Location of Pathogen Inoculum in the Potting Substrate Influences Damage by Globisporangium ultimum, Fusarium culmorum and Rhizoctonia solani and Effectiveness of Control Agents in Maize Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize Seeds
2.2. Fungal Pathogens and Preparation of Inocula
2.3. Seed Treatment
2.4. Growth Chamber Trials
2.5. Statistical Analysis
3. Results
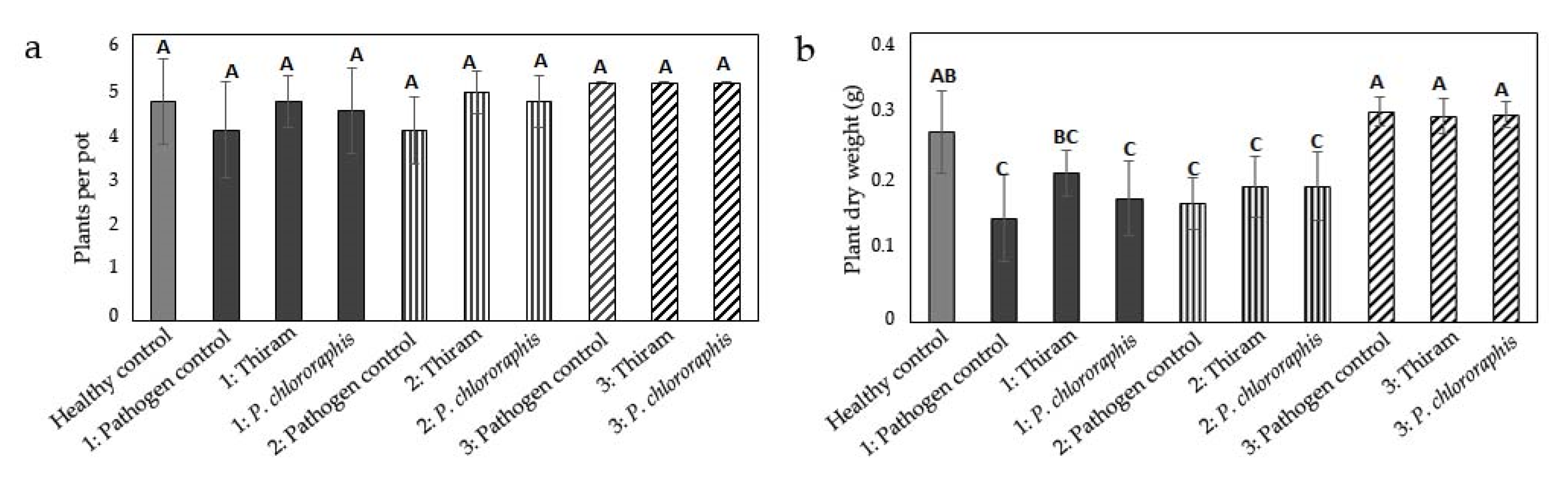
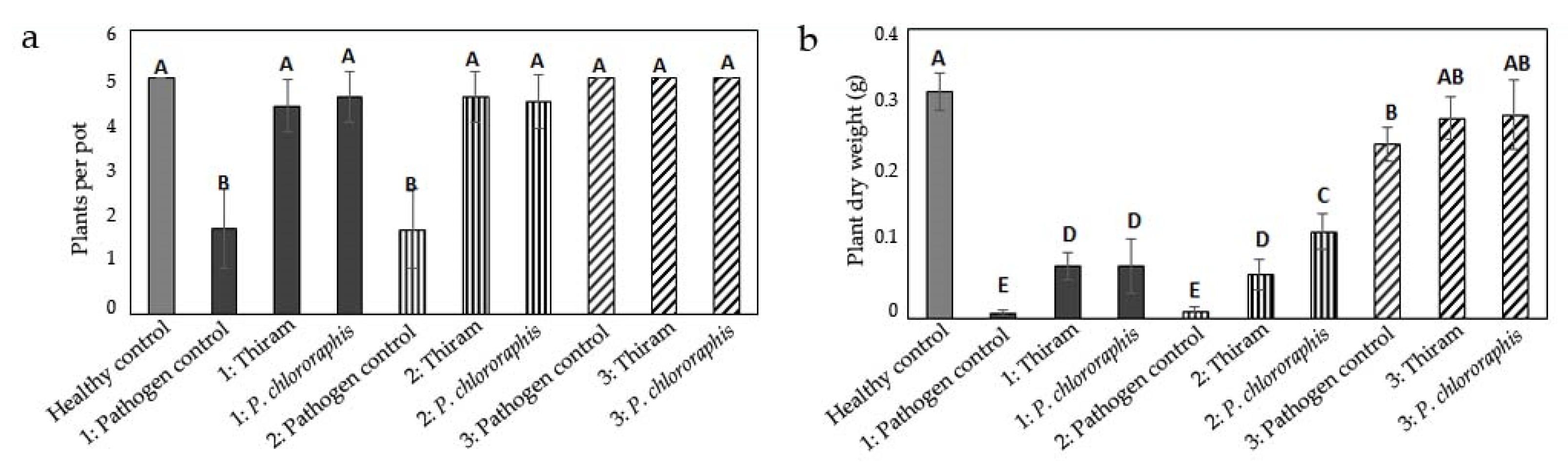
3.1. Influence of Inoculum Location on Damage by Globisporangium ultimum
3.2. Influence of Inoculum Location on Damage by Fusarium culmorum
3.3. Influence of Inoculum Location on Damage by Rhizoctonia solani
3.4. Influence of Inoculum after Transfer from Healthy Soil
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, A.; Messéan, A.; Aubertot, J.N. Integrated management of damping-off diseases, a review. Agron. Sustain. Dev. 2017, 37, 1–25. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting sustainability of fungicide seed treatments for field crops. Plant. Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höfte, M. The use of Pseudomonas spp. as bacterial biocontrol agents to control plant disease. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021. [Google Scholar]
- Forsberg, G.; Johnsson, L.; Lagerholm, J. Effects of aerated steam treatment on cereal diseases and crop yield. J. Plant. Dis. Prot. 2005, 112, 247–256. [Google Scholar]
- Röder, O.; Jahn, M.; Schröder, T.; Stahl, M.; Kotte, M.; Beuermann, S. Die e-ventus Technologie–eine Innovation zur nachhaltigen Reduktion von Pflanzenschutzmitteln mit Empfehlung für Bio-Saatgut. J. Verbrauch. Lebensm. 2009, 4, 107–117. [Google Scholar] [CrossRef]
- Bänziger, I.; Kägi, A.; Vogelgsang, S.; Klaus, S.; Hebeisen, T.; Büttner-Mainik, A.; Sullam, K.E. Comparison of thermal seed treatments to control snow mold in wheat and loose smut of barley. Front. Agron. 2022, 3, 75243. [Google Scholar] [CrossRef]
- Pfeiffer, T.; von Galen, A.; Zink, P.; Hübner, S.; Linkies, A.; Felgentreu, D.; Drechsel, J.; Birr, T.; Röder, O.; Kotte, M.; et al. Selection of bacteria and fungi for control of soilborne seedling diseases of maize. J. Plant. Dis. Prot. 2021, 128, 1227–1241. [Google Scholar] [CrossRef]
