Low Temperature Rather Than Nitrogen Application Mainly Modulates the Floral Initiation of Different Ecotypes of Rapeseed (Brassica napus L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1: Floral Initiation Response to Soluble Sugar and Protein Levels in the Leaf
2.2. Experiment 2: Transcriptional Regulation of Floral Initiation in Response to Vernalization
3. Results
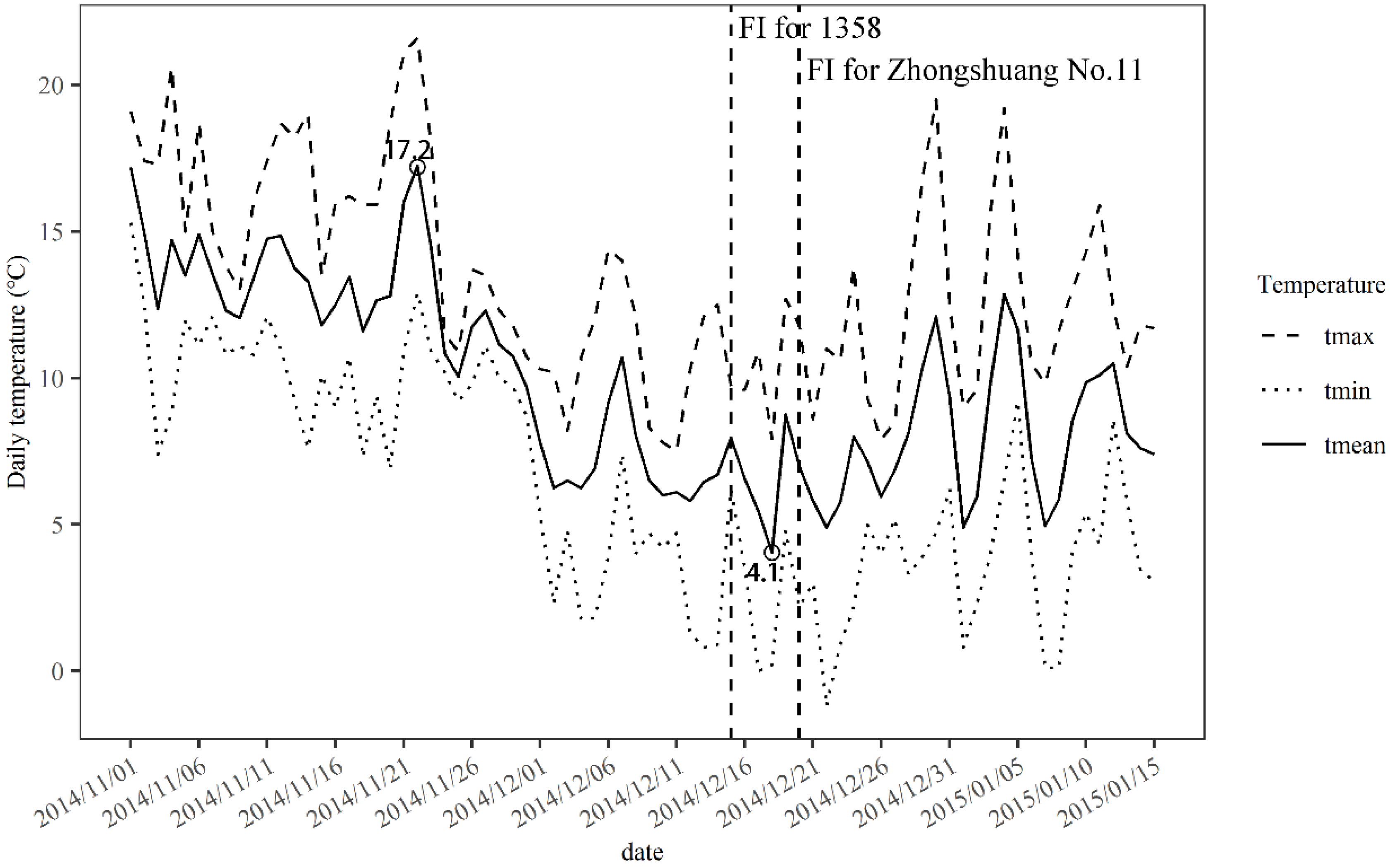
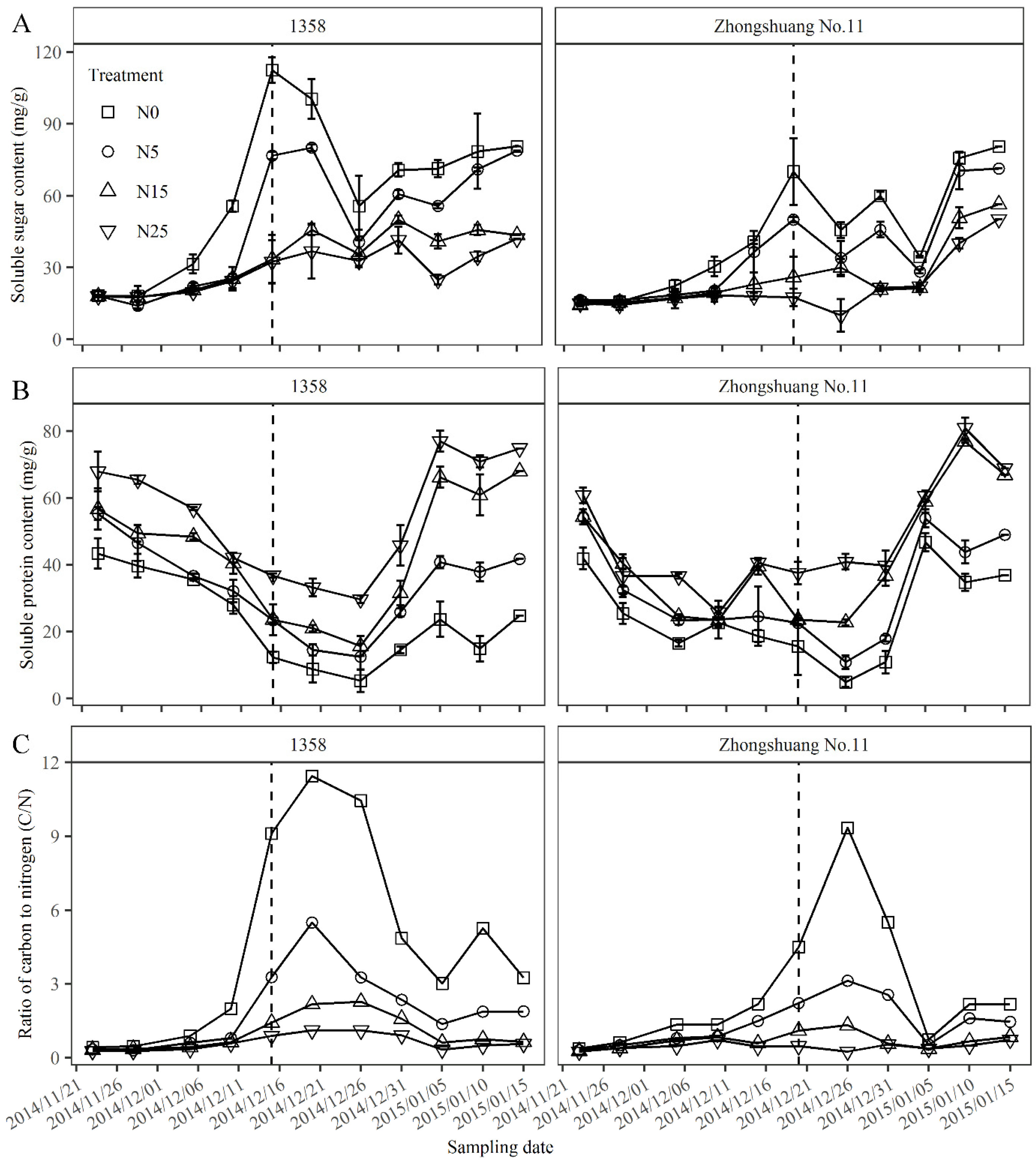
3.1. Floral Initiation Responses to Dynamic Changes of Soluble Sugar and Protein Contents of Leaf
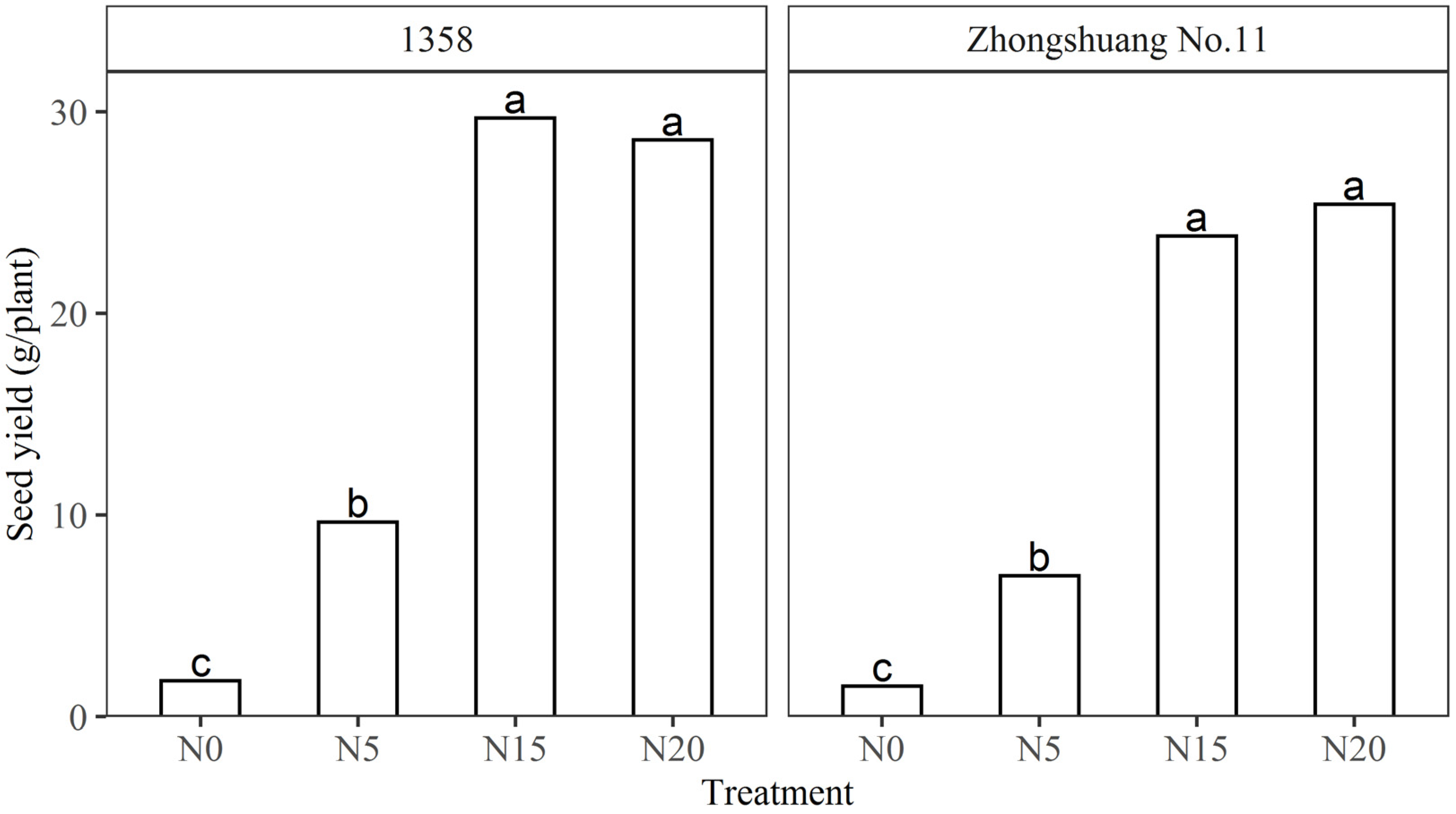
3.2. Floral Initiation Responses to Low Temperature for Spring and Semi-Winter Genotypes
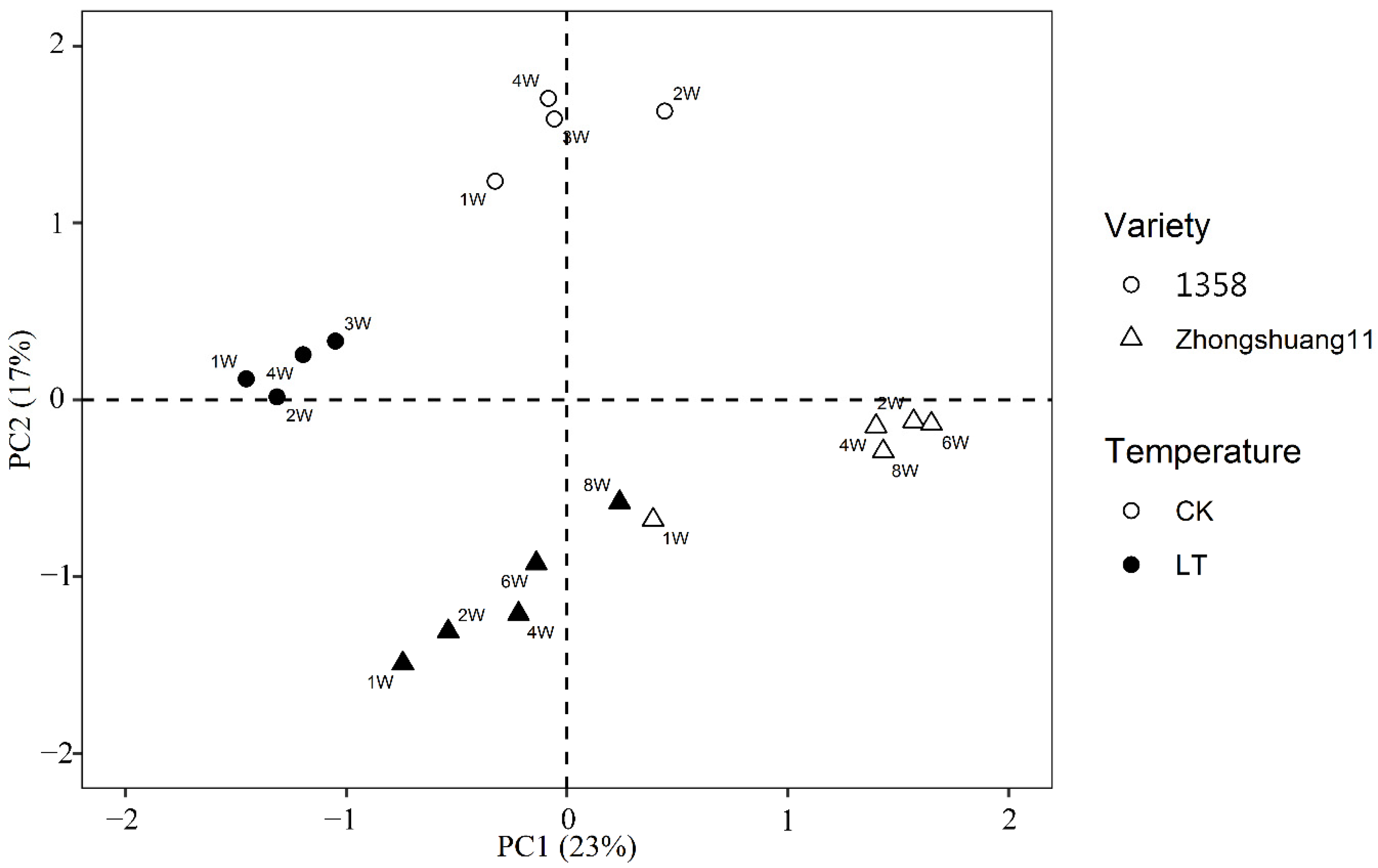
3.3. RNA Sequence Quality and Distribution of Samples under Different Treatments
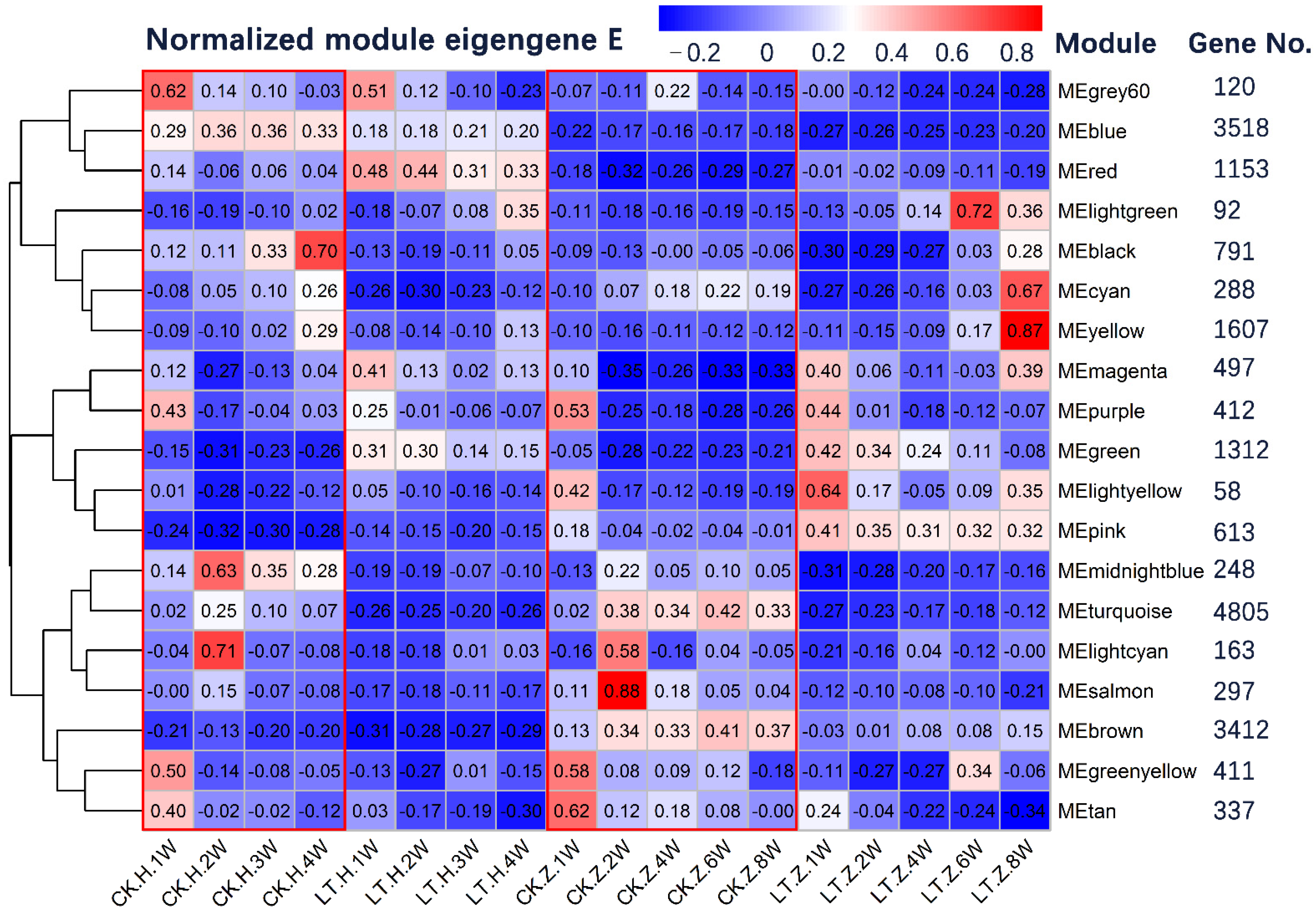
3.4. Modules Constructed by Gene Co-Expression Network Analysis
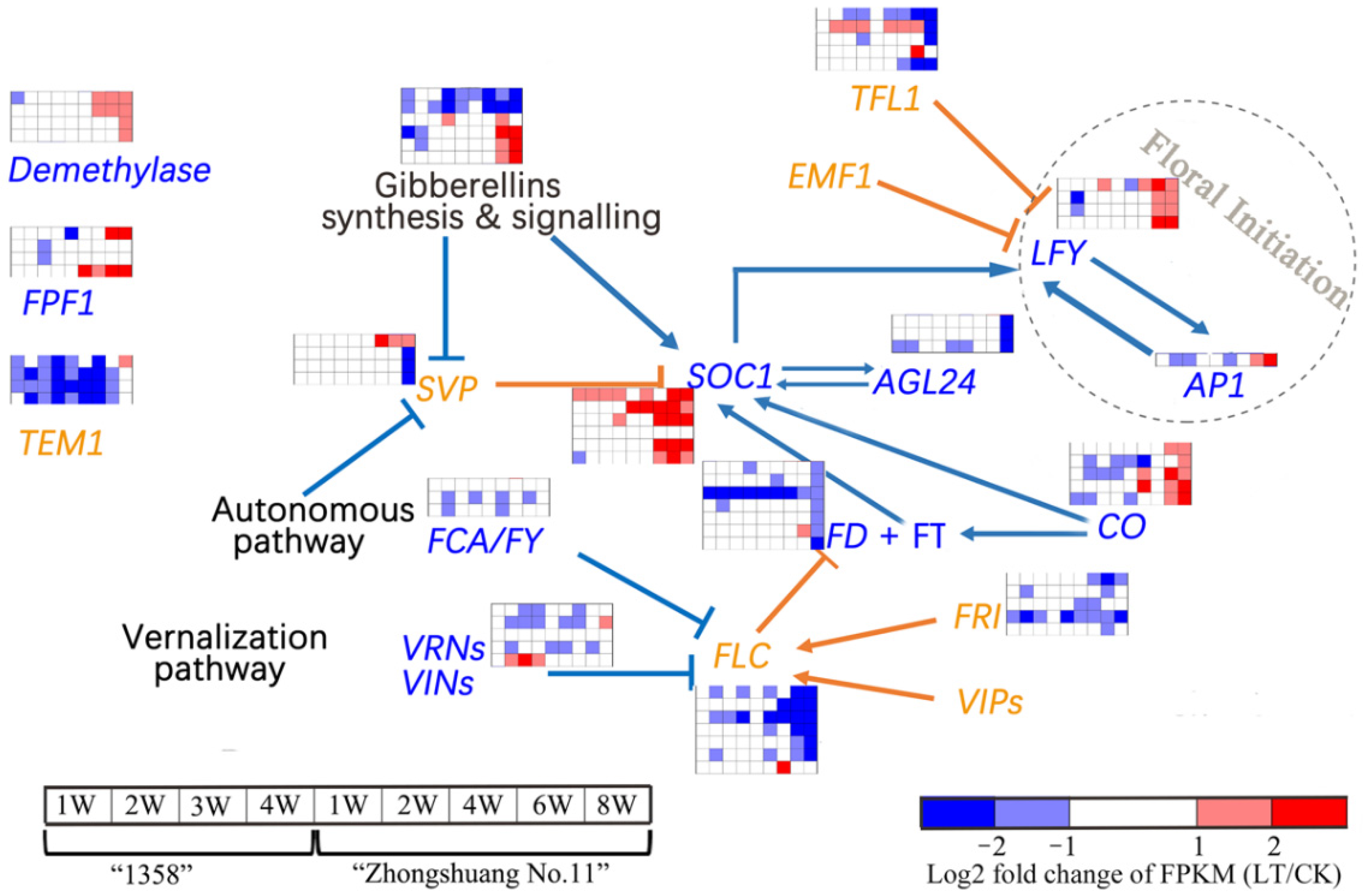
3.5. Expression of Genes Related to Regulatory Network for Floral Initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meier, E.; Lilley, J.; Kirkegaard, J.; Whish, J.; McBeath, T. Management practices that maximise gross margins in Australian canola (Brassica napus L.). Field Crops Res. 2020, 252, 107803. [Google Scholar] [CrossRef]
- Lu, K.; Wei, L.; Li, X.; Wang, Y.; Wu, J.; Liu, M.; Zhang, C.; Chen, Z.; Xiao, Z.; Jian, H.; et al. Whole-genome resequencing reveals Brassica napus origin and genetic loci involved in its improvement. Nat. Commun. 