The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy
Abstract
:1. Introduction
2. Weed Infestation of Agricultural Plantations in Europe
2.1. Main Weed Species on Plantations of the Most Economically Important Crops in Europe
2.2. Consequences of Weed Infestation of Farmlands
2.3. Mode of Action of Weeds
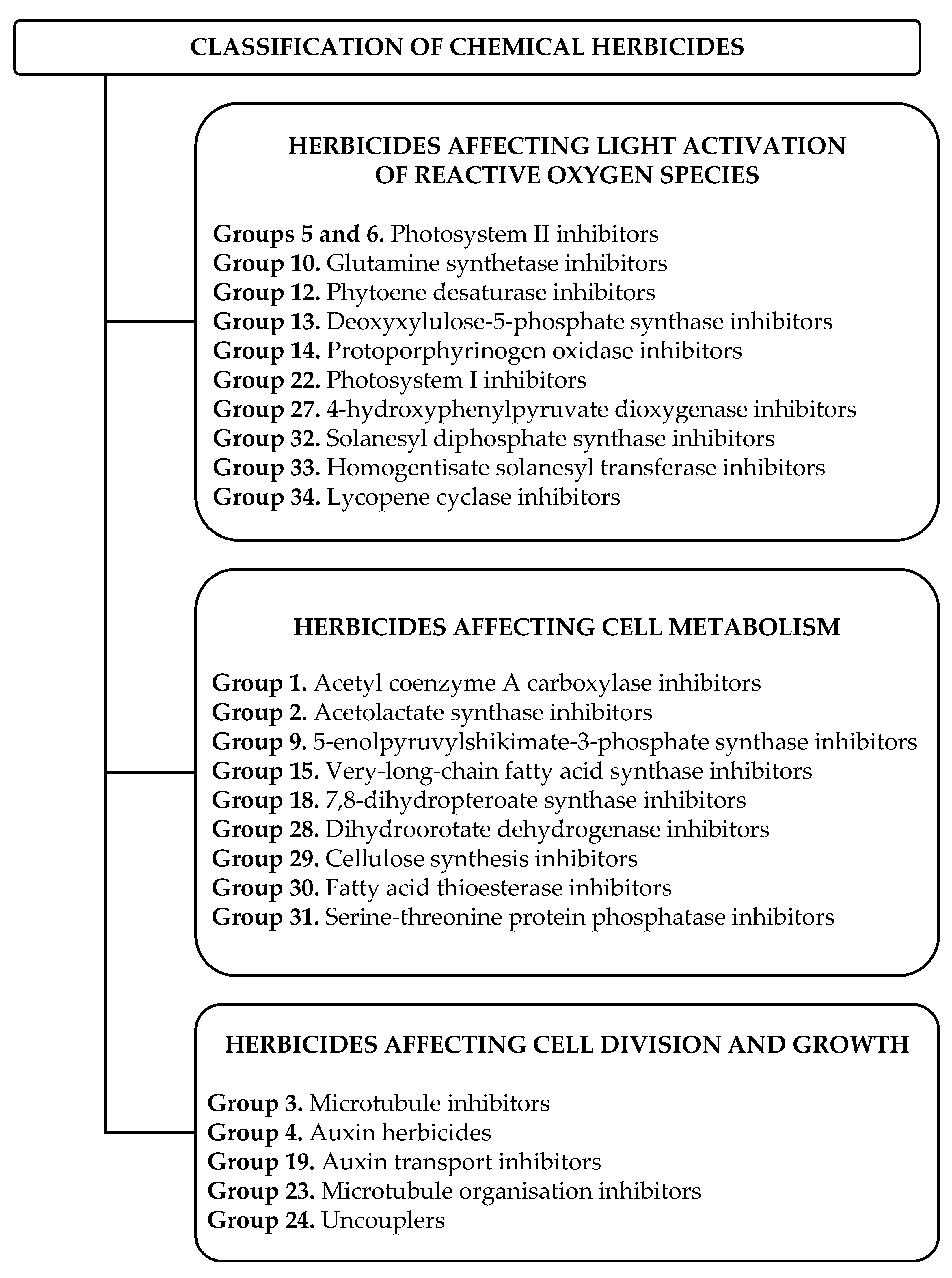
3. Chemical Herbicides
3.1. Chemical Herbicides Available on the European Market
3.2. Mode of Action of Chemical Herbicides
3.3. Mechanisms of Weed Resistance to Herbicides
3.4. Effect of Chemical Herbicides on the Environment and Human Health
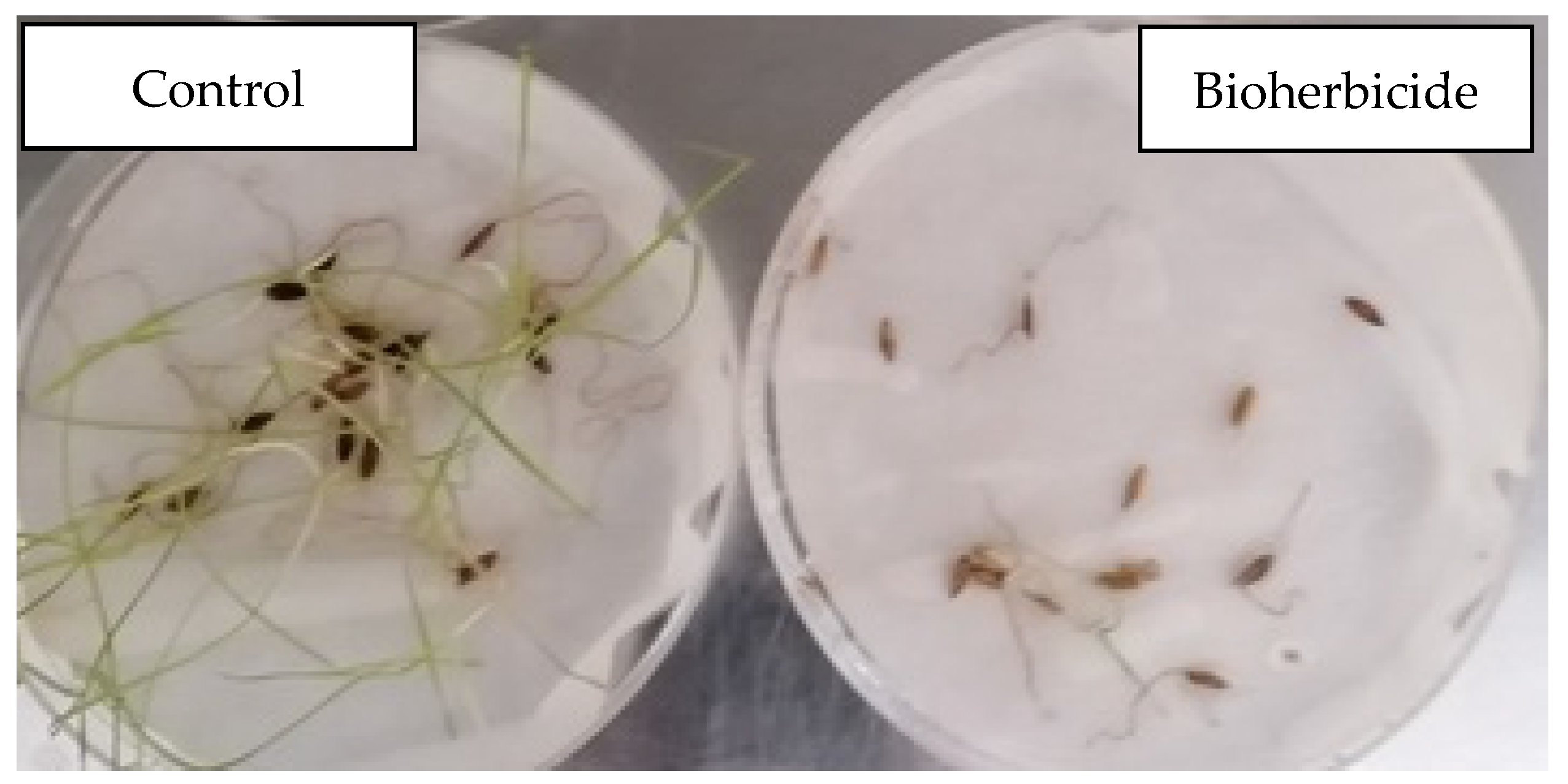
4. Bioherbicides
4.1. Mode of Action of Microorganisms on Weeds
4.2. Advantages of Bioherbicides
4.3. Commercially Available Bioherbicides
4.4. Problems with the Commercial Availability of Bioherbicides
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stokstad, E.; Grullon, G. Infographic: Pesticide planet. Science 2013, 341, 730–731. [Google Scholar] [CrossRef]
- Trognitz, F.; Hackl, E.; Widhalm, S.; Sessitsch, A. The role of plant–microbiome interactions in weed establishment and control. FEMS Microbiol. Ecol. 2016, 92, fiw138. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 245–266. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commmission to the European Parliament, The European Council, The Council, The European Economic and Social Commmittee and The Commmittee of the Regions, The European Green Deal; European Commission: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 12 June 2022).