- Lamprecht, S.C.; Tewoldemedhin, Y.T.; Botha, W.J.; Calitz, F.J. Fusarium graminearum species complex associated with maize crowns and roots in the KwaZulu-Natal province of South Africa. Plant. Dis. 2011, 95, 1153–1158. [Google Scholar] [CrossRef] [Green Version]
- Okello, P.N.; Petrović, K.; Kontz, B.; Mathew, F.M. Eight species of Fusarium caused root rot of corn (Zea mays) in South Dakota. Plant. Health Prog. 2019, 20, 38–43. [Google Scholar] [CrossRef]
- Sumner, D.R.; Bell, D.K. Root diseases induced in corn by Rhizoctonia solani and Rhizoctonia zeae. Phytopathology 1982, 72, 86–91. [Google Scholar] [CrossRef]
- Ithurrart, M.E.F.; Büttner, G.; Petersen, J. Rhizoctonia root rot in sugar beet (Beta vulgaris ssp. altissima)—Epidemiological aspects in relation to maize (Zea mays) as a host plant. J. Plant. Dis. Prot. 2004, 111, 302–312. [Google Scholar]
- Da Silva, M.P.; Tylka, G.L.; Munkvold, G.P. Seed treatment effects on maize seedlings coinfected with Rhizoctonia solani and Pratylenchus penetrans. Plant. Dis. 2017, 101, 957–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacon, C.W.; Hinton, D.M. Potential for control of seedling blight of wheat caused by Fusarium graminearum and related species using the bacterial endophyte Bacillus mojavensis. Biocontrol Sci. Technol. 2007, 17, 81–94. [Google Scholar] [CrossRef]
- Lu, Z.X.; Tu, G.P.; Zhang, T.; Li, Y.Q.; Wang, X.H.; Zhang, Q.G.; Song, W.; Chen, J. Screening of antagonistic Trichoderma strains and their application for controlling stalk rot in maize. J. Integr. Agric. 2020, 19, 145–152. [Google Scholar] [CrossRef]
- Mao, W.; Lumsden, R.D.; Lewis, J.A.; Hebbar, P.K. Seed treatment using pre-infiltration and biocontrol agents to reduce damping-off of corn caused by species of Pythium and Fusarium. Plant. Dis. 1998, 82, 294–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Palni, L.M.S.; Hebbar, K.P. Suppression of damping-off in maize seedlings by Pseudomonas corrugata. Microbiol. Res. 2001, 156, 191–194. [Google Scholar] [CrossRef]
- Munkvold, G.P.; O’Mara, J.K. Laboratory and growth chamber evaluation of fungicidal seed treatments for maize seedling blight caused by Fusarium species. Plant. Dis. 2002, 86, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.S.; Leclerque, A.; Pfeiffer, T.; Goessling, J.W.; Orlik, M.; Jamshidi, B.; Saar, K.; Sellman, J.; Siepe, I.; Koch, E. Pathogenicity of Pythium species to maize. Eur. J. Plant. Pathol. 2020, 158, 335–347. [Google Scholar] [CrossRef]
