Abstract
The main characteristic of Cannabis sativa L. is the production of compounds of medicinal interest known as phytocannabinoids. Environmental factors and crop management practices are directly related to the yield of these compounds. Knowing how these factors influence the production of phytocannabinoids is essential to promote greater metabolite yield and stability. In this review, we aim to examine current cannabis agronomic research topics to identify the available information and the main gaps that need to be filled in future research. This paper introduces the importance of C. sativa L., approaching state-of-the-art research and evaluating the influence of crop management and environment conditions on yield and phytocannabinoid production, including (i) pruning; (ii) light and plant density; (iii) ontogeny; (iv) temperature, altitude, and CO2 concentration; (v) fertilization and substrate; and (vi) water availability, and presents concluding remarks to shed light on future directions.
1. Introduction
Legal restrictions in the last decades [1] have prevented the progress of academic research involving Cannabis sativa L. These conditions have resulted in a scarcity of science-based information on C. sativa L., which is peculiar considering that it is one of our oldest crops with a rich usage history by humankind [2,3], and its domestication dates back to prehistoric times [3,4,5]. Samples of microfossil pollens indicate that its origin was in Tibet at least 19.6 million years ago [6]. Historically, several cultures and societies have reported medicinal applications [4,7], and it is currently used for the treatment of several diseases, such as epilepsy, Parkinson’s disease, and chronic pain [7], due to its therapeutic safety and efficacy, as recent clinical trials have demonstrated [8]. This species has several uses due to the production of fibers, seeds, and secondary metabolites that have industrial value for a myriad of production chains [9].
In recent years, the medicinal applications of C. sativa L. have gained repercussions worldwide. The United Nations (UN) recently removed the species from the highly dangerous substances list and of low medicinal application [10], and this change recognizes the plant’s medicinal value, which may allow additional scientific advances [11]. The medicinal value of this species is related to the production of secondary metabolites called phytocannabinoids, molecules produced in abundance by this species, which are present in their acidic form in plant tissue [12,13,14]. About 150 phytocannabinoids have been reported in the literature, and the most prevalent in many varieties are CBDA, CBGA, and THCA; this latest one, in its decarboxylated form (THC), being responsible for psychotropic effects. [12,13,15].
Cannabis sativa L., popularly known as cannabis (canábis or cânabis in Portuguese), is classified as hemp or marijuana based on its THC content. The USDA (United States Department of Agriculture) stated that marijuana contains from 3% to 15% THC (dry weight), whilst hemp has less than 1%. In the European Union (EU), although each member-state has different cannabis legislation, all of the EU members basically distinguish hemp from marijuana using the 0.2% threshold for THC concentration (Regulation (EU) nº 1307/2013), although this level of THC in cannabis is insufficient to induce psychotropic effects [9]. Although the classification between hemp and marijuana is based on legal convention, different studies indicate that the discrimination between these varieties is not limited to cannabinoid biosynthesis but can be monitored across the whole genome [15].
Cannabis is a herbaceous, annual [13,16], dioecious species that can produce monoecious plants [17]. Morphologically, the inflorescences of male dioecious plants are characterized by hanging panicles with few or no leaves, and inflorescences of female plants bear racemes with leafy bracts [17,18]. Female plants have the highest phytocannabinoid production due to a higher density of glandular trichomes, where these compounds are synthesized and stored [19,20,21,22]. In addition, male and hermaphroditic plants have reduced floral biomass and, thus, reduced phytocannabinoid yield [23].
Male and female plants of the same variety have been reported to produce similar amounts of cannabinoids [24,25,26,27,28]. However, other studies have shown the opposite [23,29,30]. According to metabolomic analyses, the female floral tissue from different genotypes averaged 3.5% phytocannabinoids, while male floral tissues from different genotypes averaged less than about 1% total phytocannabinoids [23]. According to the literature, male and female plants of some varieties may have similar concentrations of cannabinoids; however, cannabis cultivation for medicinal purposes is carried out with female plants. Furthermore, sowing male plants for phytocannabinoid production is uncommon due to the female plants’ pollination, diverting phytocannabinoid production to seed development [21,31].
There is a misunderstanding that cross-pollination changes the chemotype of the plant. However, this change only appears in seeds resulting from cross-pollination and not in the pollinated plant. The problem with pollination occurring in crops intended for the production of phytocannabinoids is that the energy is shifted to seed production, not cannabinoid production [32]. Feder et al. (2021) [31] observed that pollination resulted in a significant decrease in the overall total phytocannabinoid concentration in inflorescences. The THC-rich chemovar female exhibited an average 75% decrease, while CBD-rich females showed a 60% decrease in phytocannabinoid content after fertilization [31].
Pollination prevention stimulates the formation of new flowers, increasing phytocannabinoid production [33,34]. Although male plants tend to be larger and bloom before female plants, it is difficult to distinguish them during the vegetative phase [21]. The most common way to differentiate female plants from male plants is by analyzing the anatomy of the inflorescences, although some genotypes develop solitary internode flowers at early stages of development, making it possible for early sexual differentiation [35].
To avoid pollination, one option is to remove male plants as they appear. Another alternative is to prevent the presence of male plants by using vegetative propagation, ensuring that the mother plant is female. When cultivation is carried out under ideal conditions, it is unlikely that there is a change in the sexual expression of clones from the mother plant. However, care should be taken with the prolonged life of the mother plant due to the occurrence of mutations and somaclonal variations that can decline the vigor and phytocannabinoid content in clones compared to the original mother plant [32]. Another option is to carry out seminiferous propagation with feminized seeds [36].
Although it is practically unanimous among scientists to consider flowers or inflorescences as the product of interest for medicinal use [12,13,21], recently a paper was published reporting that these structures should be considered as fruits or infructescence, considering that the flowers senesce and turn into fruits that, in turn, ripen [37]. However, it should be noted that parthenocarpy consists of the growth of the ovary into seedless fruit without the occurrence of pollination [38]. The structures produced by this crop have complex floral structures (such as stigmas) (Figure 1) that support their identification as flowers. It should be considered that, when the flower reaches the ideal harvest point it presents an advanced stage of senescence although it does not mature.

Figure 1.
Cannabis inflorescence with emphasis on stigmas.
The majority of researchers and growers work under the common misconception that cannabis is a short-day plant [39,40,41]. However, short-day plants are ones that only blossom after being exposed to light periods shorter than a certain critical length. Tournois (1912) demonstrated that flowering in this species was hastened by short days and delayed by long days [42], which is not in accordance with the definition of short-day plants. According to Spitzer-Rimon et al. (2019) [35], the effect of short photoperiods on cannabis florogenesis is not flower induction, but rather a dramatic change in shoot apex architecture to form an inflorescence structure. The development of solitary flowers clearly indicates that the plant at this stage cannot be defined as vegetative or noninductive in the classical sense. Therefore, the flower induction of solitary flowers is probably age-dependent and is controlled by internal signals, but not by photoperiod [35].
The purpose of this study is to conduct a thorough review of the crop management and abiotic factors affecting phytocannabinoid production in cannabis. The review aims to summarize the current scientific understanding of the factors influencing cannabinoid productivity and, hopefully, to inform the development of management protocols that favor quality and productivity, as well as new studies to address current knowledge gaps. With this end, the paper is structured in five sections. Section 1 introduces the importance and main characteristics of cannabis. Section 2 approaches the methodology to develop this review and state-of-the-art research that evaluates the influence of management and the environment on the productivity of phytocannabinoids. The Section 3 assesses the factors influencing phytocannabinoid production and crop management techniques applied in cannabis cultivation, which include (i) pruning; (ii) light and plant density; (iii) ontogeny; (iv) temperature, altitude, and CO2 concentration; (v) fertilization and substrate; and (vi) water availability. Finally, Section 4 presents the concluding remarks and sheds light on future directions.
2. State-of-the-Art Research That Evaluates Crop Management and Abiotic Factors That Affect Phytocannabinoid Production
To develop this review, a search was carried out in the online databases of Google Scholar, Web of Science, and Scopus in order to access research that evaluated factors related to management and the environment in the production of phytocannabinoids. The criterion used to search for the articles was based on the following keywords: (hemp or cannabis + cannabinoids + pruning, nutrient, fertilizer, light, light quality, light intensity, water availability, substrate, ontogeny, plant density, temperature, altitude, relative humidity, CO2, management). In addition to searching the databases, the bibliographic references of all the articles found were analyzed, and the cited articles that were related to this review were included.
We selected 78 articles that evaluated phytocannabinoid production in relation to pruning, ontogeny, light, water availability, temperature, altitude, nutrition, and substrate. The criteria for including the articles in the review was based on the chemical analysis of phytocannabinoids by the study in order to access the influence of management and abiotic factors on the productivity of these compounds. However, some studies (41, 66, 88, 98 and 100) that did not evaluate the effect of management or abiotic factors on the production of phytocannabinoids were cited because the results obtained could contribute to the development of future researchers. These studies were not counted in the 78 selected articles.
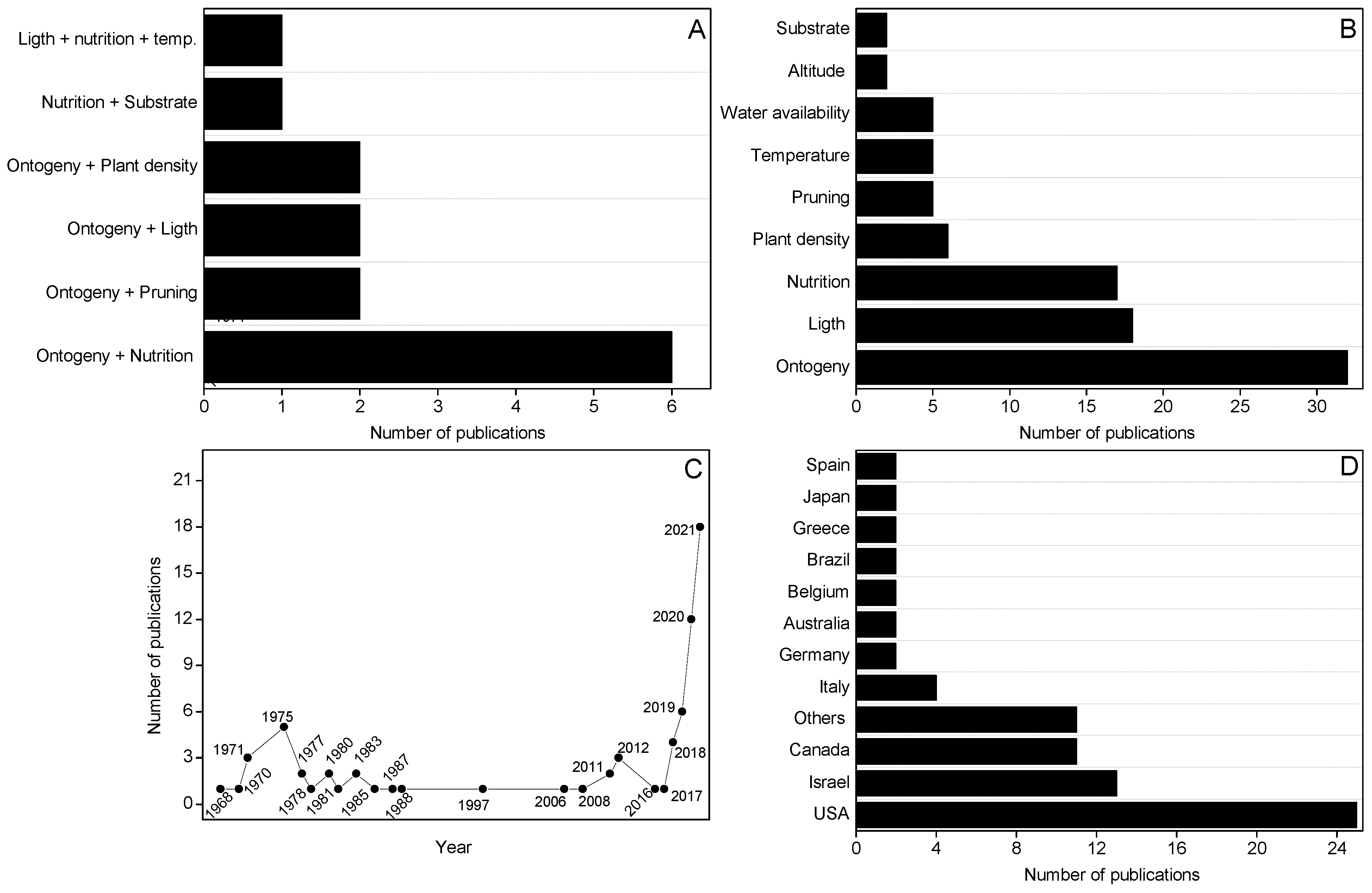
Among the 78 articles selected, 142 evaluated 2 concomitant factors (Figure 2A). The results obtained in these articles are independently reported in the respective sections of this review. It was observed that some articles carried out evaluations that were not explicit in the title or in the keywords, such as studies 114, 115 and 125, whose main focus was to evaluate the availability of nutrients in phytocannabinoid productivity but also performed analyses related to ontogeny. The Figure 2B shows the number of publications that evaluated the influence of a specific management or abiotic factor on the production of phytocannabinoids. This Figure corresponds to the evaluations observed in the 76 selected articles. According to Figure 2B, it is possible to observe that the studies that evaluated the influence of management and abiotic factors on the production of phytocannabinoids focused on evaluations of the influence of ontogeny, light, nutrition, and plant density. Figure 2C shows the number of publications related to the influences of management and abiotic factors on phytocannabinoid productivity over the years. According to the Figure, it is possible to observe that the number of publications increased significantly from 2018. In recent years, the legalization of medicinal cannabis in some countries has led to an increase in the number of publications, such as in Canada, Israel, and some states of the EUA, places that contributed the most to the number of publications (Figure 2D).