2019, 10, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Liang, Z.; Yan, T.; Xu, Y.; Xuan, L.; Tang, J.; Zhou, G.; Lohwasser, U.; Hua, S.; Wang, H.; et al. Whole-genome resequencing of a worldwide collection of rapeseed accessions reveals the genetic basis of ecotype divergence. Mol. Plant 2019, 12, 30–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, D.; Cui, Y.; He, Y.; Xiong, Q.; Qian, L.; Tong, C.; Lu, G.; Ding, Y.; Li, J.; Jung, C.; et al. A genome-wide survey with different rapeseed ecotypes uncovers footprints of domestication and breeding. J. Exp. Bot. 2017, 68, 4791–4801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Qian, W.; Suppanz, I.; Wei, L.; Mao, B.; Long, Y.; Meng, J.; Muller, A.E.; Jung, C. Flowering time variation in oilseed rape (Brassica napus L.) is associated with allelic variation in the FRIGIDA homologue BnaA.FRI.a. J. Exp. Bot. 2011, 62, 5641–5658. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.K.Y.; Wearing, A.H.; Rickert, K.G.; Birch, C.J. Detection of floral initiation in broccoli (Brassica oleracea L. var. italica Plenck) based on electron micrograph standards of shoot apices. Aust. J. Exp. Agric. 1998, 38, 313. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.S.; Kwon, S.J.; Kim, J.; Lim, M.H.; Park, Y.D.; Kim, D.Y.; Suh, S.C.; Jin, Y.M.; Ji, H.A. Delayed flowering time in Arabidopsis and Brassica rapa by the overexpression of FLOWERING LOCUS C (FLC) homologs isolated from Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant. Cell Rep. 2007, 26, 327–336. [Google Scholar] [CrossRef]
- Johanson, U.; West, J.; Lister, C.; Michaels, S.; Amasino, R.; Dean, C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 2000, 290, 344–347. [Google Scholar] [CrossRef]
- Xu, Y.; Yamaguchi, N.; Gan, E.S.; Ito, T. When to stop: An update on molecular mechanisms of floral meristem termination. J. Exp. Bot. 2019, 70, 1711–1718. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Ji, H.A. Functional conservation and diversification between rice OsMADS22/OsMADS55 and Arabidopsis SVP proteins. Plant Sci. 2012, 185–186, 97–104. [Google Scholar] [CrossRef]
