ||
| Rf | 0.53 ± 0.02 | 0.27 ± 0.01 |
| As | 1.12 ± 0.03 | 1.07 ± 0.02 |
| N/m | 3724 ± 3.79 | 4529 ± 4.68 |
| Conc. (ng/spot) | Conc. Found (ng/spot) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| Sustainable HPTLC method | |||
| Rhein | |||
| 100 | 99.64 ± 1.12 | 99.64 | 1.12 |
| 400 | 405.22 ± 4.35 | 101.30 | 1.07 |
| 1000 | 991.24 ± 9.31 | 99.12 | 0.93 |
| Aloe-emodin | |||
| 50 | 49.89 ± 0.63 | 99.78 | 1.26 |
| 400 | 404.61 ± 4.14 | 101.15 | 1.02 |
| 1000 | 988.61 ± 8.24 | 98.86 | 0.83 |
| Conventional HPTLC method | |||
| Rhein | |||
| 100 | 96.12 ± 3.13 | 96.12 | 3.25 |
| 400 | 407.14 ± 9.77 | 101.78 | 2.39 |
| 600 | 616.45 ± 13.91 | 102.74 | 2.25 |
| Aloe-emodin | |||
| 100 | 103.16 ± 3.57 | 103.16 | 3.46 |
| 400 | 415.35 ± 10.64 | 103.83 | 2.56 |
| 600 | 574.36 ± 14.61 | 95.72 | 2.54 |
| Conc. (ng/spot) | Intra-Day Precision | Inter-Day Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (ng/spot) ± SD | Standard Error | CV (%) | Conc. (ngband) ± SD | Standard Error | CV (%) | |
| Sustainable HPTLC method | ||||||
| Rhein | ||||||
| 100 | 102.31 ± 0.98 | 0.40 | 0.95 | 101.41 ± 1.04 | 0.42 | 1.02 |
| 400 | 404.31 ± 3.71 | 1.51 | 0.91 | 394.57 ± 3.81 | 1.55 | 0.96 |
| 1000 | 988.54 ± 7.84 | 3.20 | 0.79 | 1004.54 ± 8.18 | 3.34 | 0.81 |
| Aloe-emodin | ||||||
| 50 | 49.54 ± 0.42 | 0.17 | 0.84 | 51.41 ± 0.48 | 0.19 | 0.93 |
| 400 | 391.21 ± 3.12 | 1.27 | 0.79 | 403.64 ± 3.35 | 1.36 | 0.82 |
| 1000 | 992.31 ± 7.64 | 3.11 | 0.76 | 1012.64 ± 8.13 | 3.31 | 0.80 |
| Conventional HPTLC method | ||||||
| Rhein | ||||||
| 100 | 94.12 ± 3.08 | 1.25 | 3.27 | 103.25 ± 3.47 | 1.41 | 3.36 |
| 400 | 381.32 ± 9.28 | 3.78 | 2.43 | 408.54 ± 10.87 | 4.43 | 2.66 |
| 600 | 584.12 ± 13.57 | 5.54 | 2.32 | 611.25 ± 14.78 | 6.03 | 2.41 |
| Aloe-emodin | ||||||
| 100 | 91.56 ± 3.61 | 1.47 | 3.94 | 104.12 ± 4.24 | 1.73 | 4.07 |
| 400 | 378.68 ± 10.24 | 4.18 | 2.70 | 381.24 ± 11.21 | 4.57 | 2.94 |
| 600 | 614.25 ± 13.98 | 5.70 | 2.27 | 582.31 ± 15.12 | 6.17 | 2.59 |
| Conc. (ng/spot) | Mobile Phase Composition | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | Conc. (ng/spot) ± SD | % CV | Rf | ||
| Sustainable HPTLC method (ethanol/water) | ||||||
| Rhein | ||||||
| 62:38 | +2.0 | 386.41 ± 3.88 | 1.00 | 0.19 | ||
| 400 | 60:40 | 60:40 | 0.0 | 393.61 ± 4.04 | 1.02 | 0.20 |
| 58:42 | −2.0 | 405.87 ± 4.28 | 1.05 | 0.21 | ||
| Aloe-emodin | ||||||