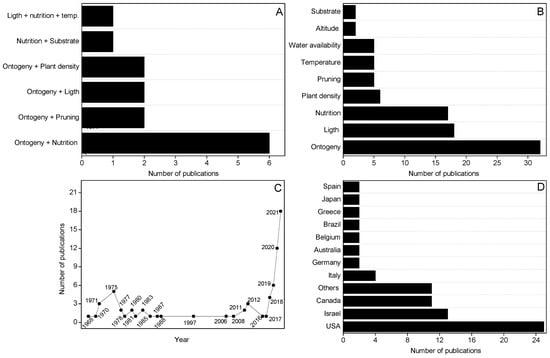
Figure 2.
(A) Number of publications that evaluated two factors related to management and/or environment in the production of cannabinoids; (B) Number of publications that evaluated the management and/or environment in the production of cannabinoids; (C) Analysis of cannabis. literature about crop management and abiotic factors that affect cannabinoid production in terms of years and number of publications published; (D) Analysis of literature in terms of country and number of publications published.
3. Phytocannabinoids Production and Management Techniques in Cannabis Cultivation
Plant secondary metabolites are a set of molecules that are part of a mechanism in plants that responds to variations in abiotic conditions. These conditions can influence the biosynthesis of secondary metabolites, inducing changes in the yield, metabolite profile, and metabolite concentration produced by the plant [43]. In addition, management techniques can be an alternative to promote an increase in secondary metabolites. However, a plant’s response to environmental factors and management varies according to the species, genotype, and metabolic route responsible for the production of secondary metabolites [43,44].
Thus, the cultivation of medicinal plants for the extraction of secondary metabolites requires knowledge of the genotype responses under different conditions, as the abiotic factors considered ideal for plant development may not be the same ones that promote an increase in secondary metabolites synthesis. It is important to emphasize that, under stress-induced conditions, the biosynthetic pathway can be stimulated, causing an increase in the production of these molecules by the plant. According to Haney and Kutscheid [45], the concentration of phytocannabinoids had a strong environmental control, and stress conditions (such as nutrient deficiency, competition with other plants, and reduced water availability) could increase phytocannabinoid production.
For centuries, humanity has explored such physiological adjustments in plants as a way to increase the biosynthesis of secondary metabolites, which are the source of pharmaceutically active ingredients used in the production of medications by the pharmaceutical industry or in the direct use of plants as herbal medicines to treat and/or cure diseases [44]. Biotechnological tools (such as omics-based methods) can contribute to the understanding of the biosynthesis of these compounds in plants cultivated under different stimuli, in addition to assisting in the development of varieties with specific chemical characteristics [11,15].
Considering the high therapeutic potential of phytocannabinoids, the knowledge of how management and abiotic factors influence the productivity of these molecules can help in the development of cultivation protocols that provide greater productivity. According to Danziger and Bernstein (2021) [46], secondary metabolite production in cannabis was very high compared to other plants. This crop presented up to 16% secondary metabolites in dry weight, equivalent to 3.2% of the fresh weight, when compared to 0.046% in roses, 0.4% in sage, 0.8% in sweet basil [46], and 0.1–2% in hops [47]. According to Bevan et al. (2021) [48], the inflorescence yield had significant correlations with the aboveground plant fresh weight, plant growth index, and root dry weight, which indicated that larger plant size could result in a higher inflorescence yield.
Despite the inflorescences having the highest concentration of cannabinoids, higher flower yield is not always related to higher yields of these compounds. When the final product is a secondary metabolite, this prediction requires greater caution, given the significant influence of the environment. There appears to be an inverse relationship between inflorescence and phytocannabinoid yield, with phytocannabinoid concentrations decreasing as the plant inflorescence yield increases [48], probably due to the dilution effect. Coffman and Gentner (1975) [49] observed that, as plant height increased, the concentration of THC in the leaf tissue generally decreased. However, other floral characteristics besides the inflorescence biomass (such as the number of flowers, length, width, uniformity, perimeter, and distribution) should be explored, as they can influence the final productivity [50].
It is important to know the maximum performance of cannabis genotypes cultivated under different environments to verify the interaction of genotypes and environments, as well as whether the applied management is conditioning an adequate yield. The production of secondary metabolites widely varies in their concentrations and phytochemical profiles, even within the same genotype cultivated under controlled conditions, as well as even between different seasons and cycles. In a study developed by Fischedick and Hazekamp (2010) [51], under strictly controlled indoor growing conditions and in a standard lot, an average variation was observed of 7.6% and 5.5% for phytocannabinoids and 11% and 4% for terpenoids in Bedrocan and Bedica cannabis plant varieties, respectively.
One main difficulty in the production of plant secondary metabolites is the standardization of these compounds. Herbal products can never be perfectly standardized for active component content; however, variability in chemical composition may also be a cause of concern for medicinal users [51] because the maintenance of the concentration and profile of secondary metabolites is important to maintain the stability of the treatment. The knowledge of how abiotic factors and management techniques influence the production of phytocannabinoids can help in the development of management protocols that provide greater productivity, quality and stability in the production of these compounds. Knowing the factors influencing the biosynthesis and formation of these compounds becomes essential to provide appropriate conditions enabling high yield.
Although genotype has a significant influence on phytocannabinoid production [26,51,52,53], its concentration is highly dependent on biotic and abiotic factors [54]. The majority of agronomic research conducted under field conditions has focused mostly on fiber production and yield [55,56]. Information that has been published on hemp production allows some parallels to be drawn. Despite being used for phytocannabinoid production [33,57], hemp is a field-grown crop that traditionally has been bred selectively for fiber or seed production, rather than for flower and secondary metabolite production [55,58]. However, despite fiber and seeds being the main products, a growing interest in the valorization of hemp phytocannabinoids has appeared [55,57], which can be extracted from the leaves and the inflorescences of plants cultivated for the production of fibers and seeds, although crop development for dual purposes initially would reduce the final concentration of cannabinoids or the fiber quality [57,59].
Cannabis can be grown in open fields (outdoor cultivation), in protected environments (such as greenhouses), or in controlled growing rooms (indoors) [21,60]. Field cultivation with or without a protected environment has been proved to be efficient in promoting increased phytocannabinoid yields, as demonstrated by García-Tejero et al. (2020) [61] in cannabis cultivation covered by a polyethylene macrotunnel. The main advantage of outdoor cultivation is the reduction in production costs, as indoor cultivation requires artificial lighting, ventilation, and temperature control, making the costs quite high for both implementation and maintenance. In protected cultivation, it is possible to produce up to six harvests per year and to scale them up [39,62], making it possible to obtain fresh material throughout the year [41]. In comparison, outdoor cultivation allows only one or two harvests per year, depending on the environmental conditions and genotype [16]. In addition, the greater stability of the chemical profile provided by controlling environmental conditions is an important advantage of protected cultivation, and this fact should be considered when the main product to be obtained is the phytocannabinoids [21].
Most commercial production is carried out in greenhouses or growth chambers with supplementary or single-source electric lighting [41,63,64]. Even though greenhouses allow the use of natural light, complementary lighting is required due to the plants’ photosensitivity, although electricity consumption is still significantly lower when compared to the opaque structures used for indoor cultivation [40]. In equatorial and tropical countries such as Brazil, the demand for artificial lighting is lower due to the greater natural light incidence, an essential factor for reducing production costs. In fact, 80% of the arable land in Brazil is suitable for producing this crop [65]. When this crop is developed under controlled conditions, it can be carried out in hydroponic cultivation, in pots of different sizes, and to a lesser extent, directly in the soil. In this case, during crop development, it is common to transplant into larger containers. The change to larger containers depends on the final size of the plant; when the objective is to produce smaller plants, cultivation is directly in the final container (<3 gallons) [66].
Among the techniques applied by industries and growers are, stand out pruning, defoliation, the use of techniques such as Sea of Green (SoG) or Screen of Green (ScroG), light supplementation, transplanting plants to larger containers, increasing CO2 and others. In addition, knowledge of basic factors related to management, such as water availability, mineral nutrition, lighting, temperature, spacing, and substrate, helps in the development of management protocols that promote the maximum productive performance of the genotypes.
The scarcity of basic information curbs the development of high-quality, standardized production for the growing of cannabis for biomedical purposes [2]. In this agreement, few studies have been focused on phytocannabinoid production, and hence, evaluating the effects of external drivers and management techniques on phytocannabinoid production is essential for understanding those agronomic treatments and methodologies that can be applied to suit the final crop and products [67].
Several books on cannabis crop management techniques have been published. However, it is important to emphasize that many of the techniques described are based on constructions of common knowledge, lacking systematic studies to prove their efficiency and efficacy. Producers have access to internet-based horticulture guides and information online, but most of this information is not based on scientific research [68].
The techniques known as Sea of Green (SoG) and Screen of Green (ScroG) are reported in several cultivation manuals and books and are commonly applied to increase yield [69]. The Screen of Green technique, also known by the acronym ScroG, is widely used by large companies and consists of using a screen on the surface of the plants to support the branches, allowing the formation of a uniform canopy. As the plants grow, the branches are guided through the screen to form a uniform cover. Flowering is induced after the shoots cover the entire screen [69]. This technique allows more uniform light distribution among the canopy of the plants since the tendency is for the lower branches to receive less incident light [69,70]. Therefore, these branches may have lower phytocannabinoid concentrations [71].
The Sea of Green technique, or SoG, consists of a higher dense cultivation [69,72] that aims to produce as many plants as possible per square meter, so small pots are used with greater depth and less width. The objective of this technique is to accelerate the vegetative phase and induce early flowering. As soon as the clones reach the desired height, they are induced to bloom [69]. The association between SoG and pruning (removal of side shoots) may favor the automatization of the harvest process (e.g., using electric tumbler blade trimmers). In this case, a lower yield per plant can be overcome by a higher yield per area [72]. The main differences between these techniques are the use of the screen, the duration of the vegetative cycle (which is longer for the ScroG technique), and the number of plants grown per area. In ScroG, larger spacing is used because the objective is to induce the formation of lateral branches [69]. However, there is practically no research that has evaluated the development of the plants and the impact on their productivity when using these techniques.
3.1. Pruning
Crop management techniques that alter the morphology of plants are commonly applied in the cultivation of cannabis, such as pruning the main stem once, twice, or even three times; the removal of leaves, which can range from the removal of old leaves up to total defoliation; the removal of branches; the use of trellises for conduction (such as the ScroG technique); or a combination of all the previously described techniques [46]. Few studies have evaluated the influence of pruning on the yield of secondary metabolites. In general, the main objectives of pruning are to promote greater development of the branches and limit the plants’ sizes [40], but it also provides other benefits (Figure 3). Pruning induces hormonal changes in sink-to-source ratios that explain the physiological and metabolic changes, alter the morphology of the plant, and consequently have an impact on microclimatic conditions [46,73].
Figure 3.
Main pruning techniques applied in cannabis cultivation (cover pruning, defoliation and branch removal) with an approach to the benefits and possible effects on productivity.
In cannabis management, it is common to perform a cover pruning (known as topping) or removal of the apical meristem (known as fimming) to increase the inflorescence yield per plant [74]. In these processes, the upper apex of the stem is removed or damaged to break the apical dominance [75]. The difference between the two techniques is the length of the pruned branch [74]. The removal of the apical meristem changes hormone balances (e.g., auxin and cytokinin) in the plant, stimulating the development of side shoots by the relieving of apical dominance [72]. The removal of all the leaves and secondary branches from the bottom third of the plant is a pruning technique widely applied by growers and is known as ’lollipopping’. This technique aims at directing the plant resources to the top inflorescences and improving the air circulation as an aid to pest management [46,76].
Folina et al. (2020) [75] observed in two European hemp cultivars (‘Fedora 23’ and ‘Futura 75’) an increase in CBD (22.7% and 18.1%, respectively) and inflorescence yields (24.5% and 12.9%, respectively) in plants that were pruned by topping. It was also observed in other research that topped plants produced significantly more inflorescence biomass (13.5%) than unpruned plants, but the total CBD concentration was not significantly affected [72]. Another study showed that pruning (topping) did not influence the productivity of flowers and phytocannabinoids [77]; however, this response may have been due to the high density of plants cultivated per area, which makes the branching of pruned plants difficult.
Danziger and Bernstein (2021) [73] investigated the effects of eight different plant architecture modulations in a ‘Topaz’ cultivar containing high CBD (8–16%) and low THC (<1%) levels. According to the results, among the eight treatments, defoliation (performed three weeks before harvest) and the removal of the branches and leaves from one-third of the plant (at the transition to short-day) promoted an increase in the CBDA, THCA, CBDVA, and THCVA concentrations in relation to the controls. However, double pruning (pruning the plants twice in the vegetative stage) was the treatment that promoted the greatest increase in inflorescence yields and in phytocannabinoid concentrations (CBDA, CBDVA, THCA, THCVA, and CBCA were about 59, 61, 50, 53, and 59%, respectively) [73]. The higher light incidence inside the canopy may have contributed to the increase in yield, as the authors observed that the double pruning technique promoted an increase in the light intensity at different heights along the plants. The technique known as ’lollipopping’ did not influence the productivity of flowers or phytocannabinoids [73].