- European Commission. Communication from the Commission to the European Parliament, The Council, The European Economic and Social Committee and The Committee of the Regions, EU Biodiversity Strategy for 2030, Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0380 (accessed on 12 June 2022).
- European Commission. Communication from the Commmission to the European Parliament, The European Council, The Council, The European Economic and Social Commmittee and The Commmittee of the Regions, Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0381 (accessed on 12 June 2022).
- European Union. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and91/414/EEC. Off. J. Eur. Union 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 12 June 2022).
- European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides, Text with EEA Relevance. Off. J. Eur. Union 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 12 June 2022).
- FAO (Food and Agriculture Organization of the United Nations). Available online: https://www.fao.org/home/en/ (accessed on 12 June 2022).
- FAOSTAT (Food and Agriculture Organization Corporate Statistical Database). Wheat, Sugar Beet, Maize Production Statistics. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 June 2022).
- Jabran, K.; Mahmood, K.; Melander, B.; Bajwa, A.A.; Kudsk, P. Weed dynamics and management in wheat. Adv. Agron. 2017, 145, 97–166. [Google Scholar] [CrossRef]
- Gaba, S.; Gabriel, E.; Chadoeuf, J.; Bonneu, F.; Bretagnolle, V. Herbicides do not ensure for higher wheat yield, but eliminate rare plant species. Sci. Rep. 2016, 6, 30112. [Google Scholar] [CrossRef] [Green Version]
- Hofmeijer, M.A.; Melander, B.; Salonen, J.; Lundkvist, A.; Zarina, L.; Gerowitt, B. Crop diversification affects weed communities and densities in organic spring cereal fields in northern Europe. Agric. Ecosyst. Environ. 2021, 308, 107251. [Google Scholar] [CrossRef]
- Pinke, G.; Pal, R.; Botta-Dukat, Z. Effects of environmental factors on weed species composition of cereal and stubble fields in western Hungary. Cent. Eur. J. Biol. 2010, 5, 283–292. [Google Scholar] [CrossRef]
- Cimalova, S.; Lososova, Z. Arable weed vegetation of the northeastern part of the Czech Republic: Effects of environmental factors on species composition. Plant Ecol. 2009, 203, 45–57. [Google Scholar] [CrossRef]
- Harasim, E.; Wesołowski, M.; Kwiatkowski, C. The effect of reduced growth retardant rates on weed infestation of a winter wheat (Triticum aestivum L.) crop. Rom. Agric. Res. 2014, 31, 271–281. [Google Scholar]
- Sawicka, B.; Krochmal-Marczak, B.; Barbaś, P.; Pszczółkowski, P.; Ćwintal, M. Biodiversity of weeds in fields of grain in South-Eastern Poland. Agriculture 2020, 10, 589. [Google Scholar] [CrossRef]
- Chitband, A.A.; Ghorbani, R.; Mohassel, M.H.R.; Abbaspoor, M.; Abbasi, R. Evaluation of broadleaf weeds control with selectivity of post-emergence herbicides in sugar beet (Beta vulgaris L.). Not. Sci. Biol. 2014, 6, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Bezhin, K.; Santel, H.J.; Gerhards, R. Evaluation of two chemical weed control systems in sugar beet in Germany and the Russian Federation. Plant Soil Environ. 2015, 61, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Gerhards, R.; Bezhin, K.; Santel, H.J. Sugar beet yield loss predicted by relative weed cover, weed biomass and weed density. Plant Protect. Sci. 2017, 53, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Heno, S.; Viou, L.; Khan, M.F. Sugar beet production in France. Sugar Tech 2018, 20, 392–395. [Google Scholar] [CrossRef]
- Domaradzki, K.; Jezierska-Domaradzka, A. Changes in weed infestations on plantations of sugar beet (Beta vulgaris L. subsp. vulgaris) cultivated on black soil near Wrocław in 1989–1995 and 2006–2012. Acta Agrobot. 2016, 69, 1647. [Google Scholar] [CrossRef] [Green Version]
- Serban, M.; Maturaru, G.; Lazar, C.; Gradila, M.; Ciontu, C. Research on the selectivity and the efficacy of herbicides in controlling weeds for the maize crop. Rom. Agric. Res. 2021, 38, 371–379. [Google Scholar]
- Fried, G.; Chauvel, B.; Munoz, F.; Reboud, X. Which traits make weeds more successful in maize crops? Insights from a three-decade monitoring in France. Plants 2020, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Gołębiowska, H.; Snopczyński, T.; Domaradzki, K.; Rola, H. Changes in weed infestation in corn crops in southwestern region of Poland in 1963–2013 years. Prog. Plant Prot. 2015, 55, 327–339. [Google Scholar] [CrossRef]
- Chauhan, B.S. Grand challenges in weed management. Front. Agron. 2020, 1, 3. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, M.; Sehrawat, N.; Atri, N.; Singh, R.; Upadhyay, S.K.; Yadav, M. Mycoherbicide control strategy: Concept, constraints, and advancements. Biopestic. Int. 2021, 17, 29–40. [Google Scholar]
- Majrashi, A.A. Preliminary assessment of weed population in vegetable and fruit farms of Taif, Saudi Arabia. Braz. J. Biol. 2022, 82, e255816. [Google Scholar] [CrossRef]
- Elkhouly, A.R.; Slama, A.T.; Al Hireereeq, E.A. Survey of Global Crop Loss. Balance J. Appl. Humanit. 2021, 2, 9–19. [Google Scholar]
- Zohaib, A.; Abbas, T.; Tabassum, T. Weeds cause losses in field crops through allelopathy. Not. Sci. Biol. 2016, 8, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Ustuner, T.; Sakran, A.; Almhemed, K. Effect of herbicides on living organisms in the ecosystem and available alternative control methods. Int. J. Sci. Res. Publ. 2020, 10, 633–641. [Google Scholar] [CrossRef]
- Singh, V.V.; Singh, S.K.; Pratap, T. Tank mix herbicide combination effect on weed and yield of wheat in North-Eastern plain zone. Pharma Innov. J. 2020, 11, 1359–1362. [Google Scholar]
- Lobmann, A.; Christen, O.; Petersen, J. Development of herbicide resistance in weeds in a crop rotation with acetolactate synthase-tolerant sugar beets under varying selection pressure. Weed Res. 2019, 59, 479–489. [Google Scholar] [CrossRef]
- Isik, D.; Akca, A. Assessment of weed competition critical period in sugar beet. Tarım Bilimleri Derg. 2018, 24, 82–90. [Google Scholar] [CrossRef]
- Bruciene, I.; Aleliunas, D.; Sarauskis, E.; Romaneckas, K. Influence of Mechanical and Intelligent Robotic Weed Control Methods on Energy Efficiency and Environment in Organic Sugar Beet Production. Agriculture 2021, 11, 449. [Google Scholar] [CrossRef]
- Abd El Lateef, E.M.; Mekki, B.B.; Abd El-Salam, M.S.; El-Metwally, I.M. Effect of different single herbicide doses on sugar beet yield, quality and associated weeds. Bull. Natl. Res. Cent. 2021, 45, 21. [Google Scholar] [CrossRef]
- Jain, L.; Maliwal, P. Growth and productivity of maize (Zea mays L.) as influenced by organic weed and nutrient management practices in Western Rajasthan. Am. Political Sci. Rev. 2022, 24, 59–64. [Google Scholar] [CrossRef]
- Pant, C.; Sah, S.K.; Marahatta, S.; Dhakal, S. Weed dynamics in no-till maize system and its management: A review. Agron. J. Nepal. 2021, 5, 168–177. [Google Scholar] [CrossRef]