- An, H.; Roussot, C.; Suárez-López, P.; Corbesier, L.; Vincent, C.; Piñeiro, M.; Hepworth, S.; Mouradov, A.; Justin, S.; Turnbull. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 2004, 131, 3615–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepworth, S.R.; Valverde, F.; Ravenscroft, D.; Mouradov, A.; Coupland, G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002, 21, 4327–4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Böhlenius, H.; Eriksson, S.; Parcy, F.; Nilsson, O. The mRNA of the Arabidopsis gene FT moves from leaf to shoot apex and induces flowering. Science 2005, 309, 1694–1696. [Google Scholar] [CrossRef] [Green Version]
- Finnegan, E.; Genger, R.K.; Kovac, K.; Peacock, W.; Dennis, E. DNA methylation and the promotion of flowering by vernalization. Proc. Natl. Acad. Sci. USA 1998, 95, 5824–5829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudor, E.H.; Jones, D.M.; He, Z.; Bancroft, I.; Trick, M.; Wells, R.; Irwin, J.A.; Dean, C. QTL-seq identifies BnaFT. A02 and BnaFLC. A02 as candidates for variation in vernalization requirement and response in winter oilseed rape (Brassica napus). Plant Biotechnol. J. 2020, 18, 2466–2481. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Amasino, R.M. Vernalization and epigenetics: How plants remember winter. Curr. Opin. Plant Biol. 2004, 7, 4–10. [Google Scholar] [CrossRef]