In another study, Danziger and Bernstein (2021) [46] evaluated how eight plant architecture modulation treatments involving leaf removal (defoliation) or pruning (topping or branch removals) affected the phytocannabinoid profiles and spatial standardization in plants of two cannabis cultivars, ‘Fuji’ and ‘Himalaya’. Both cultivars were high THC (10–16%) and low CBD (<0.1%) producers. The responses in relation to the technique applied and the phytocannabinoid productivity varied according to the variety and the evaluated phytocannabinoid. For example, the production of CBDVA increased with the application of single pruning in the ‘Fugi’ variety, while it decreased in the ‘Himalaya’ variety [46]. However, the changes in phytocannabinoid concentrations and inflorescence yields were small (except for the ‘1º branch removal’ treatment, which greatly reduced both parameters). When compared to the other treatments, the ‘1º branch removal’ treatment induced a decrease in the productivity of CBDVA, CBDA, THCA, CBGA, THCVA, THC, and CBC in both varieties. The ‘lollipopping’ technique promoted a slight increase in the production of CBDVA in the ‘Fugi’ variety and CBCA in the ‘Himalaya’ variety. The pruning techniques evaluated in this study were not recommended to promote increased flower productivity, as both varieties showed high flower productivity in the control treatment (unpruned plants) [46].
The choice of pruning technique should consider whether the objective is to increase yield, modify the microclimate, or promote greater uniformity in the production of phytocannabinoids in the plant. Thus, it is important to assess whether the operational cost (such as labor, tools, and materials) increases yield at a level that brings financial return or promotes greater final quality due to the increased uniformity in the concentration of the phytocannabinoids.
3.2. Effects of Light
Both in general and in the horticultural industry, growers use different radiation spectra and intensities to manipulate plant morphology, flowering, and secondary metabolite production [78]. Therefore, studies evaluating the effects of radiation on different genotypes are essential to provide a suitable environment for growth, ensuring the best genotype performance and avoiding the superfluous use of electricity for artificial lighting while still obtaining high yields.
The modification of the light spectrum during the development of cannabis is a widespread technique among growers that has been shown as an alternative to manipulate the production of phytocannabinoids [79,80,81]. Table 1 summarizes the most relevant studies in relation to the effects, advantages, and disadvantages of different sources of light and radiation for phytocannabinoid production.

Table 1.
The most relevant studies in relation to the effects, advantages, and disadvantages of different sources of light and radiation for phytocannabinoid production (LI: light intensity).
Research has shown that photosynthetically active radiation (PAR) can be a key modulator of biomass and phytocannabinoid production, suggesting a responsive photochemical machinery [88]. However, there is little information concerning the mechanisms involved. Khajuria et al. (2020) [88] observed through chlorophyll fluorescence that genotypes with higher CBD than THC contents provided better protection to the photosynthetic machinery with the potential to tolerate light stress conditions. High THC content had a strong negative correlation with the photochemical efficiency of PSII and reduced the values of nonphotochemical quenching (NPQ) dependent on zeaxanthin. NPQ is a protective mechanism responsible for dissipating excess energy from the photochemical machinery, protecting it from photoinhibition. According to the authors, chlorophyll fluorescence could be used as a quick tool for high-throughput cannabis-screening assays based on phytocannabinoid content [88].
For the cultivation of cannabis under protected or indoor environments, it is common to use artificial lighting to supply or supplement the light energy demand. Several lamp types are used, and the most commonly used ones are high-pressure sodium (HPS) lamps, light-emitting diodes (LEDs) [86], and fluorescent lamps [69,79].
HPS lamps are predominantly within the yellow spectrum, with low emissions in the blue and UV regions and low red/far-red ratios, allowing an excessive lengthening of the stem (etiolation) and, thus, reducing THC production [78]. HPS lamps emit high levels of radiation in the green region, negatively affecting THC production [78,79,89]. About 90% of cannabis growers in Canada for cannabinoid production use HPS lamps [90] because they have a low upfront cost and high photon output [91].
LED usage in horticulture has expanded in the last decade due to the advantages they offer over conventional light sources, such as greater energy efficiency, lower heat emission, and longer lifetimes [92,93]. An advantage of LEDs is their high efficacy and low electrical (operating) costs compared to HPS lamps [91,93]. LED lamps allow the supply of specific wavelengths, facilitating research on the impact of wavelength on secondary metabolite production [94], but there is relatively little information available about different radiation intensity and quality effects on cannabis secondary metabolite composition [92], and some of the results are contradictory (for example, the influence of blue light on productivity).
Westmoreland et al. (2021) [91] investigated the effect of the blue photon fraction using different ranges (the lowest fraction of blue photons was 4% from HPS lamps, and it increased to 9.8, 10.4, 16, and 20% for LEDs). The treatments did not influence the THC and CBD yields. According to the authors, as the percent of blue light increased from 4 to 20%, the flower yield decreased by 12%. This showed that flower yield increased by 0.77% per 1% decrease in blue photons, but the potential that changed in the fraction of other wavelengths could have contributed to the results [91].
Namdar et al. (2019) [92] evaluated the development and characteristics of two cannabis varieties, ‘CS12’ and ‘CS14’ (both strains producing high amounts of THCA), grown under fluorescent lamps in the vegetative phase and HPS lamps in the reproductive phase (standard growing conditions) compared to production when using LED lamps (blue:red ratio equal to 4:1). According to the authors, the plants that bloomed under LEDs accumulated much higher amounts of CBGA (a precursor of other phytocannabinoids), with a ratio of CBGA:THCA of 1:2. At the same time, those cultivated using HPS lamps showed a CBGA:THCA ratio of 1:16. The authors observed pronounced inhibition of flowering under LED light, with a decline of 40% in the total flower mass per plant as compared with HPS lamps [92]. However, the variation in these results may have been due to the difference in PAR among the treatments, which was 90 μmol m−2 s−1 for the LED lamps and 500 μmol m−2 s−1 for the HPS lamps, which explains the higher concentration of phytocannabinoids due to the lower production of flowers in the LED treatment (lower intensity).
Similar results were obtained by Danziger and Bernstein (2021) [80] regarding the increase in CBGA production. The authors also observed an increase in CBGA accumulation (up to 400%) in treatments with LED lamps (with the highest proportions of blue ≅ 47%) compared to HPS lamps (with lower proportions of blue ≅ 4.5%). In contrast, the concentrations of other phytocannabinoids (such as THCA, CDBA, and CBCA) decreased by up to 40% under LED culturing, exhibiting a specific metabolite response to light spectra and a potential for the inhibition of enzymatic activity under LED light [80]. Another study found a 2.5% increase in THC content in plants supplemented with LED light with a blue and redder spectrum [85]. However, this variation in THC production may have been caused by the higher light intensity compared to the control treatment.
Light supplementation at the bottom of the plant canopy with red–blue (R–B) and red–green–blue (RGB) spectra impacted the phytocannabinoid, terpene, and inflorescence yields [64]. Both treatments promoted changes in the metabolomic profiles of the plants, and higher flower yields than the control treatment (without additional light) were observed. Plants submitted to R–B supplementation showed greater homogeneity of phytocannabinoids in the lower and upper parts of the canopy, and plants under the RGB treatment showed greater changes in the terpene profiles. Future studies should be carried out to quantify the pools of phytocannabinoid precursors (isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP)) to better understand the influence of spectral quality on terpene and phytocannabinoid biosynthesis [64].
Magagnini et al. (2018) [79] evaluated the effects of three light sources (one HPS and two types of LED) with different radiation spectra and the same PAR (450 µmol m−2 s−1) on the morphology and the phytocannabinoid content of the ‘G-170’ cannabis variety (high THC content). According to the authors, the wavelengths in the blue and UV-A regions could positively affect THC and CBG synthesis [79]. Magagnini et al. (2018) [79] and Namdar et al. (2019) [92] both observed lower flower yields under LED lights than under HPS lamps. Namdar et al. (2019) [92] found a significant difference in the total phytocannabinoid content in plants grown with LEDs (>60% compared to HPS). Nevertheless, Magagnini et al. (2018) [79] did not observe variation in the total phytocannabinoid concentration. The results obtained in the two studies may have been related to LEDs of different spectra, different light intensities, or different plant genotypes.
Wei and colleagues [95] observed higher flower and CBD yields when using two types of LEDs than when using HPS lamps, but the LED light intensity was higher (540 and 552 μmol m−2 s−1) than the HPS intensity (191 μmol m−2 s−1). In another study, higher THC production was observed in plants grown under LED light than HPS lamps [96]. One of the difficulties in evaluating the influence of light quality is the need to standardize the light intensity and spectra (only varying the spectrum that is evaluated). This standardization is essential to evaluate the effect of the specific spectrum.
Islam et al. (2021) [81] observed variations in phytocannabinoid concentrations in a cannabis genotype grown under different light spectra. The THC concentration was higher in all the treatments than under natural light, while the CBD concentrations showed higher values under different spectra. The low PAR intensity of the treatments (300 µmol m−2 s−1, except for the treatment with natural light, which was not informed) may have influenced the synthesis of phytocannabinoids and the responses to different light spectra. According to the authors, green light played a significant role in the synthesis of CBD and CBDA, as the far-red and UV-A spectrum (along with green) played positive and negative roles in this process, respectively [81]. It is important to note that, in this study, the analyses of the phytocannabinoids were performed on the leaves during the vegetative phase.
There are some indications that UV-B radiation influences phytocannabinoid concentration [97]. According to Pate (1983) [97], plants tended to accumulate more THC at high-altitude locations due to higher UV-B radiation. Lydon et al. (1987) [82] evaluated the influence of UV-B radiation on net CO2 assimilation rate, growth, and phytocannabinoid production in two cannabis varieties with different chemical profiles. The authors observed that the production of THC was significantly higher in the variety with high THC. In contrast, for the variety that predominantly produced CBD, no modifications in the CBD levels were observed. Thus, a positive correlation between UV-B radiation and THC production was observed [82].
Recent works have evaluated the influence of UV light supplementation and have presented contradictory results regarding the production of phytocannabinoids. Rodriguez-Morrison and colleagues (2021) [83] evaluated the responses of two cannabis varieties, ‘Low Tide’ (LT) and ‘Breaking Wave’ (BW), grown under supplemental UV radiation. The authors observed that the total dry inflorescence yield derived from apical tissues decreased in both cultivars with increasing UV exposure levels, but the total dry inflorescence yield only decreased in LT. The apical inflorescence dry weight decreased linearly by 78% and 69% in LT and BW, respectively, from the lowest to highest UV levels. In another study, it was also observed that there was no effect in phytocannabinoid concentration and inflorescence yield in the genotype ‘Meridian’ (high THC content) with supplemental UV-A spectra [84]. However, Jenkins (2021) [85] observed a 4.1% increase in the THC concentration in two genotypes (‘Larry OG’ and ‘Super White’) and no changes in another genotype (‘Pootie Tang’). The UV supplementation decreased the inflorescence yield of ‘Larry OG’ [85]. Further research with different genotypes is required to determine if there is a combination of UV spectrum, intensity, and time of application that would bring beneficial commercial effects for cannabis production [83,84].
The effects of PAR levels ranging from 120 to 1800 μmol m−2 s−1 were evaluated by Rodriguez-Morrison and colleagues (2021) [86]. The authors observed that the dry inflorescence yield, the density of the apical inflorescence, and the harvest index increased linearly with increasing canopy-level PPFD of up to 1800 μmol m−2 s−1, while the leaf-level net CO2 assimilation rate saturated well below 1800 μmol m−2 s−1. Moreover, the inflorescence yield and apical inflorescence density increased linearly as the PAR levels increased from 120 to 1800 μmol m−2 s−1 [86]. These researchers did not observe effects on the production of any of the phytocannabinoids measured. Even under ambient CO2, the linear increases in yield and the net CO2 assimilation rate indicated that the availability of PAR was still limited with whole-canopy net CO2 assimilation rates at levels as high as 1800 μmol m−2 s−1 [78].
Other works have shown that the increase in PAR intensity is positively correlated with the net CO2 assimilation rate [98] and yield [84,90,99]. Llewellyn and colleagues (2021) [84] evaluated the effects of PAR intensities ranging from 350 to 1400 μmol m−2 s−1 on yield and potency in the ‘Meridian’ genotype (high THC content). The authors observed that the inflorescence yield increased linearly as the PAR intensity increased (Table 1). Eaves et al. (2020) [90] observed a direct linear correlation between increased yield and PAR up to 1500 μmol m−2 s−1. The correlation between the increase in PAR intensity and yield can be explained by the corresponding increase in the net CO2 assimilation rate due to the high PAR saturation point of cannabis, which corresponds to the PAR intensity at which the net CO2 assimilation rate begins to decrease. As observed by Chandra et al. (2015) [100], the net CO2 assimilation rate increased from 44% to 140% when exposed to 400–2000 µmol m−2 s−1 according to the evaluated genotype, confirming the high light saturation point of the plant.
Potter and Duncombe (2012) [99] observed that the average flower yields in the total radiation intensity regime of 600 W m−2, 400 W m−2, and 270 W m−2 were 0.9 g W−1, 1.2 g W−1, and 1.6 g W−1, respectively, with a higher yield at the lowest total radiation intensity. However, in the seven varieties evaluated, the total flower yield in the lowest total radiation intensity regime ranged from 280 to 470 g m−2 (average 422 g m−2). The ranges were 350–630 g m−2 (average 497 g m−2) and 470–630 g m−2 (average 544 g m−2) in greater intensity regimes [99]. With this study, it is possible to observe the relevance of the genotype as a response to management. Potter and Duncombe (2012) [99] also observed that a higher total radiation intensity caused an increase in the total THC content. However, it was due to the higher production of female flowers, which contain higher THC concentrations than the leaves.