- Simon, A.; Bardas, M.; Popa, A. Research on the weeds control in maize crop. Agrarian Economy and Rural Development-Realities and Perspectives for Romania. In Agrarian Economy and Rural Development—Realities and Perspectives for Romania. International Symposium, 10th ed.; The Research Institute for Agricultural Economy and Rural Development (ICEADR): Bucharest, Romania, 2019. [Google Scholar]
- Kołodziejczyk, M.; Antonkiewicz, J.; Kulig, B. Effect of living mulches and conventional methods of weed control on weed occurrence and nutrient uptake in potato. Int. J. Plant Prod. 2017, 11, 275–284. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Grzywacz, K.; Niewęgłowski, M. Marketable yield of potato and its quantitative parameters after application of herbicides and biostimulants. Agriculture 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Shen, S.; Chen, H. Bio-herbicidal potential of wheat rhizosphere bacteria on Avena fatua L. grass. Bioengineered 2021, 12, 516–526. [Google Scholar] [CrossRef]
- Kocira, A.; Staniak, M. Weed ecology and new approaches for management. Agriculture 2021, 11, 262. [Google Scholar] [CrossRef]
- Aneja, K.R.; Khan, S.A.; Aneja, A. Bioherbicides: Strategies, challenges and prospects. In Developments in Fungal Biology and Applied Mycology; Satyanarayana, T., Deshmukh, S.K., Johri, B.N., Eds.; Springer: Singapore, 2017; pp. 449–470. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Barbaś, P.; Sawicka, B.; Krochmal-Marczak, B. Biological and agrotechnical aspects of weed control in the cultivation of early potato cultivars under cover. Agriculture 2020, 10, 373. [Google Scholar] [CrossRef]
- Massenssini, A.M.; Bonduki, V.H.A.; Melo, C.A.D.; Tótola, M.R.; Ferreira, F.A.; Costa, M.D. Soil microorganisms and their role in the interactions between weeds and crops. Planta Daninha 2014, 32, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Barbaś, P.; Sawicka, B.; Marczak, B.K.; Pszczółkowski, P. Effect of mechanical and herbicide treatments on weed densities and biomass in two potato cultivars. Agriculture 2020, 10, 455. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Nakielska, M.; Jończyk, K.; Berbeć, A.K.; Kopiński, J. Assessment of the suitability of 10 winter triticale cultivars (x Triticosecale Wittm. ex A. Camus) for organic agriculture: Polish case study. Agronomy 2020, 10, 1144. [Google Scholar] [CrossRef]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a changing climate: Vulnerabilities, consequences, and implications for future weed management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar] [CrossRef]
- Vila, M.; Beaury, E.M.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Laginhas, B.B.; Trillo, A.; Dukes, J.; Sorte, C.; Ibanez, I. Understanding the combined impacts of weeds and climate change on crops. Environ. Res. Lett. 2021, 16, 034043. [Google Scholar] [CrossRef]
- Błażewicz-Woźniak, M.; Patkowska, E.; Konopiński, M.; Wach, D. Effect of cover crops and ploughless tillage on weed infestation of field after winter before pre-sowing tillage. Rom. Agric. Res. 2016, 33, 185–194. [Google Scholar]
- Woźniak, A.; Soroka, M. Effects of a 3-year reduced tillage on the yield and quality of grain and weed infestation of spring triticale (Triticosecale Wittmack). Int. J. Plant Prod. 2014, 8, 231–241. [Google Scholar]
- Gawęda, D.; Woźniak, A.; Harasim, E. Weed infestations of winter wheat depend on the forecrop and the tillage syste. Acta Agrobot. 2018, 71, 1744. [Google Scholar] [CrossRef]
- Nwosisi, S.; Nandwani, D.; Hui, D. Mulch treatment effect on weed biomass and yields of organic sweetpotato cultivars. Agronomy 2019, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Karkanis, A.; Ntatsi, G.; Alemardan, A.; Petropoulos, S.; Bilalis, D. Interference of weeds in vegetable crop cultivation, in the changing climate of Southern Europe with emphasis on drought and elevated temperatures: A review. J. Agric. Sci. 2018, 156, 1175–1185. [Google Scholar] [CrossRef]
- Bufford, J.L.; Hulme, P.E. Increased adaptive phenotypic plasticity in the introduced range in alien weeds under drought and flooding. Biol. Invasions 2021, 23, 2675–2688. [Google Scholar] [CrossRef]
- Sharma, G.; Barney, J.N.; Westwood, J.H.; Haak, D.C. Into the weeds: New insights in plant stress. Trends Plant Sci. 2021, 26, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. In Co-Evolution of Secondary Metabolites; Merillon, J.M., Ramawat, K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Khan, S.; Ali, K.W.; Shinwari, M.I.; Khan, R.A.; Rana, T. Environmental, ecological and evolutionary effects of weeds allelopathy. Int. J. Bot. Stud. 2019, 4, 77–84. [Google Scholar]
- Xuan, T.D.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Minh, T.N.; Khanh, T.D.; Trung, K.H. Weed allelochemicals and possibility for pest management. Int. Lett. Nat. Sci. 2016, 56, 25–39. [Google Scholar] [CrossRef] [Green Version]
- Zohaib, A.; Anjum, S.A.; Jabbar, A.; Tabassum, T.; Abbas, T.; Nazir, U. Allelopathic effect of leguminous weeds on rate, synchronization and time of germination, and biomass partitioning in rice. Planta Daninha 2017, 35, 1–11. [Google Scholar] [CrossRef]
- Nornasuha, Y.; Ismail, B.S. Sustainable weed management using allelopathic approach. Malays. J. Appl. Biol. 2017, 46, 1–10. [Google Scholar]
- Zohaib, A.; Tabassum, T.; Anjum, S.A.; Abbas, T.; Nazir, U. Allelopathic effect of some associated weeds of wheat on germinability and biomass production of wheat seedlings. Planta Daninha 2018, 35, 1–13. [Google Scholar] [CrossRef]
- Radivojević, L.; Sarić-Krsmanović, M.; Gajić-Umiljendić, J.; Santrić, L. Allelopathic effects of invasive weed species Abutilon theophrasti Medik., Ambrosia artemisiifolia L., Datura stramonium L. and Xanthium strumarium L. on tomato. Pestic. Fitomedicina 2019, 34, 183–191. [Google Scholar] [CrossRef]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Syed, S.; Zohaib, A.; Farooq, N.; Shehzad, M.A. Allelopathic influence of aquatic weeds on agro-ecosystems: A review. Planta Daninha 2017, 35, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical composition and phytotoxicity of essential oil from invasive plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. 2020, 10, 11332. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an Invasive Plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Zohaib, A.; Tanveer, A.; Khaliq, A.; Safdar, M.E.; Tahir, M. Water soluble phenolics in five winter season leguminous weeds and their phytotoxicity against wheat. Pak. J. Weed Sci. Res. 2016, 22, 511–525. [Google Scholar]
- Duke, S.O.; Dayan, F.E. Herbicides. In eLS; Wiley: Hoboken, NJ, USA, 2018; Volume 1, pp. 1–9. [Google Scholar] [CrossRef]
- Rejestr Środków Ochrony Roślin Dopuszczonych Do Obrotu Zezwoleniem Ministra Rolnictwa I Rozwoju Wsi. Available online: https://www.gov.pl/web/rolnictwo/rejestr-rodkow-ochrony-roslin (accessed on 12 June 2022).
