Abstract
Thaumatin-like proteins (TLPs), which belong to pathogenesis-related (PR) protein family 5 (PR5), are involved in the plant host defense and developmental processes. Detailed information regarding the TLP gene family in rice remains elusive. Here, we identified 43 OsTLP genes from the rice genome database. The in silico analysis comprised of the evolutionary tree, gene structure, conserved motifs, and chemical properties highlighted the key aspects of the OsTLP genes. By phylogenetic analysis, the OsTLP genes were classified into ten groups (OsTLPI-OsTLPX). Gene ontology (GO) analysis showed that OsTLP genes regulate plant growth and response to various environmental stimuli. Moreover, most of the OsTLP genes are expressed in almost all tissues. Microarray data showed differential expression trends of OsTLP genes under salinity stress and hormonal treatments, whereas under BPH, SSB, and RFL, only OsTLP1, OsTLP2 and OsTLP27 were expressed. The qRT-PCR analysis showed OsTLP27 as the prominent gene, displayed by its upregulated expression under JGM. Our results provide detailed knowledge of OsTLP gene resistance in rice plants, and we believe the current study will facilitate the development of cultivars resistant to biotic/abiotic stress, particularly stress caused by the chewing and sucking of insect pests.
1. Introduction
The static nature of plants entails the frequent endurance of numerous environmental stresses, such as drought, salinity, heat, and other biotic stressors [1]. Plants exhibit numerous key factors that provide response to various environmental stimuli. Among these are antioxidants enzymes, ROS (reactive oxygen species), anti-microbial compounds (e.g.,phytoalexins), proline, sugars, and pathogenesis-related (PR) proteins [2]. The serological and biochemical properties of PR proteins have been classified into 17 different families, including b-1,3-glucanases (PR-2), chitinases (PR-3, 4, 8, and 11), thaumatin-like proteins (TLPs), or osmotin (PR-5), proteinase-inhibitor (PR-6), endoproteinase (PR-7), peroxidase (PR-9), defense (PR-12), thionins (PR-13), and lipid transfer proteins (PR-14) [3].
Plant thaumatin-like proteins (TLPs) belong to the PR-5 proteins family, a sweet-tasting protein discovered as a combination of proteins isolated from the katemfe fruit, Thaumatococcus daniellii (Bennett), in West Africa [3,4]. The TLPs have 16 cysteine residues, which may evolve from eight disulfide linkages [5]. Further, these structures stabilize the protein that resists high pH, proteases, and temperature-induced denaturation of various biomolecules [5]. The small TLPs, with 10 conserved cysteine residues, were identified in various monocotyledonous and coniferous plant species [6,7].
TLPs play various key roles in the plant defense system against biotic/abiotic stresses and in various physiological and developmental processes [7]. For example, the over-expression of the Camellia sinensis (CsTLP) gene induced stress resistance in transgenic potatoes against late blight disease caused by the pathogenic fungi Phytophthora infestans and Macrophomina phaseolina [8]. TLP also plays a role in plant stress biology, such as that the TLP produces antifreeze protein in leaves of cereals during cold weather and collectively provides cold resistance [9]. However, the TLPs were also reported to have a vital role in biological processes such as seed germination [10], fruit ripening [11], and antimicrobial activity [12].
Rice (Oryza sativa L.) is one of the world’s staple foods and is widely cultivated, feeding almost half of the world’s population [13]. Rice is cultivated in more than 100 countries, with more than 700 million tons produced each year [14]. Because its easy availability and nutritional value lead to high consumption, it is estimated that rice production must be increased by 1% annually to fulfill the growing population’s needs [15]. However, the rice crop is exposed to various environmental stresses, and the combined effects lead to a huge yield loss [16]. The abiotic stresses include high/low temperature, drought, high salinity, low nutrient availability, flooding, etc., and biotic stresses include insect pests, herbicide toxicity, and bacterial, viral, and fungal infections that result in drastic yield loss [17].
More than 800 insect pests feed on rice, causing direct and indirect infections [18]. Among these, 65 species of planthoppers were recorded to be associated with the rice ecosystem in which the brown planthopper (BPH) Nilaparvata lugens Stål (Hemiptera: Delphacide) and small brown planthopper (SBPH) Laodelphax striatellus (Hemiptera: Delphacidae) are recognized as key pests [19,20]. The BPH and SBPH are severe pests in rice, causing a huge loss in annual rice production through direct infestation and transmission of several viruses, such as rice ragged stunt virus (RRSV), Southern rice black-streaked dwarf virus (SRBSDV), and rice grassy stunt virus (RGSV) [21,22,23]. The antibiotic jinggangmycin (JGM) was developed in China and is commonly applied 2–3 times per crop cycle in rice fields for controlling rice sheath blight disease (Rhizoctonia solani) [24]. However, JGM is also reported to induce BPH fecundity. In addition, several spraying treatments, such as root zone application, leaf spray, and topical application, among the JGM foliar sprays, are highly effective in inducing changes in rice physiology and biochemistry that lead to enhanced BPH fecundity [25]. In addition, JGM also enhances BPH flight capacity, body weight, thermotolerance, protein, and lipids contents [24].
Owing to the importance of the TLP gene family linked with developmental, physiological, various defense responses, and the diversity of TLP gene members in many plant species, it is critical to investigate the global status and evolution of the TLP gene family in rice and other cereals crops to maintain the food and brewing industry supply chain. However, little information exists regarding the role of the TLP gene family in rice, particularly the OsTLP genes response against the BPH and SBPH infestation and the JGM applications that stimulate the rice’s immune response. Herein, we performed comprehensive bioinformatics and expression analyses to investigate the involvement of OsTLP genes in rice growth and developmental processes. Additionally, the participation of OsTLP genes in boosting rice plant immunity against various stresses has been studied.
2. Materials and Methods
2.1. OsTLP Gene Family Member Identification and Sequence Analysis
The protein sequence of the TLP genes family was retrieved from the Arabidopsis thaliana [26] and rice [27] using the Hidden Markov model (HMM). Further, the extracted rice protein sequences were evaluated using the CD-search server (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, http://www.ebi.ac.uk/interpro/search/sequence/) (accessed on 10 February 2022) along with SMART databases (http://smart.embl-heidelberg.de/) (accessed on 10 February 2022). Protein that did not display thaumatin-like proteins (TLPs) was disregarded. Additionally, the chemical properties of OsTLP protein were examined by employing the (Swiss Institute of Bioinformatics, Zurich, Switzerland) originated Expasy online server (http://web.expasy.org/protparam/) (accessed on 10 February 2022). Finally, the OsTLP gene predicted subcellular location was displayed using the online server CELLO2GO [28].
2.2. Phylogenetic Tree, Motif, and Digital Expression Analysis
We used theClustalW 2.0 European Molecular Biology Laboratory, Cambridgeshire, CB10 1SD, UK (http://www.genome.jp/tools/clustalw/) (accessed on 16 February 2022) originated 01 July 2003 at Bioinformatics Center, Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011, Japan, to analyze the sequences and construct the maximum-likelihood phylogenetic, and MEGA (Version 7.0) [29]. The coding sequences of each OsTLP gene were obtained from the rice genome database. The conserved motif of OsTLP genes was predicted with the online MEME server (Version 4.12.0) (http://meme-suite.org/tools/meme) (accessed on 16 February 2022). Further, for the heatmap, the TBtools developed by South China Agricultural University, Guangzhou, China, were used, and the transcriptomic data was retrieved from the rice genome database and GEO dataset platform in NCBI [27].