- Nelson, E.B. Exudate molecules initiating fungal responses to seeds and roots. In The Rhizosphere and Plant Growth; Keister, D.L., Cregan, P.B., Eds.; Springer: Doordrecht, The Netherlands, 1991. [Google Scholar]
- Nelson, E.B. Rapid germination of sporangia of Pythium species in response to volatiles from germinating seeds. Phytopathology 1987, 77, 1108–1112. [Google Scholar] [CrossRef]
- Osburn, R.M.; Schroth, M.N.; Hancock, J.G.; Hendson, M. Dynamics of sugar beet seed colonization by Pythium ultimum and Pseudomonas species: Effects on seed rot and damping-off. Phytopathology 1989, 79, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Murillo, I.; Cavallarin, L.; Segundo, B.S. Cytology of infection of maize seedlings by Fusarium moniliforme and immunolocalization of the pathogenesis-related PRms protein. Phytopathology 1990, 89, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Campo, S.; Carrascal, M.; Coca, M.; Abián, J.; San Segundo, B. The defense response of germinating maize embryos against fungal infection: A proteomics approach. Proteomics 2004, 4, 383–396. [Google Scholar] [CrossRef]
- Sneh, B.; Ichielevich-Auster, M.; Shomer, I. Comparative anatomy of colonization of cotton hypocotyls and roots by virulent and hypovirulent isolates of Rhizoctonia solani. Can. J. Bot. 1989, 67, 2142–2149. [Google Scholar] [CrossRef]
- Benhamou, N.; Broglie, K.; Chet, I.; Broglie, R. Cytology of infection of 35S-bean chitinase transgenic canola plants by Rhizoctonia solani: Cytochemical aspects of chitin breakdown in vivo. Plant. J. 1993, 4, 295–305. [Google Scholar] [CrossRef]
- Lawrence, E.B.; Nelson, P.E.; Ayers, J.E. Histopathology of sweet corn seed and plants infected with Fusarium moniliforme and F. oxysporum. Phytopathology 1981, 71, 379–386. [Google Scholar] [CrossRef]
- Chérif, M.; Benhamou, N.; Bélanger, R.R. Ultrastructural and cytochemical studies of fungal development and host reactions in cucumber plants infected by Pythium ultimum. Physiol. Mol. Plant. Pathol. 1991, 39, 353–375. [Google Scholar] [CrossRef]