In a study developed by Bouchard (2009) [101], an average of 0.5 g of flowers per watt was observed. However, this metric (installed wattage) has the advantage of negating the effects of different fixture efficacies (μmol J−1). Since photosynthesis is considered a quantum phenomenon, crop yield may be more appropriately related to incident or absorbed photons and integrated over the entire production cycle (i.e., light integral; mol m−2) in a yield metric that is analogous to the maximum quantum yield (QY: g mol−1) [86].
The effects of radiation quality on phytocannabinoid production were evaluated by Mahlberg and Hemphill (1983) [89] in a Mexican variety using colored filters to alter the radiation spectrum. The authors concluded that the THC content in the young leaves of plants grown in a shaded environment under solar radiation, both under red light and under blue light, did not differ significantly from the THC content of the control plants (grown under sunlight). However, the leaves of plants grown under green light contained significantly lower THC levels than those grown under full solar radiation [89].
Finally, in addition to light quality and intensity, photoperiod can influence flower and phytocannabinoid yields (Table 1) [67,87]. The number of days a plant remains under a long photoperiod influences the size and number of flowers, as well as phytocannabinoid yield [41,102,103]. There is a lack of scientific information to determine the number of days a plant should spend in long-day lighting to maximize yield [102]. According to a meta-analysis carried out by Dang et al. (2022) [102], the increases in floral biomass and phytocannabinoid productivity responded differently according to the duration of the vegetative phase (long photoperiod). Floral productivity and the duration of the vegetative phase showed a negative linear relationship, suggesting that shorter vegetative growth periods are preferable to increase flower productivity. In contrast, THC and CBD showed a negative quadratic relationship with long day length durations, suggesting that intermediate periods of vegetative growth maximize phytocannabinoid concentrations [102]. This information is important because it can be used to define management methods, such as ScroG and SoG techniques, depending on the purpose of the crop (flower or phytocannabinoid).
3.3. Plant Density
Plant density can affect the final yields of different crops. When cannabis cultivation is for phytocannabinoid production, it is desirable to lower densities, easing the plants’ lateral branch development and increasing the number of leaves and lateral flowering [55]. Toonen et al. (2006) [62] evaluated 77 indoor plantations (intended for inflorescence production) in the Netherlands and observed an average of 15 plants m−2. The plant density used for inflorescence production is highly variable. Studies have reported an average of 10 to 20 plants m−2 [104], although it is also common to cultivate only one or two plants m−2 [105]. It is important to consider that small and nondense plants support homogenous light distribution and less microclimatic variations within the crop and, consequently, allow greater stability in the concentration of phytocannabinoids in the plant (less variation between the apical and basal parts of the plant) [80].
Vanhove and colleagues (2021) [63] evaluated density (16 or 20 plants m−2) and light intensity (400 W m−2 or 600 W m−2) on the yields of four cannabis genotypes and observed that the yield per plant of all the genotypes was higher at a density of 16 plants m−2. However, there was no significant difference in yields between the two densities when this yield was expressed m−2 [63]. This finding indicated that light interception is a determining factor of yield. The lower density of plants provided greater light absorption throughout the canopy, promoting greater net CO2 assimilation rates and yields. However, only certain genotypes (those with a plant structure based on pronounced lateral shoot development) showed increased flower yield when subjected to higher light intensity [63].
Vanhove et al. (2012) [106] also found no significant difference in flower yield per area (g m−2) at different densities. However, they observed higher flower yield per plant at a lower density (12 plants m−2) compared to a higher density (16 plants m−2), corroborating the results obtained by Vanhove et al. (2011) [63] and Benevenute et al. (2021) [107]. According to the results of Vanhove et al. (2012) [106], the yield m−2 did not differ significantly between different plant densities, but significant differences did occur between different varieties. Benevenute et al. (2021) [107] observed that the phytocannabinoid concentration in flowers was not affected by plant density, and increasing the plant density decreased the flower yield per plant but increased the total flower (per area) and CBD yields.
García-Tejero and colleagues (2019) [108] evaluated the effect of plant density (33,333, 16,667, and 11,111 plants ha−1) on the yields of two hemp varieties (‘Ermes’ and ‘Carma’). The highest density (33,333 plants ha−1) promoted greater yields of CBG and CBD in the ‘Carma’ variety. In comparison, the ‘Ermes’ variety showed greater production of CBG with intermediate spacing (16,667 plants ha−1) and CBD in both the denser- and the higher-spaced regimes [108]. Additionally, García-Tejero et al. (2020) [61] evaluated both the phytocannabinoid and dry matter yields of three cannabis varieties with CBD chemotypes (‘Sara’, ‘Pilar’, and ‘Theresa’) and two with CBG chemotypes (‘Aida’ and ‘Juani’) grown at three densities (PD1, 9.777; PD2, 7.333; and PD3, 5.866 plants ha−1). These authors concluded the importance of the genotypes, as well as the advantages of PD1, to obtain the highest total phytocannabinoid yield due to the larger number of plants per hectare and the capacity of the plants to produce side branches under these conditions [61]. Backer et al. (2019) [104] carried out a meta-analysis study of factors determining cannabis yield. The authors observed that increasing plant density reduced yield per watt and THC per square meter. According to the authors, plant density was not an effective predictor of yield per square meter [104].
Taking into consideration the diversity of the information, more research is needed to evaluate the influences of plant density and plant architecture on the cultivation of inflorescence and phytocannabinoid production. The development of research to determine the impact of plant density on the productivity of phytocannabinoids and flowers is important, but it is also necessary to evaluate the influence on the duration of the production cycle of different varieties to know the costs involved in maintaining plants with longer cycles (generally less densely cultivated) because the increase in productivity must compensate for the costs involved in production.
3.4. Ontogeny
Ontogeny is the entire sequence of events involved in the development of an organism. It starts from the seed and goes through different developmental stages, such as the seedling stage, vegetative stage, and reproductive stage, and ends in the senescence stage [74]. Chemical and physiological plant variability are key factors in regulating secondary metabolite production related to medicinal product standardization that have not been studied as of yet. This variation can be regulated both in the organs and during development at the ontological level [71]. The concentration of phytocannabinoids changes with the development of the plant [28,30,53,109,110,111,112,113]. Table 2 summarizes the most relevant studies in relation to ontogeny cannabis research and the results about the influence of phytocannabinoid production.

Table 2.
Nonexhaustive summary about the most relevant studies in relation to the effects of ontogeny on phytocannabinoid production.
In 1971, two research projects were developed to evaluate the concentrations of phytocannabinoids in different plant organs [25,26]. According Fetterman and collaborators, all the hemp plant parts contained phytocannabinoids, and they showed that the decrease in THC content in different parts of the plants followed the order of bracts, flowers, leaves, smaller stems, larger stems, roots, and seeds [26], with similar results in other works [27,113]. Another study confirmed that bracts were the organ with the highest concentration of phytocannabinoids [52]. Ohlsson and collaborators reported that phytocannabinoids were most abundant in flowering tops and the young, small leaves surrounding the flowers [25]. Other research reported a lack of detectable levels of phytocannabinoids in the seeds (intact or crushed) and in pollen grains [116]. According to Jin et al. (2020) [117], the phytocannabinoid content decreased from inflorescences to leaves, stem bark, and roots.
Guiroud et al. [34] investigated the evolution of the phytocannabinoid content during the growth of seven varieties of different chemotypes (based on the CBDA/THCA ratio): three of each from chemotype I (THCA/CBDA ratio: >1.0) and chemotype III (THCA/CBDA ratio: 0.5–2.0), and one from chemotype II (THCA/CBDA ratio: <1.0). The authors observed that the plants from chemotypes II and III needed more time to reach peak production of THCA and CBDA than chemotype I (ninth flowering week). Chemotype III plants synthetized CBDA continuously until the end of the experiment with eleven weeks of flowering, while CBGA reached a maximum concentration around five weeks of flowering and decreased afterwards [53]. Similarly, Massuela et al. (2022) [72] also observed an increase in CBDA at a later harvest time in a chemotype III genotype. The harvest time was statistically significant for the biomass of inflorescence and the total CBDA concentration [72]. According to these authors, the ideal harvest time for the evaluated genotype (high CBD content) was around nine weeks of flowering; however, after this period (eleventh week), a slight increase in CBD concentration was observed. Thus, it is important to carry out more studies that evaluate the production of phytocannabinoids throughout the development of the plant until the period of decline in phytocannabinoid production.
Stack et al. (2021) [110] observed the rapid accumulation of phytocannabinoids in different varieties about 3.5 weeks after the onset of flowering. Hammami et al. (2021) [59] evaluated the phytocannabinoid production of 12 varieties (intended for fiber or grain production) at different stages of the reproductive phase and observed that the synthesis of these compounds varied among the genotype and during cannabis ontogenic development. The phytocannabinoid content in glandular trichomes varies with trichome age, trichome type, and trichome location on the plant [118,119].
Indeed, the concentration of phytocannabinoids varies during the reproductive phase [37,58], and there may be a relationship between the chemotype and the sensitivity of the genotype to the photoperiod [120]. In agreement, Yang et al. (2020) [120] found that the maximum concentration of CBG in three photoperiod-sensitive genotypes was highest in the fourth week of flowering and subsequently decreased. In the photoperiod-neutral genotypes, the maximum concentration of CBG was in the second and third weeks of flowering, and after this production peak, a significant drop in CBG production was observed, but in the twelfth week, the contraction returned to the maximum value. These authors also observed that, in the sixth week, all the genotypes showed maximum concentrations of CBDA and THCA, but the photoperiod-sensitive genotypes showed higher total phytocannabinoid concentrations in the second week of flowering, while the maximum total phytocannabinoid concentration in the photoperiod-neutral varieties was in the eighth and ninth weeks of flowering.
Although the reproductive phase is characterized by the highest production of phytocannabinoids, these compounds are also produced in lower concentrations in leaves during the vegetative phase [60,81] and in seedlings [121]. It is important to consider that each phytocannabinoid presents a dynamic according to the development of the plant due to the biosynthesis mechanism involved in the production and the speed of degradation of each compound. Although the concentration of phytocannabinoids varies during plant development, the CBD/THC ratio (related to the chemotype of the plant) remains stable, given that it is a characteristic of greater genetic control [122]. However, although there are reports that the CBD/THC ratio changes during ontogeny, this change does not alter the chemotype of the plant. [120].
Since the 1970s, many studies have been published that show the difference in the concentration of phytocannabinoids between the apical and basal parts of the plant so that the apical part tends to accumulate higher concentrations of certain phytocannabinoids, such as THC [71,119,123], with recent studies confirming this assumption [30,61,71,111,115,124,125]. Bernstein et al. (2019) [71] observed that the THC content in the flowers closest to the apex was 12% compared to only 6% in the flowers located lower (Table 2). For CBD, CBG, and CBC, the increase was three times higher from the base to the top of the plant. The THC and CBD concentration levels in leaves close to inflorescences were about half of those found in flowers from the median and basal parts, while the THC, CBD, THCV, CBG, and CBN concentrations in fully developed leaves represented about 10% of those found in the flowers.
The light interception differences of the leaves are partly attributable to the intercanopy attenuation of PAR from self-shading, which may have influenced the variations in phytocannabinoid concentrations observed by Bernstein et al. (2019) [71]. The leaves located at the bottom of the plant receive lower radiation intensities than the upper leaves, and this difference in PAR interception influences the net CO2 assimilation rate. This parameter tends to diminish on the lower leaves compared to the upper ones (more exposed to light), as observed by Danziger and Bernstein (2021) [46] in a medical variety and Bauerle et al. (2020) [126] in a hemp variety. Hawley et al. (2014) [64] supported this hypothesis since they observed greater homogeneity in the phytocannabinoid and terpene profiles in the upper and lower canopy parts of plants subjected to light supplementation. Danziger and Bernstein (2021) [46] also observed that defoliation provided greater uniformity in phytocannabinoid content in the upper and lower canopy parts of plants, which could be the result of increased light uniformity due to penetration to the bottom parts of the plants.
In addition, different factors can influence the concentrations of phytocannabinoids in different organs of the plant. For example, Shiponi and Bernstein (2021) [115] observed that the availability of phosphorus (P) influenced the concentration of phytocannabinoids in apical and basal inflorescences. Bernstein et al. (2019) [114] also observed that nutritional supplementation influenced the concentrations of phytocannabinoids in different plant organs and locations along the plant height. Danziger and Bernstein (2021) [80] observed that different light spectra influenced the biomass of the plant organs and phytocannabinoid concentrations in top inflorescences and bottom inflorescences when observing three medical cannabis varieties (‘CS10’, ‘CS12’, and ‘CS14’). However, similar phytocannabinoid concentrations were seen in the two different parts. These studies have demonstrated the potential of management techniques (such as nutritional availability and light quality) for regulating the concentrations of specific secondary metabolites in defined parts in the cannabis plant.
3.5. Temperature, Altitude and CO2 Concentration
The optimum temperature for cannabis development ranges between 25–30 °C, depending on the plant’s genotype [21]. One study evaluated wild plants collected from two regions in northern India with different altitudes and temperatures [127]. It concluded that plants grown in drier regions (higher altitude and temperature) presented an increased density and augmented trichomes. However, the author stated that these variations may also be related to genotype differences [117].