- EUROSTAT (European Statistical Office). Available online: https://ec.europa.eu/eurostat (accessed on 12 June 2022).
- Das, S.K.; Mondal, T. Mode of action of herbicides and recent trends in development: A reappraisal. Int. J. Agric. Soil Sci. 2014, 2, 27–32. [Google Scholar]
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; InTech: London, UK, 2015; pp. 165–186. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E.; Barker, A.; Bough, R.; Ortiz, M.; Takano, H.; Duke, S.O. Herbicide mechanisms of action and resistance. Compr. Biotechnol. 2019, 3, 36–48. [Google Scholar] [CrossRef]
- HRAC (Herbicide Resistance Action Committee). Available online: https://hracglobal.com/ (accessed on 12 June 2022).
- Jugulam, M.; Shyam, C. Non-target-site resistance to herbicides: Recent developments. Plants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayan, F.E.; Zaccaro, M.L.D.M. Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pestic. Biochem. Physiol. 2012, 102, 189–197. [Google Scholar] [CrossRef]
- WSSA (Weed Science Society of America). Available online: https://wssa.net/wssa/weed/herbicides/ (accessed on 12 June 2022).
- Kraehmer, H.; Laber, B.; Rosinger, C.; Schulz, A. Herbicides as weed control agents: State of the art: I. Weed control research and safener technology: The path to modern agriculture. Plant Physiol. 2014, 166, 1119–1131. [Google Scholar] [CrossRef] [Green Version]
- Grzanka, M.; Sobiech, Ł.; Idziak, R.; Skrzypczak, G. Effect of the Time of Herbicide Application and the Properties of the Spray Solution on the Efficacy of Weed Control in Maize (Zea mays L.) Cultivation. Agriculture 2022, 12, 353. [Google Scholar] [CrossRef]
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide resistant weeds: A call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest. Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Hwang, J.I.; Norsworthy, J.K.; Gonzalez-Torralva, F.; Piveta, L.B.; Barber, L.T.; Butts, T.R. Cross-resistance of barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] to aryloxyphenoxypropionate herbicides. Pestic. Biochem. Physiol. 2022, 184, 105089. [Google Scholar] [CrossRef] [PubMed]
- Yanniccari, M.; Gigón, R.; Larsen, A. Cytochrome P450 Herbicide Metabolism as the Main Mechanism of Cross-Resistance to ACCase- and ALS-Inhibitors in Lolium spp. Populations From Argentina: A Molecular Approach in Characterization and Detection. Front. Plant Sci. 2020, 11, 600301. [Google Scholar] [CrossRef]
- LeClere, S.; Wu, C.; Westra, P.; Sammons, R.D. Cross-resistance to dicamba, 2,4-D, and fluroxypyr in Kochia scoparia is endowed by a mutation in an AUX/IAA gene. Proc. Natl. Acad. Sci. USA 2018, 115, 2911–2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez-Garcia, J.G.; Alcantara-de la Cruz, R.; Palma-Bautista, C.; Rojano-Delgado, A.M.; Cruz-Hipólito, H.E.; Torra, J.; Barro, F.; de Prado, R. Accumulation of Target Gene Mutations Confers Multiple Resistance to ALS, ACCase, and EPSPS Inhibitors in Lolium Species in Chile. Front. Plant Sci. 2020, 11, 553948. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Li, X.; Li, D.; Li, Z.; Cui, H. Pro-197-Ser Mutation in ALS and High-Level GST Activities: Multiple Resistance to ALS and ACCase Inhibitors in Beckmannia syzigachne. Front. Plant Sci. 2020, 11, 572610. [Google Scholar] [CrossRef]
- Tahmasebi, B.K.; Alcantara-de la Cruz, R.; Alcantara, E.; Torra, J.; Dominguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; Rojano-Delgado, A.M.; de Prado, R. Multiple Resistance Evolution in Bipyridylium-Resistant Epilobium ciliatum After Recurrent Selection. Front. Plant Sci. 2018, 9, 695. [Google Scholar] [CrossRef]
- International Herbicide-Resistant Weed Database. Available online: https://www.weedscience.org/Home.aspx (accessed on 12 June 2022).