- Bouché, F.; Lobet, G.; Tocquin, P.; Périlleux, C. FLOR-ID: An interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Res. 2016, 44, D1167–D1171. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, B.; Ma, N.; Liu, X.; Qin, M.; Zhang, Y.; Wang, K.; Guo, N.; Zuo, K.; Liu, X. Quantitative trait locus mapping and identification of candidate genes controlling flowering time in Brassica napus L. Front. Plant Sci. 2021, 11, 626205. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, C.; Du, D.; Fu, Z.; Yao, Y.; Xu, C.; Zhang, H. Quantitative trait analysis of flowering time in spring rapeseed (B. napus L.). Euphytica 2014, 200, 321–335. [Google Scholar] [CrossRef]
- Huang, L.; Min, Y.; Schiessl, S.; Xiong, X.; Jan, H.U.; He, X.; Qian, W.; Guan, C.; Snowdon, R.J.; Hua, W. Integrative analysis of GWAS and transcriptome to reveal novel loci regulation flowering time in semi-winter rapeseed. Plant Sci. 2021, 310, 110980. [Google Scholar] [CrossRef]
- Scholefield, P.; Sedgley, M.; Alexander, D.M. Carbohydrate cycling in relation to shoot growth, floral initiation and development and yield in the avocado. Sci. Hortic. 1985, 25, 99–110. [Google Scholar] [CrossRef]
- Guiyu, L.; Zaifan, H.; Cuihua, Z.; Chengshu, Z. Changes in ultraweak luminescence intensity, respiration rate and physiological metabolism of Chrysanthemum during floral differentiation. Acta Hortic. Sin. 2008, 35, 1819–1824. [Google Scholar]
- Raper, C.D., Jr.; Thomas, J.F.; Tolley-Henry, L.; Rideout, J.W. Assessment of an apparent relationship between availability of soluble carbohydrates and reduced nitrogen during floral initiation in tobacco. Bot. Gaz. 1988, 149, 289–294. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Maruyama, Y.; Chiba, Y.; Kobayashi, M.J.; Joseph, B.; Shimizu, K.K.; Mochida, K.; Hiura, T.; Kon, H.; Satake, A. Nitrogen as a key regulator of flowering in Fagus crenata: Understanding the physiological mechanism of masting by gene expression analysis. Ecol. Lett. 2014, 17, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Zhang, J.; Khan, M.N.; Liu, J.; Xu, Z.; Hu, L. Temperature variation caused by sowing dates significantly affects floral initiation and floral bud differentiation processes in rapeseed (Brassica napus L.). Plant Sci. 2018, 271, 40–51. [Google Scholar] [CrossRef]
- Ebell, L. Variation in total soluble sugars of conifer tissues with method of analysis. Phytochemistry 1969, 8, 227–233. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Langfelder, P.; Horvath, S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst. Biol. 2007, 1, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putterill, J.; Laurie, R.; Macknight, R. It’s time to flower: The genetic control of flowering time. BioEssays News Rev. Mol. Cell. Dev. Biol. 2004, 26, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wellmer, F.; Riechmann, J.L. Gene networks controlling the initiation of flower development. Trends Genet. 2010, 26, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Rideout, J.W.; Raper, C.D., Jr.; Miner, G.S. Changes in ratio of soluble sugars and free amino nitrogen in the apical meristem during floral transition of tobacco. Int. J. Plant Sci. 1992, 153, 78–88. [Google Scholar] [CrossRef]
- Sachs, R. Source-sink relationships and flowering. In Strategies of Plant Reproduction; Allanheld, Osmun: Totowa, NJ, USA, 1983; pp. 263–272. [Google Scholar]
- Xiaohong, Z.; Liangjie, F.; Tewu, Y.; Zhenghua, X.; Liyong, H. Effects of chilling stress on physiological characteristics of rapeseed seedlings in winter. Plant Physiol. J. 2015, 51, 737–746. [Google Scholar] [CrossRef]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 274–279. [Google Scholar] [CrossRef]