Environmental conditions influenced by altitude have been proved to be important factors inducing variations in the secondary metabolite composition of cannabis inflorescences. Giupponi et al. (2020) [128] evaluated the influence of altitude on the productivity of secondary metabolites in the inflorescences of four cannabis genotypes. The authors noted that, in plants cultivated at high altitudes, the production of terpenes was significantly higher, and increases of 46, 36, and 39% in the concentrations of CBDA, CBCA, and CBGA, respectively, were observed. In contrast, Bruci et al. (2012) [30] observed that plants grown in lower-altitude regions in Albania showed higher THC production.
According to Paris et al. (1975) [129], temperature and relative air humidity significantly influenced THC content. The authors observed that the THC content was 30 times higher with increased temperature (22 °C and 24 °C) and reduced relative humidity (50% compared to 80%). In this experiment, the humidity variation was considerably higher than the temperature variation, leading to a greater influence of relative humidity on the THC content.
The few studies relating the influence of temperature to phytocannabinoid production are conflicting. Whilst one study showed that higher temperatures (32° C vs. 22 °C) increased phytocannabinoid content [54,130], other works have shown that phytocannabinoid content decreases with increasing temperature (32 °C vs. 23 °C) [54,130,131]. Indeed, temperature can influence the production of phytocannabinoids, and lower temperatures can delay the time to flowering, but the response varies depending on genetics and other environmental conditions, such as photoperiod [67].
Bazzaz and colleagues (1975) [131] evaluated phytocannabinoid production in varieties from temperate and tropical climates grown at low (23 °C day and 16 °C night) and high (32 °C day and 23 °C night) temperatures. The authors observed higher THC yields under low-temperature conditions. However, the same varieties grown in both environments showed wide qualitative variation (CBD and THC) in the phytocannabinoid composition. This variation may be due to using seeds collected for the study, probably with no genetic stability, so it would have been more appropriate to employ clones obtained through vegetative propagation. Therefore, this type of stress response is likely to be more complex, involving several factors and varying according to the genetics of the plants.
Chandra et al. [132] evaluated gas exchange in seven cannabis varieties (different chemotypes) subjected to different temperatures. The maximum net CO2 assimilation rate for each variety ranged from 25 °C to 35 °C, where the varieties with the highest net CO2 assimilation rates had superior chlorophyll content. It was also found that the relative THC content was related to the carotenoid content so that the varieties with high THC contents presented higher carotenoid contents than those varieties with a low capacity for THC synthesis. High temperature conditions above 35 °C could cause a decrease in the net CO2 assimilation rate [9,132] due to reduced stomatal conductance.
Although some authors reported that the net CO2 assimilation rate of cannabis was limited at temperatures above 35 °C [132], research comparing plant development under varying temperatures can shed light on how different genetic characteristics result in physiologically different responses to these conditions and provide concrete data on the flower and the influence on phytocannabinoid yield.
Despite the lack of research evaluating the influence of CO2 supplementation on cannabinoid productivity, this is a relevant issue considering that yield and plant growth always reflect an interplay between different factors, such as light, temperature, nutrients, and CO2 concentration [64]. A high CO2 concentration increases net CO2 assimilation and accelerates plant growth, with the potential to increase yield [98]. Plants grown under ideal conditions and at higher CO2 concentrations may show a 50% increase in net CO2 assimilation rate compared to plants grown under normal CO2 concentration [133].
In this species, an increase in the CO2 concentration in a specific area around the leaf can cause gains in net CO2 assimilation rate [98]. Chandra et al. (2008) [98] evaluated the photosynthetic response of a specific leaf area of a cannabis variety under different temperatures, PAR intensities, and CO2 concentrations through a portable photosynthesis system. The highest net CO2 assimilation rate was verified at 30 °C and a PAR of 1500 μmol m−2 s−1. In general, the effect of light intensity on photosynthetic carbon assimilation was more prominent than temperature [134]. The net CO2 assimilation rate decreased by about 50% when the evaluations were carried out at 250 μmol mol−1 compared with the higher CO2 environmental concentration (350 μmol mol−1) [134]. A 50% increase in the net CO2 assimilation rate at a CO2 concentration of 750 μmol mol−1 was also observed. The best performance was observed under conditions of high PAR for temperatures between 25 to 30 °C, with high potential for increased growth and yield in environments enriched with CO2 [98]. In this study, only one variety was evaluated, being relevant to evaluate other genotypes under these conditions and the impact of these variables on the flowers and the phytocannabinoid yield.
CO2 supplementation should be performed under conditions of high light intensity in which the plant reaches the point of light saturation. In this agreement, cannabis has a high light saturation point (≅1500 mol μmol m−2 s−1) [86,98], that is, a high light intensity is necessary to reach the maximum CO2 assimilation rate. However, a higher CO2 assimilation rate does not always reflect an increase in yield because the source–sink balance determines whether this type of management causes an increase in yield, as the photoassimilates can be directed to the production of roots, stems, or leaves instead of to flowers and phytocannabinoids.
An increase in CO2 concentration can also influence secondary metabolite production in plant species such as peppers (Capsicum chinense Jacq.), with increased levels of capsaicin, or reductions in citric acid in strawberries (Fragaria × ananassa Dutch. ‘Camarosa’) and polyphenols in grape (Vitis vinifera L.’ Red Tempranillo’) [135]. Thus, research must be conducted to test the hypothesis that carbon fertilization can be used to manipulate the chemical profile of cannabis, promoting greater yields.
3.6. Nutrition and Substrate
An understanding of the nutritional requirements and the physiological and morphological responses of cultivars to mineral nutrition can improve nutritional management for adequate nutrient supply [136], promoting increased phytocannabinoid and flower yields. There is some evidence supporting the influence of mineral nutrition on phytocannabinoid production [137]. While some studies report that an increase in macronutrient mineral content could result in an increase in inflorescence and phytocannabinoid yields [115], others have reported that the increased availability of NPK decreased THC and other phytocannabinoids in inflorescences [68,114,115,125] and leaves [114,115] In fact, nutritional availability influences phytocannabinoid production with specific effects on organs and compounds [114,115].
The first study that evaluated the influence of nitrogen on the production of phytocannabinoids presented contradictory results. Coffman and Gentner (1977) [137] observed that N supply in the range of 0–125 mg L−1 N did not affect the THC content in inflorescences. On the other hand, results obtained by Bócsa et al. (1997) [138] showed that the THC content in leaves declined in response to a high-nitrogen regime. However, the difference in these results may have been related to the availability of other nutrients, plant genotypes, ontogeny, and environmental conditions. De Prato et al. (2022) [67] observed that plants grown at the lowest (50 N kg ha−1) and highest (150 N kg ha−1) N rates produced higher concentrations of THC than the mid-level N plants (100 N kg ha−1), but a significant difference was found between the varieties.
More recently, in another study, de Prato et al. (2022) [139] observed differences in the concentrations of phytocannabinoids in a variety of industrial hemp (‘ECO-GH15’) cultivated with different doses of K (11, 43, and 129 ppm) with the conventional, fast-release potassium K of potassium sulphate (K2SO4) and a slow-release form (131 ppm of K) containing soil microbes (SRK). The authors observed a significant increase in Δ9-THC, Δ8-THC, and THCA concentrations in the SRK treatment compared with the three conventional K treatments. CBD and CBDV showed an opposite pattern, being smaller in plants receiving SRK than the conventional K treatments, with the highest level (129 ppm) of application resulted in the highest concentration of CBDV.
Excessive fertilization during the growing season can result in a decrease in THC concentration and flower yield [115,140]. During the vegetative phase and when using two organic substrates based on coconut fiber, it was suggested that 389 mg of N L−1 could be considered adequate via the application of liquid organic fertilizer (4.0 N:1.3 P:1.7 K) [140]. During the reproductive phase, the ideal rate of this organic fertilizer was around 225 mg of N L−1 and ranged according to the substrate characteristics [68]. According to Caplan et al. (2017) [68], organic fertilizer rate increasing from 57 to 283 mg of N L−1 (2.0 N:0.87 P:3.3 K) increased the inflorescence biomass by more than 100% in the ‘WP:Med (Wappa)’ variety. However, it caused a decrease in the THC, THCA, and CBGA concentrations in the flowers probably due to the dilution effect.
Saloner and Bernstein (2020) [125] evaluated the physiological response of an ‘Annapurna’ cultivar (containing about 7% THC and 7% CBD) during the vegetative phase that was submitted to different nitrogen levels (30, 80, 160, 240, and 320 mg of N L−1). The authors reported that a physiological response considered as adequate was achieved at 160 mg L−1. When using 30 mg L−1, there was a decrease in the net CO2 assimilation rate and plant biomass with visual symptoms of nutritional deficiency. In the reproductive phase, the concentrations of THCA, CBDA, THCVA, CBGA, and CBCA, decreased significantly in the inflorescences with the increase in N supply, and the concentrations of THCA and CBDA decreased by 69% and 63%, respectively, with increased N supply from 30 to 320 mg L−1 N so that greater phytocannabinoid yields were under lower concentrations (30 mg L−1 N) [125]. Phytocannabinoid concentrations in the inflorescence leaves were generally not altered by N supply, but the inflorescence yield increased with the increase in N supply up to 160 mg L−1 N and was not affected by the increased availability of N [125]. According to Bevan et al. (2021) [48], the inflorescence yield responded to N best in the range of 160–230 mg L−1. Caplan et al. (2017) [68] and Saloner and Bernstein (2020) [125] reported contrasting optimal values. This response was probably due to differences in other nutrient concentrations and the response associated with the genotype evaluated in each study.
It is important to consider that the rations of ammonium (NH4−) and nitrate (NO3−) is a factor determining the impact of N nutrition on plant function and metabolic responses [141]. Indeed, the ratios of NH4/NO3 influences primary and secondary metabolism and inflorescence productivity in cannabis. The highest inflorescence yield was obtained under sole NO3 supply but ratios of 10–30% NH4+ (20–60 ppm NH4+) did not substantially impair cannabinoids yield but produce smaller inflorescences and lower inflorescence yield compared with only NO3 nutrition [141].
Recently, Bernstein et al. (2019) [114] reported that P and NPK supplementation did not alter the THC or CBD levels in flowers. However, these nutrients caused the THC and CBD contents to decrease in leaves close to inflorescences. The opposite effect was observed for CBG so that the treatments with +P and +NPK did not alter the CBG content in inflorescence leaves but caused a 70% increase in the CBG in flowers. Supplementation with humic acid decreased the contents of most of the evaluated phytocannabinoids. In general, except for increased CBG, in the treatment with NPK supplementation (80% increase when compared to controls), the additions of N, P, and NPK caused reductions in the phytocannabinoid content [114].
Shiponi and Bernstein (2021) [115] evaluated two genotypes, ‘Royal Medic’ (balanced THC and CBD) and ‘Desert Queen’ (high THC and low CBD), grown under five P concentrations containing 5, 15, 30, 60, and 90 mg L−1 (ppm). The phytocannabinoid concentrations decreased linearly with increasing inflorescence yield so that P supplies higher than 5 mg L−1 reduced THCA and CBDA concentrations in the inflorescences by up to 25%, consistent with a yield dilution effect, but the total phytocannabinoid content per plant increased with increasing P supply. These results revealed contrasting trends regarding the effects of P supply on phytocannabinoid concentration, which were highest under 30 mg L−1 P, while the inflorescence biomass was highest under 30–90 mg L−1 P. This value was within the range estimated (40–80 mg L−1 P) by Bevan et al. (2021) [48]. These results demonstrated that the effect of P availability on the phytocannabinoid profile may be genotype-specific. More research needs to be conducted on specific genotypic responses to P addition above the optimal dosage [115]. Another study suggested a wide optimum range for P at the vegetative growth stage, with a minimum requirement of 15 mg L−1 P and a recommended application of 30 mg L−1 [142].
The effects of different potassium levels (15, 60, 100, 175, and 240 ppm K) on two cannabis varieties (‘Royal Medic’: RM and ‘Desert Queen’: DQ), which showed different responses [2]. Growth reduction under low K availability (15 ppm) was mainly due to the impact of this nutrient on water tissue relations and transpiration of the RM variety and on water relations and carbon fixation of the DQ variety. A 100 µL L−1 concentration of K promoted a higher net CO2 assimilation rate in the DG cultivar. The RM variety showed no differences in the net CO2 assimilation rate when the K supply was above 15 µL L−1. The variability inflorescence production under K availability in the responses of 60-240 mg L−1 K did not differ substantially but decreased under 15 mg L−1 K [143]. The cannabinoid content decreased with the elevation of K supply, and considering that no beneficial effects were observed for the elevation of K supply from 60 to 175 mg L−1, 60 mg L−1 K was considered the optimal supply level to maintain high function, yield, and cannabinoids profile. However, it is important to consider that ins this research not all cannabinoids and organs were affected similarly by K availability [143]. Bevan et al. (2021) [48] observed that the inflorescence yield did not respond to K within the tested range (60 to 340 mg L−1) and estimated the highest average inflorescence yield of 144 g/plant with N and P concentrations of 194 and 59 mg L−1, respectively, but the phytocannabinoid concentrations in the floral tissues did not respond to nutrient solution NPK concentrations.