2.3. Cis-Elements and Gene Ontology of OsTLP Genes
For each OsTLP gene, 1.5 kb of upstream genomic DNA sequence with the starting codon (ATG) was obtained from the rice genome sequence database. We used the plantCARE database, Gent, Belgium (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (accessed on 18 February 2022)to identify the cis-regulatory elements in the promoters of 10 OsTLP genes. Further, the gene ontology (GO) analysis of OsTLP protein sequences was obtained from the Blast2GO program, Valencia–ES-Spain (Version 2.7.2) (http://www.blast2go.com) (accessed on 18 February 2022); the resulting three groups, molecular functions, biological process, and cellular component GO classification, were recovered.
2.4. Interactive Protein Network Analysis of OsTLP Genes
The Swiss Institute of Bioinformatics, Zurich, Switzerland, developed online server string (https://string-db.org) was (accessed on 3 March 2022) to identify the interactive proteins with OsTLP proteins [30]. The Oryza sativa protein (OsTLP1) was selected as a reference protein, and the interactive proteins network was recovered.
2.5. Insect Rearing, Chemical and Stress Treatment
In the current study, we used the rice Ninjing4 and Wuyujing-3 varieties of Oryza sativa. These varieties are commonly planted in Jiangsu Province of China because they have no resistance to the Nilaparvata lugens (Stål) brown planthopper (BPH) and the Laodelphax striatellus (Fallén) small brown planthopper (SBPH) [31,32]. The seeds were sown indoors in a plastic tray in a standard size of ¼ 60 cm H _ 100 cm W _ 200 cm L), and after germination, the six-leaf seedlings were transplanted into plastic pots in dimensions R ¼ 16 cm. Next, the rice plants were used for further experiments at the tillering stage (40 ± 2 days).
We used the BPH and SBPH strains in the study, obtained from the China National Rice Research Institute Insect Repository (Hangzhou, China). The BPH strain was reared on the rice plants in cement tanks, covered with fine mesh, under natural conditions from April to October. Further, the BPH strain was overwintered in an insectary at the Ecological Laboratory of Yangzhou University on Ninjing4 variety rice seedlings under standard conditions of 26 ± 2 °C with the 16 h l: 8 h d in relative humidity of 80 ± 10% [32].
The SBPH strain was reared on 10–15 days old seedlings of the Wuyujing-3 rice variety in a laboratory. A plastic frame cage, with dimensions of 50 cm in length and width (L:W), was used in a growth chamber with standard temperature conditions of 26 ± 2 °C, 70–80% relative humidity (RH), and a 10:14 photoperiod. Further, a nylon mesh cloth with a zipper prevented the SBPH from escaping. The rice seedlings were replaced every 10–14 days to ensure sufficient nutrition for SBPH feeding [31].
Technical grade fungicide jinggangmycin (JGM) (61.7%) was purchased from the Qianjiang Biochemistry Co. Ltd. (Haining, Zhejiang Province, China). The 200 parts per million (PPM) JGM solution was prepared with Tween 20, purchased from Sinopsin Group Chemical Reagent Co. (Shanghai, China). As previously reported by Ge et al. (2010), the third instar of both BPH and SBPH was used in the experiments [32].
2.6. Expression Profiling of TLP Genes in Oryza sativa
Forty-day-old rice plants were exposed to BPH and SBPH stress, and samples were taken at 2, 4, and 8 days after infestation. Similarly, JGM was sprayed on the rice plants, and samples were taken at 2, 4, and 8 days after treatment. Following that, RNA was extracted from the samples using Trizol reagent (Invitrogen, Carlsbad, CA, USA). First, the DNA was removed using DNase I, the concentration and purity was measured with a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Rockford, IL, USA), and the integrity was checked using 1.5% agarose gel electrophoresis. Following the manufacturer’s instructions, 1 μg of total RNA was reverse-transcribed on a thermocycler programmed at 37 °C for 15 min using the PrimeScriptTM RT Master Mix (TaKaRa, Dalian, China) containing oligo dT primer.
Two μL aliquots of cDNA were amplified by qPCR in 20 μL reaction volumes using the SYBR Premix Ex TaqTM II (TaKaRa, Dalian, China). The cDNAs were amplified at 95 °C for 2 min, followed by 35 cycles of 10 s at 95 °C for 30 s, and 72 °C for 30 s, with a final extension step of 72 °C for 10 min in a CFX96 real-time PCR system (Bio-Rad Co. Ltd., Hercules, CA, USA). The mRNA amounts of all genes were separately quantified with the stable expression of the constitutive reference gene, actin. The specific primers are detailed in Table S1. After amplification, the target gene cycle threshold (Ct) values were normalized to the reference gene using the 2−ΔΔCT method [33]. The data mean values of three biologically independent replicates were used for the final graphs.
2.7. Statistical Analysis
The SPSS software (Version 25.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis (ANOVA) and statistical significance, and a 95% confidence interval (p ≤ 0.05) was used. The data were analyzed and expressed as means ± standard deviation (SD) of the three biologically independent replicates in all measured parameters, and GraphPad Prism (Version 8.0.2) (GraphPad Software, Inc., LA Jolla, CA, USA) was used for graphical representation.
3. Results
3.1. Identification and Sequence Analysis of TLP Genes in Oryza sativa
We retrieved 43 OsTLP genes encoding the TLPs protein sequence from the rice genome database [27]. Genes that are not possessing THAUMATIN domain were excluded from the list. The domain structure of OsTLP genes has been presented in (Table S2). All the genes were nomenclatured as OsTLP1 to OsTLP43 (Table 1). Among 43 OsTLP genes, 37 OsTLP genes were located extracellularly, four resided in the nucleus, and only two were found in the plasma membrane. Additionally, various other features of Oryza sativa OsTLP protein were identified, such as chromosomal coordinates, molecular weight, chemical properties, and isoelectric point (PI) and are listed in Table 1.

Table 1.
The gene and protein features of TLP gene family members in Oryza sativa.
3.2. Sequence Alignment and Evolutionary Relationship of TLP Genes in Oryza sativa
A maximum-likelihood phylogenetic tree was created following alignment to learn more about the evolutionary relationship between Oryza sativa and Arabidopsis thaliana thaumatin-like proteins (Figure 1). The protein sequence of 43 OsTLP and 28 AtTLP [26,27] genes constructed the phylogenetic tree. First, all the sequences were aligned using ClustalX software, with default parameters, then the phylogenetic tree was constructed using MEGA6 software, and the final tree was programmed using the Biobyte Solutions Company; Heidelberg, Germany, developed Interactive Tree Of Life (iTOL) (version 5) https://itol.embl.de/ (accessed on 10 March 2022) [34]. Additionally, the OsTLP protein was clustered into ten subgroups based on their phylogenetic relationships (Group I, Group II, Group III, Group IV, Group V, Group VI, Group VII, Group VIII, Group IX, and Group X). We found that among the ten sub-groups, the rice TLP protein was present in all subgroups, with different numbers of proteins, such as Group X, which contained 8 proteins, followed by Group I, Group III, and Group IX, which had 6 proteins. Further, Group II and Group VII had the third-highest number, at 5 proteins, whereas Group V, Group VI, and Group VII were found to have the least 2, while Group IV had only a single protein.
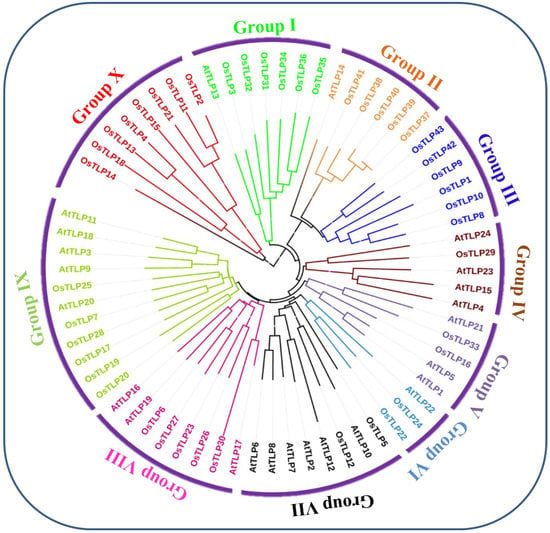
Figure 1.