- Swanton, C.J.; Huang, J.Z.; Shrestha, A.; Tollenaar, M.; Deen, W.; Rahimian, H. Effects of temperature and photoperiod on the phenological development of barnyardgrass. Agron. J. 2000, 92, 1125–1134. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Yu, H. Florigen trafficking integrates photoperiod and temperature signals in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 1385–1398. [Google Scholar] [CrossRef]
- Robertson, M.J.; Watkinson, A.R.; Kirkegaard, J.A.; Holland, J.F.; Potter, T.D.; Burton, W.; Walton, G.H.; Moot, D.J.; Wratten, N.; Farre, I.; et al. Environmental and genotypic control of time to flowering in canola and Indian mustard. Aust. J. Exp. Agric. 2002, 53, 793–809. [Google Scholar] [CrossRef]
- Sheldon, C.C.; Rouse, D.T.; Finnegan, E.J.; Peacock, W.J.; Dennis, E.S. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc. Natl. Acad. Sci. USA 2000, 97, 3753–3758. [Google Scholar] [CrossRef]
- Tadege, M.; Sheldon, C.C.; Helliwell, C.A.; Stoutjesdijk, P.; Dennis, E.S.; Peacock, W.J. Control of flowering time by FLC orthologues in Brassica napus. Plant J. 2001, 28, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nobutoshi, Y. The epigenetic mechanisms regulating floral hub genes and their potential for manipulation. J. Exp. Bot. 2021, 73, 1277–1287. [Google Scholar]
- Burn, J.E.; Bagnall, D.; Metzger, J.; Dennis, E.; Peacock, W. DNA methylation, vernalization, and the initiation of flowering. Proc. Natl. Acad. Sci. USA 1993, 90, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, C.C.; Finnegan, E.J.; Rouse, D.T.; Tadege, M.; Bagnall, D.J.; Helliwell, C.A.; Peacock, W.J.; Dennis, E.S. The control of flowering by vernalization. Curr. Opin. Plant Biol. 2000, 3, 418–422. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wang, Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef]