Yep et al. (2020) [144] evaluated the phytocannabinoid and inflorescence yields in two varieties (‘Sensi Star’ and ‘Nordle’) grown in hydroponic and aquaponic systems. The highest flower and phytocannabinoid yields were observed in the hydroponic system. The greater availability of N, P, K, and certain micronutrients in the hydroponic system may have caused a difference in the yield [144]. The ‘Nordle’ variety had higher phytocannabinoid and terpene concentrations in the aquaponic system, probably due to stress such as the limited size of the container, nutritional toxicity and deficiency, or possible salinity stress. These results indicated the influence of genotype characteristics on cannabis management. In another study, Yep et al. (2020) [145] observed that the effects of salt stress caused by high concentration of NaCl (above 5 mM) could decrease the yield and concentration of phytocannabinoids. However, plants grown in the aquaponic system showed greater tolerance to higher concentrations of NaCl than plants grown in the hydroponic system, although in both systems there was a decrease in the concentration of phytocannabinoids.
Other factors that can influence nutrient availability and absorption from the soil are nitrification and the usage of urease inhibitors in nitrogen fertilization. Kakabouki et al. (2021) [146] evaluated four different fertilization treatments (control, urea, urea + urease inhibitor, and urea + nitrification inhibitor + urease inhibitor) in two hemp varieties. They observed that applying urea with both the inhibitors significantly affected the qualitative and agronomic characteristics, such as increased CBD content, in the two varieties evaluated. Amino acid biostimulant supplements may help standardize the content of secondary metabolites in hemp plants for biomedical purposes, increasing phytocannabinoid production, as observed by Malik et al. (2022) [147].
In relation to the different substrates that can be used for hemp cultivation, there is no consensus among growers on the most suitable substrate for growing cannabis with biomedical purposes. However, some characteristics are considered important by producers when choosing the substrate, such as high porosity, fast draining, maintaining moderate water content between irrigations, the capability of fast dry-downs to reduce the risk of root pathogens, well-balanced physical and chemical properties, high consistency, and free of weeds and pathogens; among these, perlite, vermiculite, coconut fiber, peat, and humus are among the most commonly used [66].
Studies evaluating the influence of these substrates on cannabis production are scarce. Two substrates based on coconut fiber in soilless cultivation were evaluated: the substrate with better drainage resulted in greater THCA and CBGA yields and higher growth rates [68]. The authors reported that the elevated values of fertigation frequency and higher root oxygenation might have contributed to increased secondary metabolite production. According to the authors, the coconut-fiber-based substrate was effective for the production of cannabis during the vegetative stage [140]. In the flowering phase, the substrate with less container capacity provided yield increases for the flowers and THC. According to this research, substrates with high porosity could contribute to increased flower and THC productivity. Another study evaluated the influence of different substrates on two cannabis genotypes (namely, ‘Kanada’ and ‘0.2x’). According to the results, the total CBD yield did not differ between the substrates, but the flower yield was higher in the peat-based substrate only in the ‘Kanada’ genotype, while for the ‘0.2x’ genotype the yield did not vary among the substrates [148]. The difference in the observed variables may have been related to the physical characteristics of the substrates, such as the porosity. Coconut fibers have an extremely high air capacity, but a lower water capacity. Peat has a high-water holding capacity due to low porosity while green fibers allow high aeration and good drainage due to high porosity. The combination of these materials can provide a favorable environment for the development of cannabis [148].
3.7. Water Availability
There are some reports claiming that cannabis is a culture of high-water demand [149,150], that exceeds the needs of several agricultural crops, such as maize, soybeans and wheat [151]. However, this demand is highly dependent on genotype, agronomic practices and the edaphoclimatic factors of the growing area. To date, there are few studies evaluating the water demand and the effect of water stress on the production of phytocannabinoids.
Adequate water availability is essential for plant development and growth, considering that cellular expansion occurs through the turgor pressure exerted by the vacuole. However, conditions of light or moderate water limitation can increase secondary metabolite yield because, under stress conditions, the production of these compounds can be stimulated [152]. It is important to consider that, depending on the severity of water stress and the tolerance of the genotype, the flower yield (dry matter) may decrease [153]. In addition, severe water deficit can result in stomatal closure, reducing the net CO2 assimilation rate and causing metabolic disorders.
According to Caplan et al. (2019), controlled water restriction did not influence the inflorescence yield. However, the yields of THC, CBD, THCA, and CBDA per unit of cultivation area increased significantly [153]. Sheldon et al. (2021) [154] observed that hemp cultivars that had higher evapotranspiration thresholds under water deficit stress produced higher percentages of CBD than those cultivars with low evapotranspiration thresholds. Thus, controlled water stress can be an effective management technique to increase phytocannabinoid yield. The response to this type of stress can range according to the genotype of the plants, the water restriction intensity, the frequency, and the evaluated metabolite.
García-Tejero et al. (2014) [155] evaluated the response of two hemp cultivars (‘Ermes’ and ‘Carma’) submitted to water restrictions. These authors observed that high irrigation frequency and high oxygenation of the root zone optimized the phytocannabinoid yield. However, additional studies are needed to investigate these variables [153]. In a study developed by Sikora et al. (2011) [156] was observed that precipitation affected the CBD content (R2 = 0.55) more significantly than it did the THC content (R2 = 0.31); however, other environmental factors may have influenced this response. In another study, it was observed that the sum of rainfall from sowing to full bloom was negatively correlated (−62%) with the CBD content at full bloom, but the same correlation was positive in senescence (76%). The THC concentration at full bloom was positively related (72%) with the mean air humidity from sowing to full bloom [57]. According to Campbell [77], greater water availability increased the THC production; however, other edaphoclimatic factors may have influenced this response. Toth et al. (2021) [157] evaluated the effects of different stresses, including excess water (flooding). The authors did not observe any influence on the production of THC, CBD, or CBG in relation to the control treatment [157].
The amount and frequency of irrigation depends on the cultivation environment (indoor or outdoor), on the characteristics of the substrate, on the size of the cultivation container [66], on the environmental conditions (such as temperature and humidity), and on the response of the genotype, given that some are more tolerant than others. Future works focused on evaluating different levels of water availability in cannabis cultivation could provide more solid data regarding the influence of water stress and irrigation requirements for phytocannabinoid production.
4. Conclusions and Future Directions
International narcotic conventions and associated legislation have constrained the establishment, characterization, and use of cannabis genetic resource collections. This has resulted in the underutilization of genepool variability in cultivar development. Moreover, a limited amount of research on the management and influence of environmental conditions on phytocannabinoid yield is evident. Prohibitionist legislation has discouraged scientists from studying cannabis production, the main factor responsible for the current lack of research. In several countries, legislation is changing due to recognition of the medicinal and agricultural value of cannabis plants.
In recent years, the number of works on the topics covered in this review has increased significantly. However, they are not enough to determine the influence of management techniques and abiotic factors on phytocannabinoid production. Thus, it is necessary to develop basic research to evaluate how nutritional availability, water availability, harvest time, CO2 concentration, light (intensity and quality), different types of stress, pruning, and other management methodologies could ultimately influence the phytocannabinoid yields for different genotypes. Epigenetic and biotechnology tools (such as omics-based methods) can explain how these compounds are synthesized according to the physiological responses under crop management techniques and different environmental conditions.
Additionally, the fibers produced by plants grown for the production of phytocannabinoids can be used as raw material for several industries. However, there is little research on the production of fibers and phytocannabinoids. There is a need to explore the concomitant productive potential of these raw materials to ease circular bioeconomy practices. Detailed knowledge of the biological and physiological processes of Cannabis sativa plants is important in order to support the development of efficient cultivation methods aiming to provide higher yields and consistent quality.
Author Contributions
Conceptualization, I.T. and E.C.; methodology, software, validation, formal analysis, resources, investigation, I.T., P.R.d.S., G.A.R.d.S., R.M.d.S.N.d.M., A.L.P.M.d.S., K.D.d.S. and D.Z.S.; data curation, I.T., I.F.G.-T. and E.C.; writing—original draft preparation, I.T.; writing—review and editing, I.T., P.R.d.S., D.Z.S., I.F.G.-T. and E.C.; visualization, I.T., P.R.d.S., K.D.d.S., D.Z.S., I.F.G.-T. and E.C.; supervision, I.F.G.-T. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported a CNPq fellowships (PQ fellowship 303166/2019-3 for E.C.) and Fundação Carlos Chagas de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ) grants (E-26/202.759/2018, E-26/210.309/2018, and E-26/210.037/2020 for E.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Cannabidiol (CBD); cannabidiolic acid (CBDA); tetrahydrocannabinol (THC), tetrahydrocannabinolic acid (THCA); cannabigerol (CBG); cannabigerolic acid (CBGA); cannabichromene (CBC), cannabichromenic acid (CBCA); cannabinol (CBN); cannabicitran (CBT); tetrahydrocannabivarinic acid (THCVA); cannabidivarinic acid (CBDVA); cannabichromene (CBC).
References
- Musto, D.F. The Marihuana Tax Act of 1937. Arch. Gen. Psychiatry 1972, 26, 101. [Google Scholar] [CrossRef] [PubMed]
- Saloner, A.; Sacks, M.M.; Bernstein, N. Response of Medical Cannabis (Cannabis sativa L.) Genotypes to K Supply under Long Photoperiod. Front. Plant Sci. 2019, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L. An Archaeological and Historical Account of Cannabis in China. Econ. Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Singh Chopra, G. Man and Marijuana. Int. J. Addict. 1969, 4, 215–247. [Google Scholar] [CrossRef]
- Mechoulam, R.; Carlini, E.A. Toward Drugs Derived from Cannabis. Naturwissenschaften 1978, 65, 174–179. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Mechoulam, R. Cannabis—The Israeli Perspective. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 181–187. [Google Scholar] [CrossRef]
- Treister-Goltzman, Y.; Freud, T.; Press, Y.; Peleg, R. Trends in Publications on Medical Cannabis from the Year 2000. Popul. Health Manag. 2019, 22, 362–368. [Google Scholar] [CrossRef]
- Schluttenhofer, C.; Yuan, L. Challenges towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef]
- UNODC WHO Scheduling Recommendations on Cannabis and Cannabis-Related Substances. Available online: https://www.unodc.org/unodc/en/commissions/CND/Mandate_Functions/current-scheduling-recommendations.html (accessed on 24 May 2021).
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis sativa Research Trends, Challenges, and New-Age Perspectives. iScience 2021, 24, 103391. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Small, E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- de Meijer, E.P.M.; Bagatta, M.; Carboni, A.; Crucitti, P.; Moliterni, V.M.C.; Ranalli, P.; Mandolino, G. The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics 2003, 163, 335–346. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Phineas Jones, A.M. Recent Advances in Cannabis Biotechnology. Ind. Crops Prod. 2020, 158, 113026. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Faux, A.M.; Draye, X.; Flamand, M.C.; Occre, A.; Bertin, P. Identification of QTLs for Sex Expression in Dioecious and Monoecious Hemp (Cannabis sativa L.). Euphytica 2016, 209, 357–376. [Google Scholar] [CrossRef]
- Cristiana Moliterni, V.M.; Cattivelli, L.; Ranalli, P.; Mandolino, G. The Sexual Differentiation of Cannabis sativa L.: A Morphological and Molecular Study; Kluwer Academic Publishers: Norwell, MA, USA, 2004; Volume 140. [Google Scholar]
- Dayanandan, P.; Kaufman, P.B. Trichomes of Cannabis sativa L. (Cannabaceae). Am. J. Bot. 1976, 63, 578–591. [Google Scholar] [CrossRef]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The Hexanoyl-CoA Precursor for Cannabinoid Biosynthesis Is Formed by an Acyl-Activating Enzyme in Cannabis sativa Trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef]
- Upton, R.; Craker, L.; ElSohly, M.; Romm, A.; Russo, E.; Sexton, M. American Herbal Pharmacopoeia; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–60. [Google Scholar]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis Glandular Trichomes: A Cellular Metabolite Factory. Front. Plant Sci. 2021, 12, 1923. [Google Scholar] [CrossRef]
- Welling, M.T.; Deseo, M.A.; Bacic, A.; Doblin, M.S. Untargeted Metabolomic Analyses Reveal Chemical Complexity of Dioecious Cannabis Flowers. Aust. J. Chem. 2021, 74, 463–479. [Google Scholar] [CrossRef]
- Valle, J.R.; Lapa, A.J.; Barros, G.G. Pharmacological Activity of Cannabis According to the Sex of the Plant. J. Pharm. Pharmacol. 1968, 20, 798–799. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Abou-Chaar, C.I.; Agurell, S.; Nilsson, I.M.; OIofsson, K.; Sandberg, F. Cannabinoid Constituents of Male and Female Cannabis sativa. Bull. Narc. 1971, 23, 29–32. [Google Scholar]
- Fetterman, P.S.; Keith, E.S.; Waller, C.W.; Guerrero, O.; Doorenbos, N.J.; Quimby, M.W. Mississippi-grown Cannabis sativa L.: Preliminary Observation on Chemical Definition of Phenotype and Variations in Tetrahydrocannabinol Content versus Age, Sex, and Plant Part. J. Pharm. Sci. 1971, 60, 1246–1249. [Google Scholar] [CrossRef]
- Doorenbos, N.J.; Fetterman, P.S.; Quimby, M.W.; Turner, C.E. Cultivation, Extraction, and Analysis of Cannabis sativa L. Ann. N. Y. Acad. Sci. 1971, 191, 3–14. [Google Scholar] [CrossRef]
- Kushima, H.; Shoyama, Y.; Nishioka, I. Cannabis. XII. Variations of Cannabinoid Contents in Several Strains of Cannabis sativa L. with Leaf-Age, Season and Sex. Chem. Pharm. Bull. 1980, 28, 594–598. [Google Scholar] [CrossRef][Green Version]
- Kimura, M.; Okamoto, K. Distribution of Tetrahydrocannabinolic Acid in Fresh Wild Cannabis. Experientia 1970, 26, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Bruci, Z.; Papoutsis, I.; Athanaselis, S.; Nikolaou, P.; Pazari, E.; Spiliopoulou, C.; Vyshka, G. First Systematic Evaluation of the Potency of Cannabis sativa Plants Grown in Albania. Forensic Sci. Int. 2012, 222, 40–46. [Google Scholar] [CrossRef]
- Lipson Feder, C.; Cohen, O.; Shapira, A.; Katzir, I.; Peer, R.; Guberman, O.; Procaccia, S.; Berman, P.; Flaishman, M.; Meiri, D. Fertilization Following Pollination Predominantly Decreases Phytocannabinoids Accumulation and Alters the Accumulation of Terpenoids in Cannabis Inflorescences. Front. Plant Sci. 2021, 12, 753847. [Google Scholar] [CrossRef]
- Olejar, K.J.; Park, S.-H. Industry-Based Misconceptions Regarding Cross-Pollination of Cannabis spp. Front. Plant Sci. 2022, 13, 793264. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Giroud, C. Analysis of Cannabinoids in Hemp Plants. Chimia 2002, 56, 80–83. [Google Scholar] [CrossRef]
- Spitzer-Rimon, B.; Duchin, S.; Bernstein, N.; Kamenetsky, R. Architecture and Florogenesis in Female Cannabis sativa Plants. Front. Plant Sci. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis Cultivation: Methodological Issues for Obtaining Medical-Grade Product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Riboulet-Zemouli, K. ‘Cannabis’ Ontologies I: Conceptual Issues with Cannabis and Cannabinoids Terminology. Drug Sci. Policy Law 2020, 6, 205032452094579. [Google Scholar] [CrossRef]
- Gorguet, B.; van Heusden, A.W.; Lindhout, P. Parthenocarpic Fruit Development in Tomato. Plant Biol. 2005, 7, 131–139. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Cannabis Production and Markets in Europe; Publications Office of the European Union: Luxembourg, 2012; p. 268.