- Delye, C.; Duhoux, A.; Pernin, F.; Riggins, C.; Tranel, P. Molecular Mechanisms of Herbicide Resistance. Weed Sci. 2015, 63, 91–115. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, H.; Wei, S.; Cui, H.; Li, X.; Zhang, C. Glyphosate resistance in Eleusine indica: EPSPS overexpression and P106A mutation evolved in the same individuals. Pestic. Biochem. Physiol. 2020, 164, 203–208. [Google Scholar] [CrossRef]
- Hada, Z.; Menchari, Y.; Rojano-Delgado, A.M.; Torra, J.; Menedez, J.; Palma-Bautista, C.; de Prado, R.; Souissi, T. Point Mutations as Main Resistance Mechanism Together With P450-Based Metabolism Confer Broad Resistance to Different ALS-Inhibiting Herbicides in Glebionis coronaria From Tunisia. Front. Plant Sci. 2021, 12, 626702. [Google Scholar] [CrossRef]
- Zhao, N.; Ge, L.; Yan, Y.; Bai, S.; Wang, D.; Liu, W.; Wang, J. Trp-1999-Ser mutation of acetyl-CoA carboxylase and cytochrome P450s-involved metabolism confer resistance to fenoxaprop-P-ethyl in Polypogon fugax. Pest. Manag. Sci. 2019, 75, 3175–3183. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.; Tranel, P.J.; Kupper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Gaines, T.A.; Jhala, A.J.; Knezevic, S.Z. Inheritance of Mesotrione Resistance in an Amaranthus tuberculatus (var. rudis) Population from Nebraska, USA. Front. Plant Sci. 2018, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Brunharo, C.; Streisfeld, M. Multiple evolutionary origins of glyphosate resistance in Lolium multiflorum. Evol. Appl. 2022, 15, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Ruzmi, R.; Moosa, S.; Asib, N.; Zulperi, D.; Ismail, S.I.; Ahmad-Hamdani, M.S. Asp-376-Glu substitution endows target-site resistance to AHAS inhibitors in Limnocharis flava, an invasive weed in tropical rice fields. Physiol. Mol. Biol. Plants 2021, 27, 969–983. [Google Scholar] [CrossRef]
- Yanniccari, M.; Vazquez-Garcia, J.G.; Gigón, R.; Palma-Bautista, C.; Vila-Aiub, M.; de Prado, R. A novel EPSPS Pro-106-His mutation confers the first case of glyphosate resistance in Digitaria sanguinalis. Pest. Manag. Sci. 2022, 78, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, Q.; Han, H.; Yu, C.; Nyporko, A.; Tian, X.; Beckie, H.; Powles, S. A naturally evolved mutation (Ser59Gly) in glutamine synthetase confers glufosinate resistance in plants. J. Exp. Bot. 2022, 73, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Kuepper, A.; Malone, J.; Petrovic, T.; Figueiredo, A.; Campagnola, G.; Peersen, O.; Prasad, K.; Patterson, E.; Reddy, A.; et al. An in-frame deletion mutation in the degron tail of auxin co-receptor IAA2 confers resistance to the herbicide 2,4-D in Sisymbrium orientale. Proc. Natl. Acad. Sci. USA 2021, 119, e2105819119. [Google Scholar] [CrossRef]
- Vital Silva, V.; Mendes, R.; Suzukawa, A.; Adegas, F.; Marcelino-Guimaraes, F.; Oliveira, R., Jr. A Target-Site Mutation Confers Cross-Resistance to ALS-Inhibiting Herbicides in Erigeron sumatrensis from Brazil. Plants 2022, 11, 467. [Google Scholar] [CrossRef]
- Widianto, R.; Kurniadie, D.; Widayat, D.; Umiyati, U.; Nasahi, C.; Sari, S.; Juraimi, A.S.; Kato-Noguchi, H. Acetolactate Synthase-Inhibitor Resistance in Monochoria vaginalis (Burm. f.) C. Presl from Indonesia. Plants 2022, 11, 400. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Fernandez-Moreno, P.T.; Alcantara-de la Cruz, R.; Sanchez-Gonzalez, E.; Cruz-Hipolito, H.E.; Dominguez-Valenzuela, J.A.; de Prado, R. Pro-106-Ser mutation and EPSPS overexpression acting together simultaneously in glyphosate-resistant goosegrass (Eleusine indica). Sci. Rep. 2017, 7, 6702. [Google Scholar] [CrossRef] [Green Version]
- Delye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef]
- Mahmood, K.; Mathiassen, S.K.; Kristensen, M.; Kudsk, P. Multiple Herbicide Resistance in Lolium multiflorum and Identification of Conserved Regulatory Elements of Herbicide Resistance Genes. Front. Plant Sci. 2016, 7, 1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Bai, S.; Zhao, N.; Jia, S.; Li, W.; Zhang, L.; Wang, J. Non-target site-based resistance to tribenuron-methyl and essential involved genes in Myosoton aquaticum (L.). BMC Plant Biol. 2018, 18, 225. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Non-target site mechanisms of resistance to herbicides. Crit. Rev. Plant Sci. 2017, 36, 24–34. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Gaines, T.A.; Dayan, F.E.; Patterson, E.L.; Jhala, A.J.; Knezevic, S.Z. Reversing resistance to tembotrione in an Amaranthus tuberculatus (var. rudis) population from Nebraska, USA with cytochrome P450 inhibitors. Pest. Manag. Sci. 2018, 74, 2296–2305. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Li, J.; Shen, J.; Xu, Y.; Liu, H.; Deng, W.; Li, X.; Zheng, M. Metabolic Resistance to Acetolactate Synthase Inhibiting Herbicide Tribenuron-Methyl in Descurainiasophia L. Mediated by Cytochrome P450 Enzymes. J. Agric. Food Chem. 2018, 66, 4319–4327. [Google Scholar] [CrossRef]
- Guo, F.; Iwakami, S.; Yamaguchi, T.; Uchino, A.; Sunohara, Y.; Matsumoto, H. Role of CYP81A cytochrome P450s in clomazone metabolism in Echinochloa phyllopogon. Plant Sci. 2019, 283, 321–328. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Q.; Wang, J.; Han, H.; Mao, L.; Nyporko, A.; Maguza, A.; Fan, L.; Bai, L.; Powles, S. An ABCC-type transporter endowing glyphosate resistance in plants. Proc. Natl. Acad. Sci. USA 2021, 118, e2100136118. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, V.; Osborne, C.; Freckleton, R. Drought exposure leads to rapid acquisition and inheritance of herbicide resistance in the weed Alopecurus myosuroides. Ecol. Evol. 2022, 12, e8563. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Markus, C.; Pecinka, A.; Merotto, A., Jr. Insights into the Role of Transcriptional Gene Silencing in Response to Herbicide-Treatments in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 3314. [Google Scholar] [CrossRef] [PubMed]
- Tyczewska, A.; Gracz-Bernaciak, J.; Szymkowiak, J.; Twardowski, T. Herbicide stress-induced DNA methylation changes in two Zea mays inbred lines differing in Roundup resistance. J. Appl. Genet. 2021, 62, 235–248. [Google Scholar] [CrossRef]
- El-Nahhal, I.; El-Nahhal, Y. Pesticide residues in drinking water, their potential risk to human health and removal options. J. Environ. Manag. 2021, 299, 113611. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lim, W.; Song, G. Reproductive toxicity due to herbicide exposure in freshwater organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109103. [Google Scholar] [CrossRef]