| 62:38 | +2.0 | 392.13 ± 3.65 | 0.93 | 0.85 | ||
| 400 | 60:40 | 60:40 | 0.0 | 397.41 ± 3.87 | 0.97 | 0.86 |
| 58:42 | −2.0 | 408.16 ± 4.18 | 1.02 | 0.87 | ||
| Conventional HPTLC method (chloroform/methanol) | ||||||
| Rhein | ||||||
| 72:28 | +2.0 | 390.54 ± 10.28 | 2.63 | 0.52 | ||
| 400 | 70:30 | 70:30 | 0.0 | 398.34 ± 10.66 | 2.67 | 0.53 |
| 68:32 | −2.0 | 416.37 ± 11.54 | 2.77 | 0.54 | ||
| Aloe-emodin | ||||||
| 72:28 | +2.0 | 388.41 ± 11.31 | 2.91 | 0.26 | ||
| 400 | 70:30 | 70:30 | 0.0 | 396.58 ± 11.89 | 2.99 | 0.27 |
| 68:32 | −2.0 | 413.65 ± 12.13 | 2.93 | 0.28 | ||
| Samples | TE | UBE |
|---|---|---|
| Sustainable HPTLC method | ||
| Amount of rhein (mg/g) | ||
| Commercial Rhubarb | 2.78 ± 0.102 | 4.33 ± 0.110 |
| Rhubarb plant extract | 2.51 ± 0.092 | 3.81 ± 0.101 |
| Amount of aloe-emodin (mg/g) | ||
| Commercial Rhubarb | 4.61 ± 0.112 | 5.74 ± 0.121 |
| Rhubarb plant extract | 3.84 ± 0.108 | 4.71 ± 0.114 |
| Conventional HPTLC method | ||
| Amount of rhein (mg/g) | ||
| Commercial Rhubarb | 0.016 ± 0.001 | 0.036 ± 0.003 |
| Rhubarb plant extract | 0.008 ± 0.000 | 0.026 ± 0.002 |
| Amount of aloe-emodin (mg/g) | ||
| Commercial Rhubarb | 0.523 ± 0.005 | 0.609 ± 0.008 |
| Rhubarb plant extract | 0.461 ± 0.004 | 0.504 ± 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, M.H.; Alam, P.; Shakeel, F.; Alam, A.; Salkini, M.A.; Muharram, M.M. Simultaneous Estimation of Rhein and Aloe-Emodin in Traditional and Ultrasound-Based Extracts of Rheum palmatum L. (Rhubarb) Using Sustainable Reverse-Phase and Conventional Normal-Phase HPTLC Methods. Agronomy 2022, 12, 1295. https://doi.org/10.3390/agronomy12061295
Alqarni MH, Alam P, Shakeel F, Alam A, Salkini MA, Muharram MM. Simultaneous Estimation of Rhein and Aloe-Emodin in Traditional and Ultrasound-Based Extracts of Rheum palmatum L. (Rhubarb) Using Sustainable Reverse-Phase and Conventional Normal-Phase HPTLC Methods. Agronomy. 2022; 12(6):1295. https://doi.org/10.3390/agronomy12061295
Chicago/Turabian StyleAlqarni, Mohammed H., Prawez Alam, Faiyaz Shakeel, Aftab Alam, Mohammad A. Salkini, and Magdy M. Muharram. 2022. "Simultaneous Estimation of Rhein and Aloe-Emodin in Traditional and Ultrasound-Based Extracts of Rheum palmatum L. (Rhubarb) Using Sustainable Reverse-Phase and Conventional Normal-Phase HPTLC Methods" Agronomy 12, no. 6: 1295. https://doi.org/10.3390/agronomy12061295