- Clarke, R.C. Marijuana Botany: The Propagation and Breeding of Distintive Cannabis, 2nd ed.; Ronin Publishing: Berkeley, CA, USA, 1981. [Google Scholar]
- Potter, D.J. A Review of the Cultivation and Processing of Cannabis (Cannabis Sativa L.) for Production of Prescription Medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef]
- van der Werf, H.M.G.; Haasken, H.J.; Wijlhuizen, M. The Effect of Daylength on Yield and Quality of Fibre Hemp (Cannabis sativa L.). Eur. J. Agron. 1994, 3, 117–123. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Theis, N.; Lerdau, M. The Evolution of Function in Plant Secondary Metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Haney, A.; Kutscheid, B.B. Quantitative Variation in the Chemical Constituents of Marihuana from Stands of Naturalized Cannabis sativa L. in East-Central Illinois. Econ. Bot. 1973, 27, 193–203. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Plant Architecture Manipulation Increases Cannabinoid Standardization in ‘Drug-Type’ Medical Cannabis. Ind. Crops Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Lermusieau, G.; Collin, S. Hop Aroma Extraction and Analysis. In Analysis of Taste and Aroma; Springer: Berlin/Heidelberg, Germany, 2002; pp. 69–88. [Google Scholar] [CrossRef]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of Nitrogen, Phosphorus, and Potassium for Soilless Production of Cannabis sativa in the Flowering Stage Using Response Surface Analysis. Front. Plant Sci. 2021, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Coffman, C.B.; Gentner, W.A. Cannabinoid Profile and Elemental Uptake of Cannabis sativa L. as Influenced by Soil Characteristics 1. Agron. J. 1975, 67, 491–497. [Google Scholar] [CrossRef]
- Naim-Feil, E.; Breen, E.J.; Pembleton, L.W.; Spooner, L.E.; Spangenberg, G.C.; Cogan, N.O.I. Empirical Evaluation of Inflorescences’ Morphological Attributes for Yield Optimization of Medicinal Cannabis Cultivars. Front. Plant Sci. 2022, 13, 858519. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic Fingerprinting of Cannabis sativa L., Cannabinoids and Terpenoids for Chemotaxonomic and Drug Standardization Purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Glivar, T.; Eržen, J.; Kreft, S.; Zagožen, M.; Čerenak, A.; Čeh, B.; Tavčar Benković, E. Cannabinoid Content in Industrial Hemp (Cannabis sativa L.) Varieties Grown in Slovenia. Ind. Crops Prod. 2020, 145, 112082. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Gorelick, J.; Bernstein, N. Chemical and Physical Elicitation for Enhanced Cannabinoid Production in Cannabis. In Cannabis sativa L.—Botany and Biotechnology; Springer International Publishing: Cham, Switzerland, 2017; pp. 439–456. ISBN 9783319545646. [Google Scholar]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key Cultivation Techniques for Hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Van Der Werf, H.; Mathussen, E.W.J.M.; Haverkort, A. The Potential of Hemp (Cannabis sativa L.) for Sustainable Fibre Production: A Crop Physiological Appraisal. Ann. Appl. Biol. 1996, 129, 109–123. [Google Scholar] [CrossRef]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High Added-Value Compounds from Cannabis Threshing Residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Letter to the Editor: Small, Ernest. 2015. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Botanical Review 81(3): 189–294. Bot. Rev. 2015, 81, 295–305. [Google Scholar] [CrossRef]
- Hammami, N.; Privé, J.-P.; Joly, D.L.; Moreau, G. Associations between Cannabinoids and Growth Stages of Twelve Industrial Hemp Cultivars Grown Outdoors in Atlantic Canada. Ind. Crops Prod. 2021, 172, 113997. [Google Scholar] [CrossRef]
- de Backer, B.; Maebe, K.; Verstraete, A.G.; Charlier, C. Evolution of the Content of THC and Other Major Cannabinoids in Drug-Type Cannabis Cuttings and Seedlings During Growth of Plants. J. Forensic Sci. 2012, 57, 918–922. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Hernández, A.; Ferreiro-Vera, C.; Zuazo, V.H.D.; García, J.H.; Sánchez-Carnerero, C.; Casano, S. Yield of New Hemp Varieties for Medical Purposes under Semi-Arid Mediterranean Environment Conditions. Comun. Sci. 2020, 11, e3264. [Google Scholar] [CrossRef]
- Toonen, M.; Ribot, S.; Thissen, J. Yield of Illicit Indoor Cannabis Cultivation in The Netherlands. J. Forensic Sci. 2006, 51, 1050–1054. [Google Scholar] [CrossRef]
- Vanhove, W.; van Damme, P.; Meert, N. Factors Determining Yield and Quality of Illicit Indoor Cannabis (Cannabis spp.) Production. Forensic Sci. Int. 2011, 212, 158–163. [Google Scholar] [CrossRef]
- Hawley, D.; Graham, T.; Stasiak, M.; Dixon, M. Improving Cannabis Bud Quality and Yield with Subcanopy Lighting. HortScience 2018, 53, 1593–1599. [Google Scholar] [CrossRef]
- Rocha, S.B.F. Potencial Brasileiro Para o Cultivo de Cannabis sativa L.; Para Uso Medicinal e Industrial: Viçosa, Brazil, 2019. [Google Scholar]
- Nemati, R.; Fortin, J.-P.; Craig, J.; Donald, S.; Fonteno, C.; Michel, J.-C.; Jackson, B.E. Growing Mediums for Medical Cannabis Production in North America. Agronomy 2021, 11, 1366. [Google Scholar] [CrossRef]
- de Prato, L.; Ansari, O.; Hardy, G.E.S.J.; Howieson, J.; O’Hara, G.; Ruthrof, K.X. The Cannabinoid Profile and Growth of Hemp (Cannabis sativa L.) Is Influenced by Tropical Daylengths and Temperatures, Genotype and Nitrogen Nutrition. Ind. Crops Prod. 2022, 178, 114605. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal Rate of Organic Fertilizer during the Flowering Stage for Cannabis Grown in Two Coir-Based Substrates. HortScience 2017, 52, 1796–1803. [Google Scholar] [CrossRef]
- Green, G. The Cannabis Grow Bible, 2nd ed.; Green Candy Press: São Francisco, CA, USA, 2010. [Google Scholar]
- Rosenthal, E. Marijuana Grower’s Handbook; Quick American Publishing: Oakland, CA, USA, 2010; ISBN 0-932551-46-7. [Google Scholar]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between Chemistry and Morphology in Medical Cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Crispim Massuela, D.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Danziger, N.; Bernstein, N. Shape Matters: Plant Architecture Affects Chemical Uniformity in Large-Size Medical Cannabis Plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Folina, A.; Kakabouki, I.; Tourkochoriti, E.; Roussis, I.; Pateroulakis, H.; Bilalis, D. Evaluation of the Effect of Topping on Cannabidiol (CBD) Content in Two Industrial Hemp (Cannabis sativa L.) Cultivars. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2020, 77, 46. [Google Scholar] [CrossRef]
- Cervantes, J. Marijuana: Horticulture: The Indoor/Outdoor Medical Grower’s Bible, 1st ed.; Van Patten Publishing: Vancouver, WA, USA, 2007. [Google Scholar]
- Campbell, B.J.; Berrada, A.F.; Hudalla, C.; Amaducci, S.; McKay, J.K. Genotype × Environment Interactions of Industrial Hemp Cultivars Highlight Diverse Responses to Environmental Factors. Agrosyst. Geosci. Environ. 2019, 2, 180057. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.S.; Rufyikiri, A.S.; MacPherson, S.; Lefsrud, M. An Update on Plant Photobiology and Implications for Cannabis Production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light Matters: Effect of Light Spectra on Cannabinoid Profile and Plant Development of Medical Cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Islam, M.J.; Ryu, B.R.; Azad, M.O.K.; Rahman, M.H.; Cheong, E.J.; Lim, J.-D.; Lim, Y.-S. Cannabinoids Accumulation in Hemp (Cannabis sativa L.) Plants under LED Light Spectra and Their Discrete Role as a Stress Marker. Biology 2021, 10, 710. [Google Scholar] [CrossRef]
- Lydon, J.; Teramura, A.H.; Coffman, C.B. UV-B Radiation Effects on Photosynthesis, Growth and Cannabinoid Production of Two Cannabis sativa Chemotypes. Photochem. Photobiol. 1987, 46, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis Inflorescence Yield and Cannabinoid Concentration Are Not Increased with Exposure to Short-Wavelength Ultraviolet-B Radiation. Front. Plant Sci. 2021, 12, 725078. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.; Golem, S.; Foley, E.; Dinka, S.; Maxwell, A.; Jones, P.; Zheng, Y. Cannabis Yield Increased Proportionally with Light Intensity, but Additional Ultraviolet Radiation Did Not Affect Yield or Cannabinoid Content. Anat. Morphol. 2021, 1–19. [Google Scholar] [CrossRef]
- Jenkins, M.W. Cannabis sativa L. Response to Narrow Bandwidth UV and the Combination of Blue and Red Light during the Final Stages of Flowering on Leaf Level Gas-Exchange Parameters, Secondary Metabolite Production, and Yield. Agric. Sci. 2021, 12, 1414–1432. [Google Scholar] [CrossRef]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis Yield, Potency, and Leaf Photosynthesis Respond Differently to Increasing Light Levels in an Indoor Environment. Front. Plant Sci. 2021, 12, 456. [Google Scholar] [CrossRef]
- Valle, J.R.; Vieira, J.E.; Aucélio, J.G.; Valio, I.F. Influence of Photoperiodism on Cannabinoid Content of Cannabis sativa L. Bull. Narc. 1978, 30, 67–68. [Google Scholar]
- Khajuria, M.; Rahul, V.P.; Vyas, D. Photochemical Efficiency Is Negatively Correlated with the Δ9- Tetrahydrocannabinol Content in Cannabis sativa L.: Effect of THC in Cannabis. Plant Physiol. Biochem. 2020, 151, 589–600. [Google Scholar] [CrossRef]
- Mahlberg, P.G.; Hemphill, J.K. Effect of Light Quality on Cannabinoid Content of Cannabis sativa L. (Cannabaceae). Bot. Gaz. 1983, 144, 43–48. [Google Scholar] [CrossRef]
- Eaves, J.; Eaves, S.; Morphy, C.; Murray, C. The Relationship between Light Intensity, Cannabis Yields, and Profitability. Agron. J. 2020, 112, 1466–1470. [Google Scholar] [CrossRef]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Cannabis Lighting: Decreasing Blue Photon Fraction Increases Yield but Efficacy Is More Important for Cost Effective Production of Cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef]
- Namdar, D.; Charuvi, D.; Ajjampura, V.; Mazuz, M.; Ion, A.; Kamara, I.; Koltai, H. LED Lighting Affects the Composition and Biological Activity of Cannabis sativa Secondary Metabolites. Ind. Crops Prod. 2019, 132, 177–185. [Google Scholar] [CrossRef]
- Pattison, P.M.; Tsao, J.Y.; Brainard, G.C.; Bugbee, B. LEDs for Photons, Physiology and Food. Nature 2018, 563, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from Distinct Wavelength Light-Emitting Diodes Affect Secondary Metabolites in Kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, X.; Long, S.; Xiao, Q.; Guo, Y.; Qiu, C.; Qiu, H.; Wang, Y. Wavelengths of LED Light Affect the Growth and Cannabidiol Content in Cannabis sativa L. Ind. Crops Prod. 2021, 165, 113433. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Livesay, C.B. Photosynthetic Performance and Potency of Cannabis sativa L. Grown under LED and HPS Illumination. Agric. Sci. 2021, 12, 293–304. [Google Scholar] [CrossRef]
- Pate, D.W. Possible Role of Ultraviolet Radiation in Evolution of Cannabis Chemotypes. Econ. Bot. 1983, 37, 396–405. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Khan, I.A.; Elsohly, M.A. Photosynthetic Response of Cannabis sativa Photosynthetic Response of Cannabis sativa L. to Variations in Photosynthetic Photon Flux Densities, Temperature and CO2 Conditions. Physiol. Mol. Biol. Plants 2008, 14, 299–306. [Google Scholar] [CrossRef]
- Potter, D.J.; Duncombe, P. The Effect of Electrical Lighting Power and Irradiance on Indoor-Grown Cannabis Potency and Yield. J. Forensic Sci. 2012, 57, 618–622. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Mehmedic, Z.; Khan, I.A.; ElSohly, M.A. Light Dependence of Photosynthesis and Water Vapor Exchange Characteristics in Different High Δ9-THC Yielding Varieties of Cannabis sativa L. J. Appl. Res. Med. Aromat. Plants 2015, 2, 39–47. [Google Scholar] [CrossRef]
- Bouchard, M. Towards a Realistic Method to Estimate Cannabis Production in Industrialized Countries. Contemp. Drug Probl. 2008, 35, 291–320. [Google Scholar] [CrossRef]
- Dang, M.; Arachchige, N.M.; Campbell, L.G. Optimizing Photoperiod Switch to Maximize Floral Biomass and Cannabinoid Yield in Cannabis sativa L.: A Meta-Analytic Quantile Regression Approach. Front. Plant Sci. 2022, 12, 797425. [Google Scholar] [CrossRef] [PubMed]
- Lenton, S.; Frank, V.A.; Barratt, M.J.; Potter, G.R.; Decorte, T. Growing Practices and the Use of Potentially Harmful Chemical Additives among a Sample of Small-Scale Cannabis Growers in Three Countries. Drug Alcohol Depend. 2018, 192, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Eichhorn Bilodeau, S.; Lyu, D.; Ahmed, M.B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the Yield Gap for Cannabis: A Meta-Analysis of Factors Determining Cannabis Yield. Front. Plant Sci. 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Hemp as an Agricultural Commodity; Congressional Research Service: Washington, DC, USA, 2014.