- Wan, Y.; Tran, T.M.; Nguyen, V.T.; Wang, A.; Wang, J.; Kannan, K. Neonicotinoids, fipronil, chlorpyrifos, carbendazim, chlorotriazines, chlorophenoxy herbicides, bentazon, and selected pesticide transformation products in surface water and drinking water from northern Vietnam. Sci. Total Environ. 2021, 750, 141507. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Balderrama-Carmona, A.P.; Valenzuela-Rincón, M.; Zamora-Alvarez, L.A.; Adan-Bante, N.P.; Leyva-Soto, L.A.; Silva-Beltran, N.P.; Moran-Palacio, E.F. Herbicide biomonitoring in agricultural workers in Valle del Mayo, Sonora Mexico. Environ. Sci. Pollut. Res. 2020, 27, 28480–28489. [Google Scholar] [CrossRef]
- Marino, M.; Mele, E.; Viggiano, A.; Nori, S.L.; Meccariello, R.; Santoro, A. Pleiotropic Outcomes of Glyphosate Exposure: From Organ Damage to Effects on Inflammation, Cancer, Reproduction and Development. Int. J. Mol. Sci. 2021, 22, 12606. [Google Scholar] [CrossRef]
- Marin-Morales, M.A.; Ventura-Camargo, B.C.; Hoshina, M.M. Toxicity of herbicides: Impact on aquatic and soil biota and human health. In Herbicides—Current Research and Case Studies in Use; Price, A., Ed.; InTech: London, UK, 2013; pp. 399–443. [Google Scholar] [CrossRef] [Green Version]
- Suppa, A.; Kvist, J.; Li, X.; Dhandapani, V.; Almulla, H.; Tian, A.Y.; Kissane, S.; Zhou, J.; Perotti, A.; Mangelson, H.; et al. Roundup causes embryonic development failure and alters metabolic pathways and gut microbiota functionality in non-target species. Microbiome 2020, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Zaluski, A.B.; Wiprich, M.T.; de Almeida, L.F.; de Azevedo, A.P.; Bonan, C.D.; Vianna, M. Atrazine and Diuron Effects on Survival, Embryo Development, and Behavior in Larvae and Adult Zebrafish. Front. Pharmacol. 2022, 13, 841826. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Y.; Lu, K.; Xiong, H.; Zhang, Y.; Wei, W. Herbicide atrazine exposure induce oxidative stress, immune dysfunction and WSSV proliferation in red swamp crayfish Procambarus clarkii. Chemosphere 2021, 283, 131227. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zioga, E.; Kelly, R.; White, B.; Stout, J.C. Plant protection product residues in plant pollen and nectar: A review of current knowledge. Environ. Res. 2020, 189, 109873. [Google Scholar] [CrossRef] [PubMed]
- Onur, B.; Cavusoglu, K.; Yalcin, E.; Acar, A. Paraquat toxicity in different cell types of Swiss albino mice. Sci. Rep. 2022, 12, 4818. [Google Scholar] [CrossRef]
- Serra, L.; Estienne, A.; Vasseur, C.; Froment, P.; Dupont, J. Review: Mechanisms of Glyphosate and Glyphosate-Based Herbicides Action in Female and Male Fertility in Humans and Animal Models. Cells 2021, 10, 3079. [Google Scholar] [CrossRef]
- Harper, A.P.; Finger, B.J.; Green, M.P. Chronic Atrazine Exposure Beginning Prenatally Impacts Liver Function and Sperm Concentration With Multi-Generational Consequences in Mice. Front. Endocrinol. 2020, 11, 580124. [Google Scholar] [CrossRef]
- Mohammadi, K.; Sani, M.A.; Safaei, P.; Rahmani, J.; Molaee-Aghaee, E.; Jafari, S.M. A systematic review and meta-analysis of the impacts of glyphosate on the reproductive hormones. Environ. Sci. Pollut. Res. Int. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Zhang, L.; Rana, I.; Shaffer, R.M.; Taioli, E.; Sheppard, L. Exposure to glyphosate-based herbicides and risk for non-Hodgkin lymphoma: A meta-analysis and supporting evidence. Mutat. Res./Rev. Mutat. Res. 2019, 781, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, T.M.A.; Benvindo-Souza, M.; de Araujo Nascimento, F.; Woch, J.; dos Reis, F.G.; de Melo e Silva, D. Cancer and occupational exposure to pesticides: A bibliometric study of the past 10 years. Environ. Sci. Pollut. Res. 2022, 29, 17464–17475. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ma, L.; Shan, J.; Wan, X.; Hammock, B.D.; Hashimoto, K. Autism-like Behaviors in Male Juvenile Offspring after Maternal Glyphosate Exposure. Clin. Psychopharmacol. Neurosci. 2021, 19, 554–558. [Google Scholar] [CrossRef]
- Pu, Y.; Yang, J.; Chang, L.; Qu, Y.; Wang, S.; Zhang, K.; Xiong, Z.; Zhang, J.; Tan, Y.; Wang, X.; et al. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2020, 117, 11753–11759. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Harding, D.P.; Raizada, M.N. Controlling weeds with fungi, bacteria and viruses: A review. Front. Plant Sci. 2015, 6, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.S.; Nautiyal, C.S. Unravelling the beneficial role of microbial contributors in reducing the allelopathic effects of weeds. Appl. Microbiol. Biotechnol. 2013, 97, 5659–5668. [Google Scholar] [CrossRef]
- Phukan, J.; Deka, J.; Kurmi, K.; Kalita, S. Deleterious rhizobacteria as a potential bioherbicide—A review. Int. J. Agric. Environ. Sci. 2021, 8, 1–5. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumari, S.; Singh, A.; Prabha, C. Isolation and characterization of deleterious Pseudomonas aeruginosa KC1 from rhizospheric soils and its interaction with weed seedlings. J. King Saud Univ.-Sci. 2015, 27, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Lawrance, S.; Varghese, S.; Varghese, E.; Asok, A. Quinoline derivatives producing Pseudomonas aeruginosa H6 as an efficient bioherbicide for weed management. Biocatal. Agric. Biotechnol. 2019, 18, 101096. [Google Scholar] [CrossRef]
- Flores-Vargas, R.; O’Hara, G. Isolation and characterization of rhizosphere bacteria with potential for biological control of weeds in vineyards. J. Appl. Microbiol. 2006, 100, 946–954. [Google Scholar] [CrossRef]
- Zhao, L.J.; Yang, X.N.; Li, X.Y.; Mu, W.; Liu, F. Antifungal, insecticidal and herbicidal properties of volatile components from Paenibacillus polymyxa strain BMP-11. Agric. Sci. China 2011, 10, 728–736. [Google Scholar] [CrossRef]
- Verdugo-Navarrete, C.; Maldonado-Mendoza, I.; Castro-Martinez, C.; Leyva-Madrigal, K.; Martinez-Alvarez, J. Selection of rhizobacteria isolates with bioherbicide potential against Palmer amaranth (Amarathus palmeri S. Wats.). Braz. J. Microbiol. 2021, 52, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Radhakrishnan, R.; Kang, S.M.; Lee, I.J. IAA producing Enterobacter sp. I-3 as a potent bio-herbicide candidate for weed control: A special reference with lettuce growth inhibition. Indian J. Microbiol. 2015, 55, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, R.; Park, J.M.; Lee, I.J. Enterobacter sp. I-3, a bio-herbicide inhibits gibberellins biosynthetic pathway and regulates abscisic acid and amino acids synthesis to control plant growth. Microbiol. Res. 2016, 193, 132–139. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Park, J.M.; Lee, I.J.; Abd Allah, E.; Hashem, A. Bio-herbicide effect of salt marsh tolerant Enterobacter sp. I-3 on weed seed germination and seedling growth. Pak. J. Bot. 2017, 49, 1959–1963. [Google Scholar]