Phylogenetic analysis of TLP. The phylogenetic tree was generated using the amino-acid sequences of selected TLPs via NJ methods. All O. sativa TLPs and their A. thaliana counterparts were classified into 10 groups.
3.3. Gene Structure and Motif Compositions Analysis of TLPs in Oryza sativa
We identified the exons-introns distribution through CDS and genomic sequence of OsTLP genes using gene structure display (GSDS 2.0) [35]. The analysis revealed that the TLP genes family contains multiple exons and has varied intron lengths (Figure 2). Furthermore, the OsTLP11, OsTLP15, and OsTLP18 have the highest number of 5 exons and 4 introns, followed by OsTLP26, OsTLP27, OsTLP6, and OsTLP7, having 5 exons and 2 introns. In addition, OsTLP12, OsTLP19, OsTLP20, and OsTLP29 have 4 exons with a single intron, whereas OsTLP22, OsTLP24, and OsTLP25 possess 3 exons and 2 introns. Furthermore, OsTLP3, OsTLP8, OsTLP9, OsTLP10, OsTLP16, OsTLP31, OsTLP32, OsTLP35, OsTLP36, OsTLP37, OsTLP40, OsTLP41, and OsTLP42 have 3 exons without introns, whereas OsTLP28 has 3 exons with a single intron. Furthermore, the 4 OsTLP genes, such as OsTLP4, OsTLP5, OsTLP14, and OsTLP33, were found to have 2 exons and a single intron, whereas OsTLP5, OsTLP38, OsTLP39, and OsTLP43 had 2 exons. Three genes, including OsTLP13, had 4 and 3 exons-introns, followed by OsTLP17, with 4-2, and OsTLP, with 3-1 exons and introns, whereas OsTLP34 possessed only a single exon.
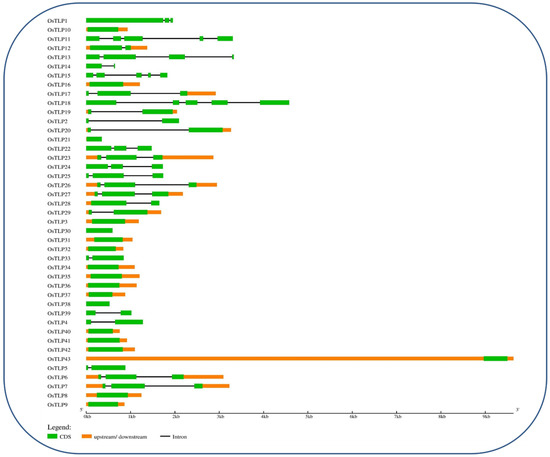
Figure 2.
Schematic representation of the gene structure of TLP genes in Oryza sativa.
To further study the phylogenetic relationship of OsTLP in Oryza sativa, conserved motifs were identified using the University of Nevada, Reno, Nevada, USA; University of Washington, Seattle, WA, USA; University of Queensland, Brisbane, Australia and University of California, San Diego, CA, USA) developed MEME server (Version 5.4.1) https://meme-suite.org/meme/ (accessed on 15 march 2022) [36] (Figure 3) and identified motif of OsTLP genes in Oryza sativa (Table S3). The motifs were variously allocated on the OsTLP genes. Among these, OsTLP25, OsTLP28, OsTLP7, OsTLP6, OsTLP27, OsTLP17, OsTLP23, OsTLP19, OsTLP26, OsTLP20, OsTLP22, OsTLP24, OsTLP33 exhibit 9 motifs each, followed by OsTLP10, OsTLP8, OsTLP1, OsTLP29, OsTLP43, and OsTLP38 with 8 of motifs. Further, OsTLP36, OsTLP32, OsTLP42, OsTLP40, and OsTLP39 were observed with 6 motifs. Several members of the OsTLP gene family were found with fewer motifs, such as OsTLP18, OsTLP13, and OsTLP4 with 4 motifs, OsTLP4 with 2 motifs, and OsTLP5 with a single motif.
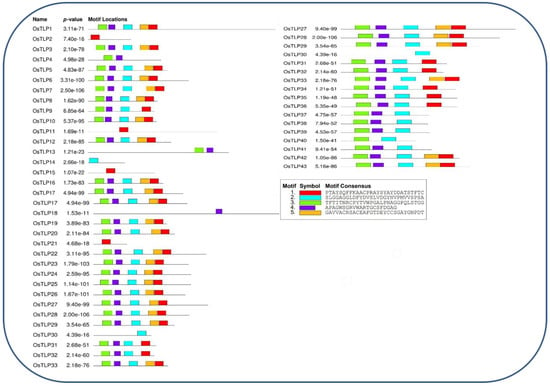
Figure 3.
Schematic representation of the conserved motif of TLP genes in Oryza sativa.
3.4. Interactive Analysis of OsTLP in Rice
The interactive protein study unfolds the numerous interacting partners of OSTLP1 (Figure 4) and their interactive protein partners (Table S4). The ERF2 had high interactions with our reference gene OSTLP1, which contributes to petunia waterlogging tolerance by suppressing programmed cell death and an alcoholic fermentation system [37]. Additionally, our reference OSTLP1 protein was highly associated with the LTP family and involved in numerous biological processes, including plant growth, development, and response to biotic and abiotic stresses [38]. Additionally, the polygalacturonase inhibiting proteins (PGIPs) are cell wall proteins that inhibit the pectin-depolymerizing activity of polygalacturonases secreted by microbial pathogens and insects [39]. These ubiquitous inhibitors have a leucine-rich repeat structure that is strongly conserved in monocot and dicot plants. Finally, the interaction between OsTLP1 with NBS-LRR proteins was observed. The NBS-LRR are plant stress-specific proteins that play a vital role in regulating immunity [40].
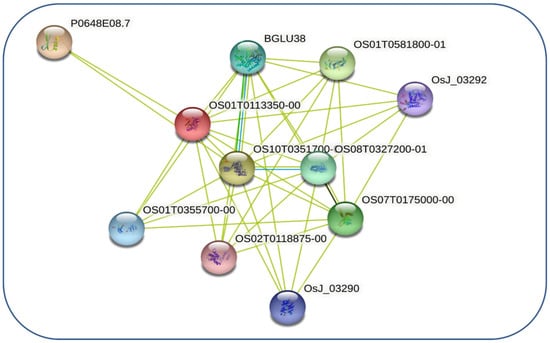
Figure 4.
Interactive network of OsTLP1 protein.
3.5. Gene Ontology (GO) Analysis
Gene ontology (GO) enrichment pathway analysis showed various key functions of OsTLP genes in Oryza sativa. Three different functional parameters were analyzed (biological, molecular, and cellular processes). According to the predicted biological processes, OsTLP genes play a crucial role in growth-related activities via hormonal and metabolic modulation (Figure 5). Additionally, the response to external stimuli and the cellular analysis proved that most of the OsTLP genes reside extracellularly, and several nuclear genes were present in OsTLP gene family and could be key to various activities. At the same time, OsTLP genes are involved in DNA binding activities, as shown in the molecular functions pie chart.
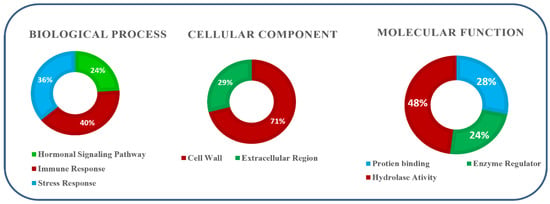
Figure 5.