- Vanhove, W.; Surmont, T.; van Damme, P.; de Ruyver, B. Yield and Turnover of Illicit Indoor Cannabis (Cannabis spp.) Plantations in Belgium. Forensic Sci. Int. 2012, 220, 265–270. [Google Scholar] [CrossRef]
- Benevenute, S.S.; Freeman, J.H.; Yang, R. How Do Pinching and Plant Density Affect Industrial Hemp Produced for Cannabinoids in Open Field Conditions? Agron. J. 2022, 114, 618–626. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán Zuazo, V.H.; Sánchez-Carnenero, C.; Hernández, A.; Ferreiro-Vera, C.; Casano, S. Seeking Suitable Agronomical Practices for Industrial Hemp (Cannabis sativa L.) Cultivation for Biomedical Applications. Ind. Crops Prod. 2019, 139, 111524. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; di Sotto, A.; di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa L. Inflorescences from Monoecious Cultivars Grown in Central Italy: An Untargeted Chemical Characterization from Early Flowering to Ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C.; et al. Season-long Characterization of High-cannabinoid Hemp (Cannabis sativa L.) Reveals Variation in Cannabinoid Accumulation, Flowering Time, and Disease Resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’Connell, M.A. Accumulation of Bioactive Metabolites in Cultivated Medical Cannabis. PLoS ONE 2018, 13, e0201119. [Google Scholar] [CrossRef]
- Turner, J.C.; Mahlberg, P.G.; Lanyon, V.S.; Pleszczynska, J. A Temporal Study of Cannabinoid Composition in Continual Clones of Cannabis sativa L. (Cannabaceae). Bot. Gaz. 1985, 146, 32–38. [Google Scholar] [CrossRef]
- Latta, R.P.; Eaton, B.J. Seasonal Fluctuations in Cannabinoid Content of Kansas Marijuana. Econ. Bot. 1975, 29, 153–163. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L.). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Shiponi, S.; Bernstein, N. The Highs and Lows of P Supply in Medical Cannabis: Effects on Cannabinoids, the Ionome, and Morpho-Physiology. Front. Plant Sci. 2021, 12, 657323. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.K.; Turner, J.C.; Mahlberg, P.G. Cannabinoid Content of Individual Plant Organs from Different Geographical Strains of Cannabis sativa L. J. Nat. Prod. 1980, 43, 112–122. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.C.; Hemphill, J.K.; Mahlberg, P.G. Interrelationships of Glandular Trichomes and Cannabinoid Content. II. Developing Vegetative Leaves of Cannabis sativa L. (Cannabaceae). Bull. Narc. 1981, 33, 63–71. [Google Scholar]
- Turner, J.C.; Hemphill, J.K.; Mahlberg, P.G. Cannabinoid Composition and Gland Distribution in Clones of Cannabis sativa L. (Cannabaceae). Bull. Narc. 1978, 30, 55–65. [Google Scholar]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis sativa L.): A Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Vogelmann, A.F.; Turner, J.C.; Mahlberg, P.G. Cannabinoid Composition in Seedlings Compared to Adult Plants of Cannabis sativa. J. Nat. Prod. 1988, 51, 1075–1079. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Carboni, A.; Moschella, A.; Mandolino, G. Time Course of Cannabinoid Accumulation and Chemotype Development during the Growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Liebmann, J.A. The Cannabinoid Content of Cannabis sativa L. Grown in England. J. Pharm. Pharmacol. 1974, 26, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen Supply Affects Cannabinoid and Terpenoid Profile in Medical Cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 167, 113516. [Google Scholar] [CrossRef]
- Bauerle, W.L.; McCullough, C.; Iversen, M.; Hazlett, M. Leaf Age and Position Effects on Quantum Yield and Photosynthetic Capacity in Hemp Crowns. Plants 2020, 9, 271. [Google Scholar] [CrossRef]
- Sharma, G.K. Altitudinal Variation in Leaf Epidermal Patterns of Cannabis sativa. Bull. Torrey Bot. Club 1975, 102, 199–200. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Pavlovic, R.; Giorgi, A. Influence of Altitude on Phytochemical Composition of Hemp Inflorescence: A Metabolomic Approach. Molecules 2020, 25, 1381. [Google Scholar] [CrossRef]
- Paris, M.; Boucher, F.; Cosson, L. The Constituents of Cannabis sativa Pollen. Econ. Bot. 1975, 29, 245–253. [Google Scholar] [CrossRef]
- Pate, D.W. Chemical Ecology of Cannabis. Int. Hemp Assoc. 1994, 2, 32–37. [Google Scholar]
- Bazzaz, F.A.; Dusek, D.; Seigler, D.S.; Haney, A.W. Photosynthesis and Cannabinoid Content of Temperate and Tropical Populations of Cannabis sativa; Pergamon Press: Oxford, UK, 1975; Volume 3. [Google Scholar]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Temperature Response of Photosynthesis in Different Drug and Fiber Varieties of Cannabis sativa L. Physiol. Mol. Biol. Plants 2011, 17, 297–303. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Does Enhanced Photosynthesis Enhance Growth? Lessons Learned from CO2 Enrichment Studies. Plant Physiol. 2011, 155, 117–124. [Google Scholar] [CrossRef]
- Murari, G.; Puccini, A.M.; de Sanctis, R.; Lombardi, S. Influence of Environmental Conditions on Tetrahydrocannabinol (Delta (′9) TCH) in Different Cultivars on Cannabis sativa L. Fitoterapia 1983, 54, 195–201. [Google Scholar]
- Li, X.; Kang, S.; Li, F.; Zhang, X.; Huo, Z.; Ding, R.; Tong, L.; Du, T.; Li, S. Light Supplement and Carbon Dioxide Enrichment Affect Yield and Quality of Off-Season Pepper. Agron. J. 2017, 109, 2107–2118. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Response of Medical Cannabis (Cannabis sativa L.) to Nitrogen Supply under Long Photoperiod. Front. Plant Sci. 2020, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Coffman, C.B.; Gentner, W.A. Responses of Greenhouse-grown Cannabis sativa L. to Nitrogen, Phosphorus, and Potassium 1. Agron. J. 1977, 69, 832–836. [Google Scholar] [CrossRef]
- Bócsa, I.; Máthé, P.; Hangyel, L. Effect of Nitrogen on Tetrahydrocannabinol (THC) Content in Hemp (Cannabis sativa L.) Leaves at Different Positions. J. Int. Hemp Assoc. 1997, 4, 80–81. [Google Scholar]
- de Prato, L.; Ansari, O.; Hardy, G.E.S.J.; Howieson, J.; O’Hara, G.; Ruthrof, K.X. Morpho-Physiology and Cannabinoid Concentrations of Hemp (Cannabis sativa L.) Are Affected by Potassium Fertilisers and Microbes under Tropical Conditions. Ind. Crops Prod. 2022, 182, 114907. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal Rate of Organic Fertilizer during the Vegetative-Stage for Cannabis Grown in Two Coir-Based Substrates. HortScience 2017, 52, 1307–1312. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen Source Matters: High NH4/NO3 Ratio Reduces Cannabinoids, Terpenoids, and Yield in Medical Cannabis. Front. Plant Sci. 2022, 13, 830224. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. Response of Medical Cannabis (Cannabis sativa L.) Genotypes to P Supply under Long Photoperiod: Functional Phenotyping and the Ionome. Ind. Crops Prod. 2021, 161, 113154. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Effect of Potassium (K) Supply on Cannabinoids, Terpenoids and Plant Function in Medical Cannabis. Agronomy 2022, 12, 1242. [Google Scholar] [CrossRef]
- Yep, B.; Gale, N.V.; Zheng, Y. Comparing Hydroponic and Aquaponic Rootzones on the Growth of Two Drug-Type Cannabis sativa L. Cultivars during the Flowering Stage. Ind. Crops Prod. 2020, 157, 112881. [Google Scholar] [CrossRef]
- Yep, B.; Gale, N.V.; Zheng, Y. Aquaponic and Hydroponic Solutions Modulate NaCl-Induced Stress in Drug-Type Cannabis sativa L. Front. Plant Sci. 2020, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kakabouki, I.; Kousta, A.; Folina, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Papastylianou, P. Effect of Fertilization with Urea and Inhibitors on Growth, Yield and Cbd Concentration of Hemp (Cannabis sativa L.). Sustainability 2021, 13, 2157. [Google Scholar] [CrossRef]
- Malík, M.; Velechovský, J.; Praus, L.; Janatová, A.; Kahánková, Z.; Klouček, P.; Tlustoš, P. Amino Acid Supplementation as a Biostimulant in Medical Cannabis (Cannabis sativa L.) Plant Nutrition. Front. Plant Sci. 2022, 13, 868350. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Graeff-Hönninger, S. Impact of Different Growing Substrates on Growth, Yield and Cannabinoid Content of Two Cannabis sativa L. Genotypes in a Pot Culture. Horticulturae 2020, 6, 62. [Google Scholar] [CrossRef]
- Carah, J.K.; Howard, J.K.; Thompson, S.E.; Short Gianotti, A.G.; Bauer, S.D.; Carlson, S.M.; Dralle, D.N.; Gabriel, M.W.; Hulette, L.L.; Johnson, B.J.; et al. High Time for Conservation: Adding the Environment to the Debate on Marijuana Liberalization. BioScience 2015, 65, 822–829. [Google Scholar] [CrossRef]
- Bauer, S.; Olson, J.; Cockrill, A.; van Hattem, M.; Miller, L.; Tauzer, M.; Leppig, G. Impacts of Surface Water Diversions for Marijuana Cultivation on Aquatic Habitat in Four Northwestern California Watersheds. PLoS ONE 2015, 10, e0120016. [Google Scholar] [CrossRef]
- Zheng, Z.; Fiddes, K.; Yang, L. A Narrative Review on Environmental Impacts of Cannabis Cultivation. J. Cannabis Res. 2021, 3, 35. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y. Increasing Inflorescence Dry Weight and Cannabinoid Content in Medical Cannabis Using Controlled Drought Stress. HortScience 2019, 54, 964–969. [Google Scholar] [CrossRef]
- Sheldon, K.; Shekoofa, A.; Walker, E.; Kelly, H. Physiological Screening for Drought-Tolerance Traits among Hemp (Cannabis sativa L.) Cultivars in Controlled Environments and in Field. J. Crop Improv. 2021, 35, 816–831. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Zuazo, V.H.D.; Pérez-Álvarez, R.; Hernández, A.; Cassano, S.; Moron, M.; Muriel, J.L. Impact of Plant Density and Irrigation on Yield of Hemp (Cannabis sativa L.) in a Mediterranean Semi-Arid Environment. J. Agric. Sci. Technol. 2014, 16, 887–895. [Google Scholar]
- Sikora, V.; Berenji, J.; Latkovic, D. Influence of Agroclimatic Conditions on Content of Main Cannabinoids in Industrial Hemp (Cannabis sativa L.). Genetika 2011, 43, 449–456. [Google Scholar] [CrossRef]
- Toth, J.A.; Smart, L.B.; Smart, C.D.; Stack, G.M.; Carlson, C.H.; Philippe, G.; Rose, J.K.C. Limited Effect of Environmental Stress on Cannabinoid Profiles in High-Cannabidiol Hemp (Cannabis sativa L.). GCB Bioenergy 2021, 13, 1666–1674. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).