- Kremer, R. Bioherbicides and nanotechnology: Current status and future trends. In Nano-Biopesticides Today and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2019; pp. 353–366. [Google Scholar] [CrossRef]
- Sindhu, S.; Khandelwal, A.; Phour, M.; Sehrawat, A. Bioherbicidal potential of rhizosphere microorganisms for ecofriendly weed management. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer: Singapore, 2018; pp. 331–376. [Google Scholar] [CrossRef]
- Phour, M.; Sindhu, S. Bio-herbicidal effect of 5-aminoleveulinic acid producing rhizobacteria in suppression of Lathyrus aphaca weed growth. BioControl 2019, 64, 221–232. [Google Scholar] [CrossRef]
- Krain, A.; Siupka, P. Fungal Guttation, a Source of Bioactive Compounds, and Its Ecological Role-A Review. Biomolecules 2021, 11, 1270. [Google Scholar] [CrossRef] [PubMed]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef]
- Idnurm, A.; Howlett, B.J. Pathogenicity genes of phytopathogenic fungi. Mol. Plant Pathol. 2001, 2, 241–255. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic Fungal Volatile Compounds as Solution for Sustainable Agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic Secondary Metabolites from Fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Triolet, M.; Guillemin, J.P.; Andre, O.; Steinberg, C. Fungal-based bioherbicides for weed control: A myth or a reality? Weed Res. 2020, 60, 60–77. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.; Chen, S.; Yang, C.; Qiang, S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest. Manag. Sci. 2019, 75, 2482–2489. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiang, S. Recent advances in tenuazonic acid as a potential herbicide. Pestic. Biochem. Physiol. 2017, 143, 252–257. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Luo, Z.; Gao, L.; Li, R.; Zhang, Y.; Kalaji, H.M.; Qiang, S.; Chen, S. Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis. J. Fungi 2022, 8, 168. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Evidente, A. Fungal phytotoxins with potential herbicidal activity to control Chenopodium album. Nat. Prod. Commun. 2015, 10, 1119–1126. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Ray, P. Mycoherbicides for the Noxious Meddlesome: Can Colletotrichum be a Budding Candidate? Front. Microbiol. 2021, 12, 754048. [Google Scholar] [CrossRef]
- Anteyi, W.O.; Klaiber, I.; Rasche, F. Diacetoxyscirpenol, a Fusarium exometabolite, prevents efficiently the incidence of the parasitic weed Striga hermonthica. BMC Plant Biol. 2022, 22, 84. [Google Scholar] [CrossRef]
- Poluektova, E.; Tokarev, Y.; Sokornova, S.; Chisty, L.; Evidente, A.; Berestetskiy, A. Curvulin and phaeosphaeride A from Paraphoma sp. VIZR 1.46 isolated from Cirsium arvense as potential herbicides. Molecules 2018, 23, 2795. [Google Scholar] [CrossRef] [Green Version]
- Evidente, M.; Cimmino, A.; Zonno, M.C.; Masi, M.; Berestetskyi, A.; Santoro, E.; Superchi, S.; Vurro, M.; Evidente, A. Phytotoxins produced by Phomachenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2015, 117, 482–488. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Avolio, F.; Berestetskiy, A.; Vurro, M.; Evidente, A. Chenopodolans A–C: Phytotoxic furopyrans produced by Phomachenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2013, 96, 208–213. [Google Scholar] [CrossRef]
- Graupner, P.R.; Carr, A.; Clancy, E.; Gilbert, J.; Bailey, K.L.; Derby, J.A.; Gerwick, B.C. The Macrocidins: Novel Cyclic Tetramic Acids with Herbicidal Activity Produced by Phomamacrostoma. J. Nat. Prod. 2003, 66, 1558–1561. [Google Scholar] [CrossRef]
- Hubbard, M.; Taylor, W.G.; Bailey, K.L.; Hynes, R.K. The dominant modes of action of macrocidins, bioherbicidal metabolites of Phomamacrostoma, differ between susceptible plant species. Environ. Exp. Bot. 2016, 132, 80–91. [Google Scholar] [CrossRef]
- Pacanoski, Z. Bioherbicides. In Herbicides, Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; InTech: London, UK, 2015; pp. 253–274. [Google Scholar] [CrossRef] [Green Version]
- Bordin, E.R.; Frumi Camargo, A.; Stefanski, F.S.; Scapini, T.; Bonatto, C.; Zanivan, J.; Preczeski, K.; Modkovski, T.; Junior, F.; Mossi, A.; et al. Current production of bioherbicides: Mechanisms of action and technical and scientific challenges to improve food and environmental security. Biocatal. Biotransform. 2021, 39, 346–359. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Pan, Z.; Bajsa-Hirschel, J.; Boyette, C.D. The potential future roles of natural compounds and microbial bioherbicides in weed management in crops. Adv. Weed Sci. 2022, 40, e020210054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Zhu, Y.; Li, L.; Zhang, Y.; Li, J.; Song, X.; Qiang, S. Biological control of Solidago canadensis using a bioherbicide isolate of Sclerotium rolfsii SC64 increased the biodiversity in invaded habitats. Biol. Control 2019, 139, 104093. [Google Scholar] [CrossRef]
- Raza, T.; Khan, M.Y.; Nadeem, S.M.; Imran, S.; Qureshi, K.N.; Mushtaq, M.N.; Sohaib, M.; Schmalenberger, A.; Eash, N.S. Biological management of selected weeds of wheat through co-application of allelopathic rhizobacteria and sorghum extract. Biol. Control 2021, 164, 104775. [Google Scholar] [CrossRef]
- IBMA (International Biocontrol Manufacturers Association). Available online: https://ibma-global.org/ (accessed on 17 July 2022).
- Uludag, A.; Uremis, I.; Arslan, M. Biological weed control. In Non-Chemical Weed Control; Elsevier: Amsterdam, The Netherlands, 2018; pp. 115–132. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Limitations of existing weed control practices necessitate development of alternative techniques based on biological approaches. Adv. Agron. 2018, 147, 239–280. [Google Scholar]
- Kennedy, A.; Johnson, B.; Stubbs, T. Host range of a deleterious rhizobacterium for biological control of downy brome. Weed Sci. 2001, 49, 792–797. [Google Scholar] [CrossRef]
- Reinhart, K.; Carlson, C.; Feris, K.; Germino, M.; Jandreau, C.; Lazarus, B.; Mangold, J.; Pellatz, D.; Ramsey, P.; Rinella, M.; et al. Weed-suppressive bacteria fail to control Bromus tectorum under field conditions. Rangel. Ecol. Manag. 2020, 73, 760–765. [Google Scholar] [CrossRef]
| Weeds | Allelochemicals | References |
|---|---|---|
| Ageratum conyzoides L. | ageratochromene, benzoic acid, coumaric acid, essential oils, gallic acid, protocatechuic acid, sinapic acid | [65,70] |
| Alternanthera philoxeroides Mart. | coumaric acid, hydroxy-methoxybenzoic acid | [33,66,70] |
| Alternanthera sessilis L. | chlorogenic acid, ferulic acid, gallic acid, vanillic acid | [33,66,70] |