Gene ontology (GO) enrichment analysis of TLP genes in Oryza sativa. The data are represented as biological processes, molecular processes, and cellular processes.
3.6. Identification of OsTLP Genes Cis-Elements
The upstream region of OsTLP genes possesses various stress, hormones, light, and growth related cis elements. Here we identified forty-three cis-regulatory elements. Among these 43 cis-elements, 4 were related to light response, 17 were related to stress response, 12 were related to hormonal response, and 10 to growth and development (Table S5).
3.7. Microarray Expression Analysis of TLP Genes in Rice Tissues in Developmental Stages Biotic/Abiotic Stresses and under Hormonal Applications
3.7.1. Expression Analysis of OsTLP Genes in Developmental Stages
We examined the different developmental stages and tissue-specific expressions to study the biological roles of OsTLP genes in rice plant growth and development based on a set of transcriptomic data retrieved from the National Institute of Agrobiological Sciences Kannondai 2-1-2, Tsukuba, Ibaraki 305-8602, Japan RiceXPro expression database (Version 3.0) https://ricexpro.dna.affrc.go.jp/ (accessed on 20 March 2022) [41]. The microarray expression data analysis of the OsTLP gene family members in twelve different tissues is presented in a heatmap, with blue to red colors reflecting the expression percentage (Figure 6). Among OsTLP 43 genes, nine members (OsTLP10, OsTLP36, OsTLP47, OsTLP38, OsTLP39, OsTLP40, OsTLP41, OsTLP42, and OsTLP43) were dominantly expressed in roots, leaf blades, and leaf sheaths, whereas no expressions were found for OsTLP21, OsTLP23, and OsTLP25 in any of the developmental stages. However, we found eight OsTLP family members (OsTLP7, OsTLP8, OsTLP9, OsTLP10, OsTLP12, OsTLP16, OsTLP40, and OsTLP43) with a high transcript in the roots. Furthermore, a deficient transcript level was detected for OsTLP3, OsTLP5, OsTLP9, OsTLP12, OsTLP16, OsTLP26, OsTLP27, and OsTLP28, among all the studied tissues. Moreover, we observed the expression profile of OsTLP3, OsTLP10, OsTLP42, and OsTLP43 among all tissues, apart from the pistil, anther, and endosperms. The expressions analysis found that most of the OsTLP gene family members were expressed in the roots, except for OsTLP19, OsTLP21, OsTLP23, and OsTLP25.
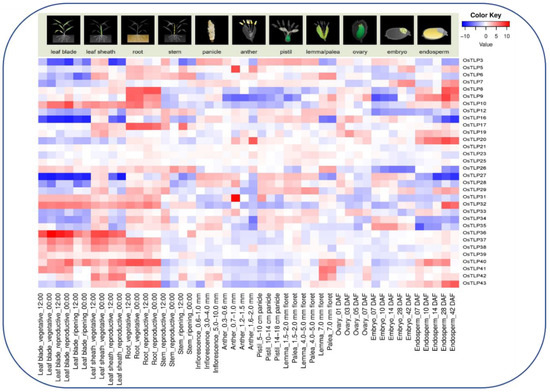
Figure 6.
Heatmap of OsTLP genes representation in developmental stages, including leaf blade, leaf sheath, root, stem, inflorescence, pistil, lemma, plea, ovary, embryo, and endosperm. The log2 transformation method normalized and converted the RPKM values displayed.
3.7.2. Expression Analysis of OsTLP Genes under Salinity
To further examine the response of OsTLP genes to saline conditions, the expression profile obtained from the publicly available NCBI database (GSE102152) [42] was analyzed in the form of a heatmap. As presented in the heatmap, various OsTLP genes displayed high/low transcription under SWR_CK (Seawater) and SWR_NaCl. The dark orange color on the scale bar represents high expression in the heatmap, whereas the light orange is moderate, and the blue color represents extremely low or no expression (Figure 7A). In the OsTLP genes family, several members such as OsTLP3, OsTLP9, OsTLP11, OsTLP12, OsTLP13, OsTLP17, OsTLP19, OsTLP26, OsTLP29, and OsTLP40 were recorded with the highest expressions, and the OsTLP4, OsTLP22, OsTLP23, OsTLP31, OsTLP32, OsTLP33, OsTLP34, OsTLP39, OsTLP41, and OsTLP42 were found with moderate expressions. Furthermore, the SWR_NaCl was found to have upregulated expressions in eight genes, including OsTLP1, OsTLP5, OsTLP6, OsTLP16, OsTLP20, OsTLP24, OsTLP25, OsTLP27; while OsTLP36, and OsTLP12, OsTLP13, OsTLP17, and OsTLP29 were found to have moderate expressions in comparison with control, whereas half of the OsTLP genes displayed no transcription in response to saline conditions.
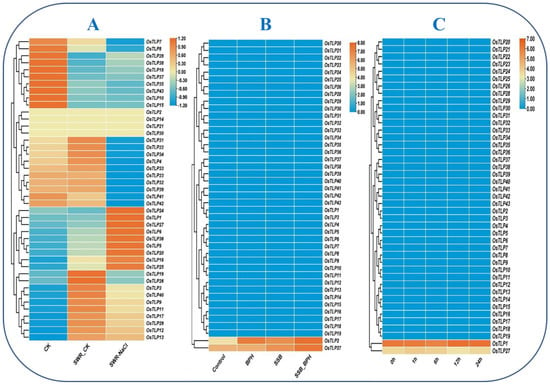
Figure 7.
Heatmap under salinity and biotic stresses. Heatmap (A) represents OsTLP gene expression in CK, SWR_CK, and SWR_NaCl. Heatmap (B) represents the OsTLP gene expressions in shoot tissues under BPH, SSB, and SSB_BPH. Heatmap (C) represents response to Cnaphalocrocis medinalis stress at different time points in Oryza sativa. The log2 transformation method normalized and converted the RPKM values displayed.
3.7.3. Expression Analysis of OsTLP Genes under Nilaparvata lugens and Chilo suppressalis
The transcriptomic expression data were obtained from NCBI (GSE167872) [43], and the analysis provided the possible role of OsTLP genes involvement in rice plant defense against brown planthopper (BPH), rice striped stem borer (SSB), Chilo suppressalis, and the combined SSB_BPH stresses (Figure 7B). Most of the OsTLP genes did not show expressions in response to the BPH infestation and SSB feeding stress. However, in response to BPH and SSB combined stress, only two genes (OsTLP2 and OsTLP27) showed dominant expressions, whereas the rest of the genes displayed extremely low or no transcriptions. The expression analysis unfolds the role of the TLP gene family in rice plants’ defense against pest infestations.
3.7.4. Expression Analysis of OsTLP Genes under Cnaphalocrocis medinalis
To explore the expression level of OsTLP genes under rice leaffolder (RLF) Cnaphalocrocis medinalis Guenée (Lepidoptera: Crambidae) feeding stress at 0, 1, 6, 12, and 24 h time points, the transcriptomic expression data were obtained from NCBI (GSE159259) [44], and the analysis unfolded that most of the OsTLP gene expression was unaffected, except OsTLP1 and OsTLP27, which show dominant expressions (Figure 7C). These expressions showed that the TLP gene family plays a vital role in the rice defense system against feeding insects.