- Hedden, P.; Gibberellins. Encycl. Appl. Plant Sci. 2017, 1, 411–420. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Koshioka, M.; Kubota, S.; Fujime, Y.; King, R.W.; Mander, L.N. The role of gibberellin biosynthesis in the control of growth and flowering in Matthiola incana. Physiol. Plant. 2000, 109, 97–105. [Google Scholar] [CrossRef]
- Wilson, R.N.; Heckman, J.W.; Somerville, C.R. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992, 100, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.R.; Hong, C.B.; Paek, N.C.; Kim, S.G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]


| Variety | Treatment | Week 1 | Week 2 | Week 3 | Week 4 | Week 6 | Week 8 |
|---|---|---|---|---|---|---|---|
| 1358 | CK | × | × | √ | √ | ||
| LT | × | × | √ | √ | |||
| Zhongshuang No. 11 | CK | × | × | × | × | × | |
| LT | × | × | × | √ | √ |
| Sample Code | Bases | Total Reads | Mapped Reads | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| CK-H-1 W | 12,346,439,739 | 42,813,771 | 33,688,652 (78.69%) | 94.83 | 48.23 |
| CK-H-2 W | 8,380,248,919 | 29,292,740 | 22,657,801 (77.35%) | 95.17 | 48.00 |
| CK-H-3 W | 8,336,941,652 | 28,895,492 | 22,374,397 (77.43%) | 94.14 | 48.06 |
| CK-H-4 W | 9,640,592,280 | 33,402,546 | 25,926,724 (77.62%) | 94.68 | 48.00 |
| CK-Z-1 W | 7,709,963,913 | 26,710,793 | 20,834,744 (78.00%) | 94.94 | 48.38 |
| CK-Z-2 W | 8,908,534,540 | 31,058,959 | 24,147,919 (77.75%) | 94.92 | 47.78 |
| CK-Z-4 W | 5,647,528,648 | 19,601,439 | 15,278,467 (77.95%) | 95.16 | 47.98 |
| CK-Z-6 W | 6,928,211,354 | 24,020,110 | 18,732,732 (77.99%) | 95.14 | 47.88 |
| CK-Z-8 W | 5,512,829,371 | 19,128,138 | 14,550,138 (76.07%) | 95.27 | 48.08 |
| LT-H-1 W | 5,948,642,517 | 20,705,540 | 16,156,386 (78.03%) | 94.89 | 48.38 |