- Rey, P.; Benhamou, N.; Tirilly, Y. Ultrastructural and cytochemical investigation of asymptomatic infection by Pythium spp. Phytopathology 1998, 88, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Hooker, A.L. Relative pathogenicity of Pythium species attacking seedling corn. Proc. Iowa Acad. Sci. 1953, 60, 163–166. [Google Scholar]
- Kondo, N.; Kodama, F.; Akai, J. Pathogenicity of Pythium species isolated from ungerminated corn seeds planted at a low temperature. Japan. J. Phytopathol. 1986, 52, 585–589. [Google Scholar] [CrossRef] [Green Version]
- Stanghellini, M.E.; Hancock, J.G. Radial extent of the bean spermosphere and its relation to the behavior of Pythium ultimum. Phytopathol. 1971, 61, 165–168. [Google Scholar] [CrossRef]
- Schiltz, S.; Gaillard, I.; Pawlicki-Jullian, N.; Thiombiano, B.; Mesnard, F.; Gontier, E. A review: What is the spermosphere and how can it be studied? J. Appl. Microbiol. 2015, 119, 1467–1481. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, E.; Zink, P.; Bernhardt, T.; Birr, T.; Linkies, A. Location of Pathogen Inoculum in the Potting Substrate Influences Damage by Globisporangium ultimum, Fusarium culmorum and Rhizoctonia solani and Effectiveness of Control Agents in Maize Seedlings. Agronomy 2022, 12, 1388. https://doi.org/10.3390/agronomy12061388
Koch E, Zink P, Bernhardt T, Birr T, Linkies A. Location of Pathogen Inoculum in the Potting Substrate Influences Damage by Globisporangium ultimum, Fusarium culmorum and Rhizoctonia solani and Effectiveness of Control Agents in Maize Seedlings. Agronomy. 2022; 12(6):1388. https://doi.org/10.3390/agronomy12061388
Chicago/Turabian StyleKoch, Eckhard, Petra Zink, Tanja Bernhardt, Tim Birr, and Ada Linkies. 2022. "Location of Pathogen Inoculum in the Potting Substrate Influences Damage by Globisporangium ultimum, Fusarium culmorum and Rhizoctonia solani and Effectiveness of Control Agents in Maize Seedlings" Agronomy 12, no. 6: 1388. https://doi.org/10.3390/agronomy12061388
APA StyleKoch, E., Zink, P., Bernhardt, T., Birr, T., & Linkies, A. (2022). Location of Pathogen Inoculum in the Potting Substrate Influences Damage by Globisporangium ultimum, Fusarium culmorum and Rhizoctonia solani and Effectiveness of Control Agents in Maize Seedlings. Agronomy, 12(6), 1388. https://doi.org/10.3390/agronomy12061388