3.7.5. Expression Analysis of OsTLP Genes under Jasmonic Acid and Brassinosteroids
Jasmonic acid (JA) and brassinosteroids (BRs) are important regulators of plant growth and developmental processes, and the role of OsTLP proteins in the hormonal signaling pathway is of great concern. To discover the potential role of OsTLP genes in response to JA and BRs, the expression data for the OsTLP genes were obtained from the National Institute of Agrobiological Sciences, Kannondai 2-1-2, Tsukuba, Ibaraki 305-8602, Japan RiceXPro expression database (Version 3.0) https://ricexpro.dna.affrc.go.jp/ (accessed on 23 March 2022) [41]. In the expression analysis of JA, among 43 members of OsTLPs, 32 genes displayed expression levels at all time points (0 h, 1 h, 6 h, and 12 h) (Figure 8). Furthermore, OsTLP6, OsTLP10, OsTLP32, and OsTLP34 showed downregulated expressions at the beginning of the treatment, but gradually increased during the treatment time. Additionally, a higher expression was detected at 6 h, followed by 12 h (OsTLP7, OsTLP9, OsTLP23, and OsTLP28), 3 h, and 1 h (OsTLP12, OsTLP13, OsTLP17, OsTLP18, OsTLP20, OsTLP24, OsTLP25, and OsTLP26) with moderate expression (OsTLP3, OsTLP12, OsTLP14, OsTLP16, OsTLP29, and OsTLP32) with extremely low expressions in 0 h.
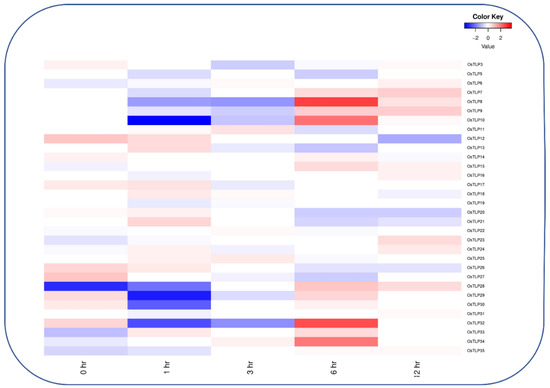
Figure 8.
Heatmap of OsTLP genes representation under jasmonic acid. The log2 transformation method normalized and converted the RPKM values displayed.
BRs are a group of steroid phytohormones with a wide range of biological activities. Here, the expression profile of the OsTLP genes under BRs unfolded the biological role of OsTLP genes (Figure 9). Among the 43 OsTLP gene family members, only 22 OsTLP showed expressions at all time points, suggesting the importance of the TLP gene family to hormonal changes. Among the OsTLP gene family, the OsTLP8, OsTLP10, OsTLP33, and OsTLP35 showed dominant indications at 6 h treatment, followed by OsTLP7, OsTLP9, OsTLP23, and OsTLP28 at 12hr, OsTLP12, OsTLP13 and OsTLP21 at 1 h, and OsTLP12, OsTLP27, OsTLP29, and OsTLP32 at 0 h, with moderate regulations. However, no transcription was observed for OsTLP10, OsTLP28, OsTLP29, OsTLP30, and OsTLP32 expressed in 1 h.
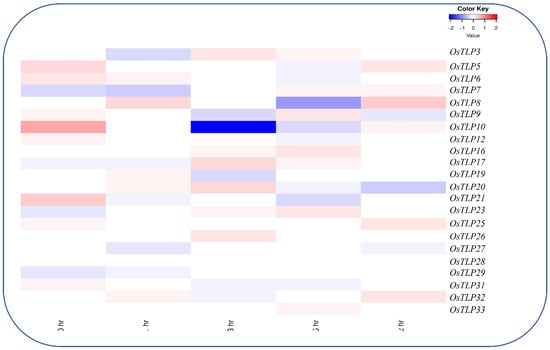
Figure 9.
Heatmap of OsTLP genes representation under brassinosteroids. The log2 transformation method normalized and converted the RPKM values displayed.
3.8. Differential Expression of OsTLP Genes in Response to Nilaparvata lugens, Laodelphax Striatellus Infestations, and JGM Spraying
To further study the response of OsTLP genes to BPH and SBPH infestations, and botanical fungicides applications, we performed the qRT-PCR to analyze the expression patterns at three time points of 2 d, 4 d, and 8 d treatments under BPH and SBPH infestation, and JGM spraying (Figure 10). The results unfolded that the response of ten candidate genes were expressed under all stress conditions. Under BPH infestation, these 10 OsTLP genes were found with moderate and high transcription under all time points. Particularly, the OsTLP36 at 4 d with high expressions of 20-fold, followed by OsTLP2, OsTLP22, and OsTLP29. Further, the OsTLP12 and OsTLP29 at 2 d infestations were observed with a dominant expressions level. However, OsTLP2, OsTLP10, OsTLP22, OsTLP27, and OsTLP33 showed moderate expressions. In addition, a similar expression pattern was observed under the SBPH infestation, in which the OsTLP2 at the 2 d time point showed high transcriptions of 11-fold, followed by OsTLP10, with a 0.5-fold expression; however, OsTLP19 at 4 d and OsTLP33 at 8 d showed upregulated expressions, and the rest of the genes were observed to have low transcriptions. Furthermore, the response of OsTLP genes was relatively higher than the BPH and SBPH at all time points, specifically of OsTLP27, OsTLP36, OsTLP22, OsTLP10, and OsTLP39, with dominant expressions, whereas the rest of the genes were observed with moderate transcription.
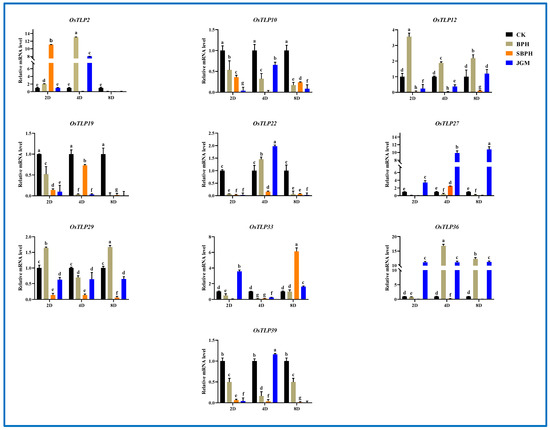
Figure 10.
Differential expression analysis of OsTLP genes (OsTLP2, OsTLP10, OsTLP12, OsTLP19, OsTLP22, OsTLP27, OsTLP29, OsTLP33, OsTLP36, and OsTLP39) under Nilaparvata lugens and Laodelphax striatellus infestations and Jinggangmycin (JGM) spraying treatment in Oryza sativa. Different letters indicate significant difference.
JGM is a synthetic antibiotic applied to treat rice sheath blight disease, and it is also reported to enhance BPH fecundity; therefore, we performed the qRT-PCR analysis to validate the OsTLP gene family response under JGM treatment (Figure 10). The obtained results revealed that the OsTLP genes, such as OsTLP36, OsTLP27, OsTLP29, and OsTLP22, presented upregulated expressions, whereas the OsTLP12 and OsTLP33 showed moderate expressions, and a single gene, OsTLP2, had a negligible expression pattern. These results suggest that the OsTLP genes participated in rice immunity regulation against the JGM spraying applications.
4. Discussion
Plants are often exposed to various harsh environmental conditions, such as biotic and abiotic stresses, during their entire life cycle, impairing their growth and development. In response to these environmental stimuli, plants activate various built-in immune machinery. Key stress marker genes are crucial in limiting the negative impact of stresses. Earlier, the thaumatin-like protein (TLPs) genes were reported to enhance plant immunity against pathogens and growth [7,16,17].