| LT-H-2 W | 6,052,433,689 | 21,111,307 | 15,589,630 (73.84%) | 94.65 | 48.73 |
| LT-H-3 W | 7,863,011,312 | 27,799,414 | 15,443,883 (55.55%) | 94.67 | 49.66 |
| LT-H-4 W | 5,625,770,861 | 19,546,507 | 15,369,155 (78.63%) | 94.55 | 48.54 |
| LT-Z-1 W | 6,504,657,487 | 22,681,919 | 17,851,101 (78.70%) | 95.01 | 48.65 |
| LT-Z-2 W | 5,888,026,230 | 20,475,304 | 16,311,490 (79.66%) | 94.63 | 48.31 |
| LT-Z-4 W | 6,179,107,275 | 21,605,985 | 16,938,907 (78.40%) | 94.99 | 48.42 |
| LT-Z-6 W | 6,065,775,007 | 21,171,760 | 16,631,248 (78.55%) | 95.04 | 48.24 |
| LT-Z-8 W | 6,965,309,530 | 24,339,956 | 19,097,804 (78.46%) | 94.97 | 48.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Lin, R.; Cheng, T.; Hu, L. Low Temperature Rather Than Nitrogen Application Mainly Modulates the Floral Initiation of Different Ecotypes of Rapeseed (Brassica napus L.). Agronomy 2022, 12, 1624. https://doi.org/10.3390/agronomy12071624
Luo T, Lin R, Cheng T, Hu L. Low Temperature Rather Than Nitrogen Application Mainly Modulates the Floral Initiation of Different Ecotypes of Rapeseed (Brassica napus L.). Agronomy. 2022; 12(7):1624. https://doi.org/10.3390/agronomy12071624
Chicago/Turabian StyleLuo, Tao, Rongmei Lin, Tai Cheng, and Liyong Hu. 2022. "Low Temperature Rather Than Nitrogen Application Mainly Modulates the Floral Initiation of Different Ecotypes of Rapeseed (Brassica napus L.)" Agronomy 12, no. 7: 1624. https://doi.org/10.3390/agronomy12071624
APA StyleLuo, T., Lin, R., Cheng, T., & Hu, L. (2022). Low Temperature Rather Than Nitrogen Application Mainly Modulates the Floral Initiation of Different Ecotypes of Rapeseed (Brassica napus L.). Agronomy, 12(7), 1624. https://doi.org/10.3390/agronomy12071624