4.1. OsTLP Genes Are Widely Distributed in the Rice Genome
We identified 43 OsTLP genes in the rice genome, which is comparatively high compared to those identified in Arabidopsis thaliana 28 AtTLP [26]. The OsTLP were further grouped into ten subgroups based on their phylogenetic relationship (Figure 1). The concurrent results were previously reported by [45]. Further, the exon and intron structural differences provide indications of the divergence and duplications of a specific gene family [46]. In this current study, we found that the intron sizes of most OsTLP genes varied across subgroups. This OsTLP gene intron size variation could be linked to tendon duplication [47]. Furthermore, the size and number of exons were shown to be conserved among OsTLP gene subgroups (Figure 2). OsTLP genes have a conserved exon size, indicating that they have undergone few structural changes and may have conserved functional characteristics [47,48].
4.2. Biological and Molecular Characteristics of OsTLP Genes
The thaumatin-like protein gene family is involved in various biological processes such as responding to immune responses and biotic/abiotic stresses, hormonal signaling pathways transduction, protein binding, enzyme regulation, and hydrolase activities.
The gene ontology revealed the importance of OsTLP genes in an array of physiological and molecular functions (Figure 4). For instance, 71% of the genes modulated the biosynthesis and development of the secondary cell wall, whereas 29% of the genes occupied the extracellular location, regulating plant immunity, providing the first line of defense in biotic/abiotic factor suppression [49,50]. The study reveals that the thaumatin-like protein gene family is important for maintaining appropriate rice plant development under both normal and stressed situations.
The cis-elements, including enhancers and promoters, have been proven to be the regulatory mechanism of gene expression and function [51,52]. By identifying the cis-element, it is critical to provide the source of morphological evolution influenced by gene function diversity [53,54]. In this study, several cis-elements of TLP genes were predicted in Oryza sativa. The majority of the key physiological activities are directly or indirectly controlled by cis-regulatory elements [55], such as fine tunning hormones or programming the trade-offs between stress and the growth of plants (Table S5). The major hormonal regulating elements, such as ABRE, ERE, TATC-box, TCA-element, ARR1, CARE, and NTBBF, were found in abundance in the promoter region of the OsTLP genes, thus implying their participation in growth-related activities. On the other hand, the presence of stress-related cis-elements such as MYC and WRKY, CTR/DRE, MYB, SEBF, SEBF, and BIHD1 increased the importance of OsTLP genes many times. These cis-elements indicate the stress and hormonal responsive nature of OsTLP genes and could be used as a potential biomarker to develop stress-resilient rice cultivars.
According to the protein–protein interaction network analysis, the OsTLP genes are homodimers and heterodimers at the same time, as they interact with other proteins to influence rice plant development. For instance, our reference gene, OsTLP1, interacts with ERF2; interestingly, ERF2 played a key role in multiple SA, JA, and ROS signaling pathways to confer stress resistance. In addition, the ERF2 may directly or indirectly regulate Pto, PR1b1, and PR-P2 expression and enhance resistance to pathogens [56]. Additionally, our reference OSTLP1 protein is highly associated with the LTP family and involved in numerous biological processes, including plant growth, development, and responses to biotic and abiotic stresses [38]. Finally, the interaction between OsTLP1 and NBS-LRR proteins was observed. The NBS-LRR are plant stress-specific proteins that play a vital role in regulating immunity [40].
4.3. OsTLP Genes Are Important to Rice Plant Growth and Development
The thaumatin-like proteins have been characterized as a highly complex protein family associated with plant host defense and developmental processes. TLPs exhibit various properties associated with their structural diversity, being mostly associated with responses against biotic stress, besides some predicted activity under drought and osmotic stresses [57]. In our study, most of the OsTLP genes showed expression in all tissues, highlighting their crucial role in plant growth and development (Figure 6). For instance, based on expression analysis, various members of the OsTLP gene family were found to be crucial in seed germination [10]. Additionally, the current study revealed that most of the OsTLP genes were dominantly expressed in root tissues, conferring their role in root developmental activity [41]. Similarly, the leaf blade, leaf sheet, and stem show regulated expressions, suggesting their critical role in development. Here, we observed most of the OsTLP genes in rice stem tissues, indicating the significant importance of this gene family in the organogenesis of leaves in rice plants. Moreover, the agronomic traits, including plant height, leaf angle, grain size, and tiller number, determine the fate of overall yield [58]. Modulations of these traits can significantly influence the yield [59]. In the present study, the majority of the OsTLP genes displayed expressions in all rice developmental stages, including the stem, panicle, anther, pistil, lemma, ovary, embryo, and endosperm; therefore, they is essential for regulating the growth and developmental activities of rice plants.
4.4. OsTLP Genes Regulate Plant Response to Chewing Insects and Other Abiotic Stresses
The TLP genes are the key players capable of rapidly accumulating to high levels in response to biotic/abiotic stress and exhibiting antifungal activity in various plant species [7]. Additionally, these biomolecules showed regulated responses to various exogenous chemicals, hormones, and biotic/abiotic stresses. However, the response of OsTLP genes against chewing and sucking insects remains elusive.
The BPH and SBPH are serious rice pests, inflicting damage on a massive scale across Asia [19,21]. Their nymphs and adults cause direct damage by feeding on phloem sap from the tillering to milking stages of rice and, in the process, transmit viral pathogens, such as rice ragged stunt virus (RRSV) and rice grassy stunt virus (RGSV) [60,61]. To study the response of OsTLP genes to BPH and SBPH infestations, we performed qRT-PCR analyses of ten candidate genes (a single gene from each subgroup) to validate their expressions. The results revealed the obvious response of OsTLP genes. Furthermore, among these ten candidate genes, OsTLP36, OsTLP12, OsTLP2, and OsTLP39 were observed with dominant expressions, and the rest of the genes with moderate expressions (Figure 10). These results uncovered the crucial role of OsTLPs in rice plant immunity in response to insect pest infestations.
The antibiotic jinggangmycin (JGM) was developed in China and is commonly applied 2–3 times in rice fields during the crop cycle for controlling rice sheath blight disease (Rhizoctonia solani) [24]. Furthermore, JGM has achieved obvious results in controlling sheath blight disease; however, it is also reported to induce BPH fecundity [24]. For example, JGM was applied to the rice plants at the rate of 200 parts per million (ppm), and the results revealed that JGM increased the resistance to rice sheath blight disease by disrupting the fungal cell wall and reducing sporulation [24]. However, JGM was also reported to enhance BPH fecundity [24]. In the current study, the OsTLP genes showed a differential expression pattern where the candidate genes from each subgroup, including OsTLP2, OsTLP22, OsTLP36, OsTLP23, OsTLP29, and OsTLP39, were dominantly expressed, whereas OsTLP10, OsTLP12, OsTLP19 and OsTLP33 were moderately expressed (Figure 10). We speculate that these OsTLP genes are essential for inducing plant immunity to fungal pathogens in rice plants. Further studies are required to elucidate the underlying mechanisms of OsTLP genes under insects pests infestations.
Jasmonic acid (JA) signaling plays a central role in plant defenses against necrotrophic pathogens and herbivorous insects, afflicting roots and shoots. This pathway is also activated following the interaction with beneficial microbes that may lead to induced systemic resistance [62]. Here, the OsTLP8, OsTLP10, OsTLP34, and OsTLP35 displayed upregulated expressions; on the other hand, most genes show a sharp decline in expression (Figure 8). Our results suggest that the OsTLP gene may be involved in JA-mediated plant growth activities; however, this area requires further study.
Brassinosteroids (BRs) are a group of polyhydroxylated steroidal phytohormones that are mandatory for plant development, growth, and productivity. Besides their significant involvement in growth-related activities, BRs are considered a key stress hormone [63,64,65]. Herein, the upregulated expressions of OsTLP8, OsTLP10, and OsTLP21 suggest the essential role of the OsTLP gene family and its possible participation in the immunity regulation of the rice plant (Figure 8).
5. Conclusions
The current study identified and analyzed 43 OsTLP genes in rice from the genome database; additionally, we performed a comprehensive analysis of OsTLPs that included gene identification, phylogenetic analysis, chromosomal location, interactive protein network analysis, cis-regulatory elements, gene differential expression analysis, etc. Based on their domain and structural characteristics, 43 TLPs from the thaumatin-like protein family were identified and classified into ten subgroups. Additionally, the heatmap analysis revealed the response of OsTLPs to biological and hormonal stress. The qRT-PCR analysis demonstrated the expression patterns under BPH and SBPH infestation and JGM applications in rice, suggesting that these genes may influence resistance to pest infestations. Stress regulation is also a complicated mechanism to understand. Moreover, stress regulation is a complex mechanism, but the in silico analysis provides valuable information for future functional studies in stress biology. As a result, further functional studies are required to fully understand the regulation and pathway mechanism of OsTLPs in Oryza sativa L.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12061297/s1, Table S1: Primer sequences for RT-qPCR; Table S2: Conserved domains of OsTLP gene family; Table S3: The identified motif of OsTLP genes in Oryza sativa; Table S4: Predictive interactive partners of OsTLP1 gene; Table S5: The predicted cis-acting elements in the 1.5 kb promoter region of OsTLP genes.
Author Contributions
S.A. and L.G. designed the research; S.A. and Y.C. conducted the experimental work; H.Z. and C.X. contributed to the preparation of biological materials; A.Z.S. performed the bioinformatics analysis, and S.A. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32072415), Agricultural Science and Technology Independent Innovation Project of Jiangsu Province (CX (20) 3123), and the Yangzhou University Interdisciplinary Research Foundation for Agronomy Discipline of Targeted Support (yzuxk202005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Rahat Sharif, Department of Horticulture, School of Horticulture and Plant Protection, Yangzhou University, for his critical manuscript revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, X.M.; Griffith, M. Antifreeze proteins in winter rye leaves form oligomeric complexes. Plant Physiol. 1999, 119, 1361–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Lv, Y.; Hou, Z.; Li, X.; Ding, L. Expansion and evolution of thaumatin-like protein (TLP) gene family in six plants. Plant Growth Regul. 2015, 79, 299–307. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, A.B.; Cho, B.H.; Næsby, M.; Gregersen, P.L.; Brandt, J.; Madriz-Ordeñana, K.; Collinge, D.B.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chakrabarti, C. Crystal structure analysis of NP24-I: A thaumatin-like protein. Planta 2008, 228, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Sturrock, R.; Ekramoddoullah, A.K. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Petre, B.; Major, I.; Rouhier, N.; Duplessis, S. Genome-wide analysis of eukaryote thaumatin-like proteins (TLPs) with an emphasis on poplar. BMC Plant Biol. 2011, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Acharya, K.; Pal, A.K.; Gulati, A.; Kumar, S.; Singh, A.K.; Ahuja, P.S. Overexpression of Camellia sinensis thaumatin-like protein, CsTLP in potato confers enhanced resistance to Macrophomina phaseolina and Phytophthora infestans infection. Mol. Biotechnol. 2013, 54, 609–622. [Google Scholar] [CrossRef]
- Chun, J.; Yu, X.; Griffith, M. Genetic studies of antifreeze proteins and their correlation with winter survival in wheat. Euphytica 1998, 102, 219–226. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, A.-K.; Xiang, F.; Park, C.-M.J.P.; Physiology, C. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: Insights into their roles in salt response of seed germination. Plant Cell Physiol. 2008, 49, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Salzman, R.A.; Tikhonova, I.; Bordelon, B.P.; Hasegawa, P.M.; Bressan, R.A. Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defense response during fruit ripening in grape. Plant Physiol. 1998, 117, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorjanović, S.; Beljanski, M.V.; Gavrović-Jankulović, M.; Gojgić-Cvijović, G.; Pavlović, M.D.; Bejosano, F. Antimicrobial activity of malting barley grain thaumatin-like protein isoforms, S and R. J. Inst. Brew. 2007, 113, 206–212. [Google Scholar] [CrossRef]
- Stallworth, S.; Schumaker, B.; Fuller, M.G.; Tseng, T.-M. Consequences and Mitigation Strategies of Biotic and Abiotic Stress in Rice (Oryza sativa L.). In Plant Stress Physiology; IntechOpen: London, UK, 2020. [Google Scholar]
- Londo, J.P.; Chiang, Y.-C.; Hung, K.-H.; Chiang, T.-Y.; Schaal, B.A. Phylogeography of Asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 2006, 103, 9578–9583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Anami, B.S.; Malvade, N.N.; Palaiah, S. Classification of yield affecting biotic and abiotic paddy crop stresses using field images. nf. Process. Agric. 2020, 7, 272–285. [Google Scholar] [CrossRef]
- Ali, M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf nitrogen determination using non-destructive techniques–A review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Barrion, A.T.; Litsinger, J.A. Taxonomy of rice insect pests and their arthropod parasites and predators. In Biology and Management of Rice Insects; Wiley Eastern Ltd.: New Delhi, India; New Age International Ltd.: New Delhi, India, 1994; pp. 13–362. [Google Scholar]
- Yoshida, K.; Matsukura, K.; Sakai, J.; Onuki, M.; Sanada-Morimura, S.; Towata, T.; Matsumura, M. Seasonal occurrence of Laodelphax striatellus (Hemiptera: Delphacidae) in a rice-forage crops mixed cropping area in central Kyushu, Japan. Appl. Entomol. Zool. 2014, 49, 475–481. [Google Scholar] [CrossRef]
- Hu, G.; Lu, F.; Zhai, B.-P.; Lu, M.-H.; Liu, W.-C.; Zhu, F.; Wu, X.-W.; Chen, G.-H.; Zhang, X.-X. Outbreaks of the brown planthopper Nilaparvata lugens (Stål) in the Yangtze River Delta: Immigration or local reproduction? PLoS ONE 2014, 9, e88973. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, H.; Zheng, H.; Zhou, T.; Zhou, Y.; Wang, S.; Fang, R.; Qian, W.; Chen, X. Massively parallel pyrosequencing-based transcriptome analyses of small brown planthopper (Laodelphax striatellus), a vector insect transmitting rice stripe virus (RSV). BMC Genom. 2010, 11, 303. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Lacombe, S.; Bangratz, M.; Ta, H.A.; Vinh, D.N.; Gantet, P.; Brugidou, C. p2 of Rice grassy stunt virus (RGSV) and p6 and p9 of Rice ragged stunt virus (RRSV) isolates from Vietnam exert suppressor activity on the RNA silencing pathway. Virus Genes 2015, 51, 267–275. [Google Scholar] [CrossRef]
- Zhou, G.; Wen, J.; Cai, D.; Li, P.; Xu, D.; Zhang, S. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Jiang, L.-B.; Zhao, K.-F.; Wang, D.-J.; Wu, J.-C. Effects of different treatment methods of the fungicide jinggangmycin on reproduction and vitellogenin gene (Nlvg) expression in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pestic. Biochem. Physiol. 2012, 102, 51–55. [Google Scholar] [CrossRef]
- Ge, L.Q.; Zheng, S.; Gu, H.T.; Zhou, Y.K.; Zhou, Z.; Song, Q.S.; Stanley, D. Jinggangmycin-induced UDP-glycosyltransferase 1-2-like is a positive modulator of fecundity and population growth in Nilaparvata lugens (Stål)(Hemiptera: Delphacidae). Front. Physiol. 2019, 10, 747. [Google Scholar] [CrossRef]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M. The Arabidopsis Information Resource (TAIR): Improved gene annotation and new tools. Nucleic Acids Res. 2012, 40, D1202–D1210. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.-S.; Cheng, C.-W.; Su, W.-C.; Chang, K.-C.; Huang, S.-W.; Hwang, J.-K.; Lu, C.-H. CELLO2GO: A web server for protein subCELlular LOcalization prediction with functional gene ontology annotation. PLoS ONE 2014, 9, e99368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Shah, A.Z.; Ma, C.; Zhang, Y.; Zhang, Q.; Xu, G.; Yang, G. Decoyinine Induced Resistance in Rice against Small Brown Planthopper Laodelphax striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef]
- Ge, L.-Q.; Wang, L.-P.; Zhao, K.-F.; Wu, J.-C.; Huang, L.-J. Mating pair combinations of insecticide-treated male and female Nilaparvata lugens Stål (Hemiptera: Delphacidae) planthoppers influence protein content in the male accessory glands (MAGs) and vitellin content in both fat bodies and ovaries of adult females. Pestic. Biochem. Physiol. 2010, 98, 279–288. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.-Y.; Zhu, Q.-H.; Chen, X.; Luo, J.-C. GSDS: A gene structure display server. Yi Chuan Hered. 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Sun, D.; Han, Z.; Ni, D.; Norris, A.; Jiang, C.-Z. PhERF2, an ethylene-responsive element binding factor, plays an essential role in waterlogging tolerance of petunia. Hortic. Res. 2019, 6, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, N.; Buonanno, M.; Ayoub, J.; Barone, A.; Monti, S.M.; Rigano, M.M. Identification of non-specific Lipid Transfer Protein gene family members in Solanum lycopersicum and insights into the features of Sola l 3 protein. Sci. Rep. 2019, 9, 1607. [Google Scholar] [CrossRef]
- Kalunke, R.M.; Tundo, S.; Benedetti, M.; Cervone, F.; De Lorenzo, G.; D’Ovidio, R. An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front. Plant Sci. 2015, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Belkhadir, Y.; Subramaniam, R.; Dangl, J.L. Plant disease resistance protein signaling: NBS–LRR proteins and their partners. Curr. Opin. Plant Biol. 2004, 7, 391–399. [Google Scholar] [CrossRef]
- Sato, Y.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Namiki, N.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Antonio, B.A.; Nagamura, Y. RiceXPro version 3.0: Expanding the informatics resource for rice transcriptome. Nucleic Acids Res. 2013, 41, D1206–D1213. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Cheng, Y.; Han, S.; Van Handel, B.; Dong, L.; Li, X.; Xie, X. Whole genome sequencing and comparative transcriptome analysis of a novel seawater adapted, salt-resistant rice cultivar-Sea rice 86. BMC Genom. 2017, 18, 655. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Hu, X.; Su, S.; Ning, Y.; Peng, Y.; Ye, G.; Lou, Y.; Turlings, T.C.; Li, Y. Cooperative herbivory between two important pests of rice. Nat. Commun. 2021, 12, 6772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Du, L.; Hallerman, E.M.; Li, Y. Transcriptomic and metabolomic responses of rice plants to Cnaphalocrocis medinalis caterpillar infestation. Insects 2020, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, J.; Zhou, X.; Luan, Y.; Luan, F. Genome-wide identification, characterization and expression analysis of the TLP gene family in melon (Cucumis melo L.). Genomics 2020, 112, 2499–2509. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.J.; Guigó, R.; Agarwal, P.; Russell, R.B. Exon structure conservation despite low sequence similarity: A relic of dramatic events in evolution? EMBO J. 2001, 20, 5354–5360. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon–Intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Sheth, N.; Roca, X.; Hastings, M.L.; Roeder, T.; Krainer, A.R.; Sachidanandam, R. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 2006, 34, 3955–3967. [Google Scholar] [CrossRef] [Green Version]
- Munis, M.F.H.; Tu, L.; Deng, F.; Tan, J.; Xu, L.; Xu, S.; Long, L.; Zhang, X. A thaumatin-like protein gene involved in cotton fiber secondary cell wall development enhances resistance against Verticillium dahliae and other stresses in transgenic tobacco. Biochem. Biophys. Res. Commun. 2010, 393, 38–44. [Google Scholar] [CrossRef]
- He, L.; Li, L.; Zhu, Y.; Pan, Y.; Zhang, X.; Han, X.; Li, M.; Chen, C.; Li, H.; Wang, C. BolTLP1, a Thaumatin-like Protein Gene, Confers Tolerance to Salt and Drought Stresses in Broccoli (Brassica oleracea L. var. Italica). Int. J. Mol. Sci. 2021, 22, 11132. [Google Scholar] [CrossRef]
- Shan, C.-M.; Shangguan, X.-X.; Zhao, B.; Zhang, X.-F.; Chao, L.-M.; Yang, C.-Q.; Wang, L.-J.; Zhu, H.-Y.; Zeng, Y.-D.; Guo, W.-Z. Control of cotton fibre elongation by a homeodomain transcription factor GhHOX3. Nat. Commun. 2014, 5, 5519. [Google Scholar] [CrossRef]
- Zhao, B.; Cao, J.F.; Hu, G.J.; Chen, Z.W.; Wang, L.Y.; Shangguan, X.X.; Wang, L.J.; Mao, Y.B.; Zhang, T.Z.; Wendel, J.F. Core cis-element variation confers subgenome-biased expression of a transcription factor that functions in cotton fiber elongation. New Phytol. 2018, 218, 1061–1075. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhu, F.; Duan, D. Function analysis and stress-mediated cis-element identification in the promoter region of VqMYB15. Plant Signal. Behav. 2020, 15, 1773664. [Google Scholar] [CrossRef] [PubMed]
- Mengarelli, D.A.; Zanor, M.I. Genome-wide characterization and analysis of the CCT motif family genes in soybean (Glycine max). Planta 2021, 253, 15. [Google Scholar] [CrossRef] [PubMed]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. PCTOC 2016, 127, 269–287. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Sun, Y.; Wang, H.; Zhao, T.; Xu, X.; Jiang, J.; Li, J. Genome-wide identification and functional analysis of the ERF2 gene family in response to disease resistance against Stemphylium lycopersici in tomato. BMC Plant Biol. 2021, 21, 72. [Google Scholar] [CrossRef]
- de Jesús-Pires, C.; Ferreira-Neto, J.R.; Pacifico Bezerra-Neto, J.; Kido, E.A.; de Oliveira Silva, R.L.; Pandolfi, V.; Wanderley-Nogueira, A.C.; Binneck, E.; da Costa, A.F.; Pio-Ribeiro, G.; et al. Plant thaumatin-like proteins: Function, evolution and biotechnological applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, M.-Y.; Chong, K. Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep. 2014, 33, 683–696. [Google Scholar] [CrossRef]
- Sharif, R.; Liu, P.; Wang, D.; Jin, Z.; Uzair, U.; Yadav, V.; Mujtaba, M.; Chen, P.; Li, Y. Genome-wide characterisation and expression analysis of cellulose synthase genes superfamily under various environmental stresses in Cucumis sativus L. N. Z. J. Crop Hortic. Sci. 2021, 49, 127–150. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [Green Version]
- Cabauatan, P.Q.; Cabunagan, R.C.; Choi, I.-R. Rice viruses transmitted by the brown planthopper Nilaparvata lugens Stål. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; International Rice Research Institute: Los Baños, Philippines, 2009; pp. 357–368. [Google Scholar]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Tyson, G.W.; Vivanco, J.M.; Schenk, P.M. Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS ONE 2013, 8, e56457. [Google Scholar] [CrossRef] [Green Version]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The physiological and molecular mechanism of brassinosteroid in response to stress: A review. Biol. Plant. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).