| Ambrosia artemisiifolia L. | caryophyllene, germacrene, limonene, pinene | [69,71] |
| Ambrosia trifida L. | carotane sesquiterpenes, essential oils, thiarubrines, thiophenes | [65] |
| Artemisia annua L. | artemisinin | [67] |
| Avena fatua L. | caffeic acid, chlorogenic acid, coumaric acid, ellagic acid, ferulic acid, hydroxy-benzoic acid, scopoletin, vanillic acid | [33] |
| Bidens pilosa L. | caffeic acid, coumaric acid, dimethoxyphenol, ethyl-benzenediol, eugenol, ferulic acid, hydroxybenzoic acid, protocatechuic acid, pyrocatechin, salicylic acid, vanillic acid, vanillin | [65] |
| Bothriochloa laguroides DC Herter | dodecane, farnesol, hexadecane, tetradecene | [33] |
| Callistemon citrinus Curtis | leptospermone | [67] |
| Centaurea maculosa Lam. | catechin | [67] |
| Chenopodia strummurale L. S. Fuentes, Uotila and Borsch | benzoic acid, coumaric acid, ferulic acid, vanillic acid | [33] |
| Chenopodium album L. | chlorogenic acid | [33] |
| Chenopodium ambrosioides L. | ascaridole, limonene, monoterpenes, sesquiterpenes, triterpenes | [33] |
| Cirsium arvense L. Scop. | caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, hydroxybenzoic acid, vanillic acid | [33] |
| Convolvulus arvensis L. | caffeic acid, chlorogenic acid, cinnamic acid, coumaric acid, ferulic acid, hydroxybenzoic acid, protocatechuic acid, pyrogallic acid, resorcinol, salicylic acid, syringic acid | [33] |
| Conyza canadensis L. | catechol, gallic acid, syringic acid, vanillic acid | [67] |
| Conyza stricta Wild. | chlorogenic acid, coumaric acid, ferulic acid | [33,70] |
| Cyperus esculentus L. | coumaric acid, ferulic acid, hydroxybenzoic acid, syringic acid, vanillic acid | [33] |
| Cyperus rotundus L. | alkaloids, catechol tannins, flavonoids, furochromones, glycosides, sesquiterpenes | [33,70] |
| Echinochloa colona L. | apigenin, chlorogenic acid, cinnamic acid, ferulic acid, protocatechuic acid, syringic acid | [70] |
| Echinochloa crus-galli L. P. Beauv. | acenaphthene, coumaric acid, benzoic acid, cinnamic acid, decanoic acid, phthalic acid, diethyl phthalate, dihydrokavain, ferulic acid, hydroxymandelic acid, lactones, fatty acids, myristic acid, phenols, stearic acid, steroids, vanillic acid | [33,65,67,70] |
| Eclipta alba L. | benzoic acid, coumaric acid, ferulic acid, vanillic acid | [33] |
| Eichhornia crassipes Mart. | dimethylcyclopentane, isocyanatoethyl acetate, propane amide | [33] |
| Imperata cylindrical L. P. Beauv. | chlorogenic acid, coumaric acid, isochlorogenic acids, scopoletin, scopolin, syringic acid, vanillic acid | [65] |
| Ipomoea chirica L. Sweet | cinnamic acid, methylphenyl benzoate | [72] |
| Lantana camara L. | coumaric acid, furano-naphthoquinones, flavonoids, iridoid glycosides, lantadenes, methylcoumarins, monoterpenes, phenylethanoid glycosides, salicylic acid, sesquiterpenes, triterpenes | [65,73] |
| Lathyrus aphaca L. | caffeic acid, coumaric acid, gallic acid, syringic acid | [33,68,74] |
| Leonurus sibiricus L. | caffeic acid, phenols | [67] |
| Medicago polymorpha L. | coumaric acid, hydroxy-methoxybenzoic acid, vanillic acid | [33,68,74] |
| Melilotus indica L. | caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, gallic acid, hydroxy-methoxybenzoic acid, syringic acid, vanillic acid | [33,68,74] |
| Mikania micrantha Kunth | benzoic acid, lactic acid | [72] |
| Nigella sativa L. | carvacrol, dithymoquinone, hederin, nigellicine, nigellidine, thymohydroquinone, thymol, thymoquinone | [33] |
| Oryza sativa L. | momilactone | [67] |
| Parthenium hysterophorus L. | anisic acid, caffeic acid, cardiac glycosides, coronopilic acid, coronopilin, coumaric acid, chlorogenic acid, ferulic acid, hydroxybenzoic acid, lactones, myrcene, ocimene, parthenin, pinene, saponins, steroids, tannins, vanillic acid, volatile compounds | [33,65] |
| Piper longum L. | sarmentine | [67] |
| Polygonum barbatum L. | acetophenone, caffeic acid, chlorogenic acid, coumaric acid, sitosterol | [33,70] |
| Ruta graveolens L. | cadinene, camphene, caryophyllene, cineol, copaene, cymene, decanol, decanone, decyl acetate, dodecanone, dodecene, eudesmol, flavonoids, furocoumarins, heptadecane, heptanone, hexadecane, hexadecanol, humulene, limonene, linalool, methyl salicylate, nonanol, nonanone, nonene, octanoicacid, octanol, octyl acetate, pentadecanol, pentadecanone, phenylethyl alcohol, pinene, terpinolene, tridecane, tridecanone, undecanol, undecanone, valeric acid, xanthotoxin | [33] |
| Sambucus nigra L. | cyaanogenins, flavonoids, lignans, phenolic glycosides | [67] |
| Sorghum bicolour L. Moench | sorgoleone | [67] |
| Sorghum halepense L. Pers. | chlorogenic acid, coumaric acid, hydroxybenzaldehyde, phenolic compounds | [33] |
| Sphenoclea zeylanica Gaertn. | epi-zeylanoxide, zeylanoxide | [33] |
| Stauranthus perforates Liebm. | asarinin, fargesin, furanocoumarins, pellitorine, pyranocoumarins, sesquiterpene | [33] |
| Stellera chamaejasme L. | chamaejasmenin, daphnodorin, dihydrodaphnodorin, genkwanol, mesoneochamaejasmin, | [33] |
| Terminalia catappa L. | coumaric acid, ferulic acid, palmitic acid, pentadecanone, stearic acid, syringic acid, vanillic acid, β-sitosterol-glucoside | [33] |
| Trigonella polycerata M. Bieb. | coumaric acid, hydroxy-methoxybenzoic acid, syringic acid | [33,68,74] |
| Vicia sativa L. | coumaric acid, hydroxy-methoxybenzoic acid, ferulic acid | [33,68,74] |
| Genus | Phytotoxins | References |
|---|---|---|
| Alternaria sp. | AAC-toxin, AAL-toxin, alternethanoxin, maculosin, tentoxin, tenuazonic acid, vivotoxin, vulculic acid | [160,164,165,166,167] |
| Ascochyta sp. | agropyrenal, agropyrenol, agropyrenone, ascaulitoxin, ascosonchine, cyperin, trans-aminoproline, triamino-hydroxyoctanoic acid | [160,168] |
| Aspergillus sp. | tenuazonic acid | [166] |
| Colletotrichum sp. | colletochlorin, dirhamnolipid, orcinol, tyrosol | [169] |
| Curvularia sp. | butyl isobutyl ester, dehydrocurvularin, phthalic acid, radicin | [160,164] |
| Diaporthe sp. | gulypyrone, hydroxybenzaldehyde, methylbenzoic acid, nectriapyrone, nitropropionic acid, phomentrioloxin, succinic acid | [160,164] |
| Drechslera sp. | drazepinone, ophiobolin, pyrenophorin | [160,164] |
| Edenia sp. | preussomerin, palmarumycin | [160] |
| Fusarium sp. | beauvericin, decalin, dehydrofusaric acid, diacetoxyscirpenol, enniatin, fumonisin, fusaric acid, moniliformin, radicin, rhodolamprometrin, tetracides, trichothecenes, zearalenone | [160,164,170] |
| Gliocladium sp. | viridiol | [164] |
| Myrothecium sp. | roridin | [164] |
| Paraphoma sp. | curvulin, phaeosphaeride | [164,171] |
| Penicillium sp. | cinnamic acid, dihydrosporogen, hydroxybenzoic acid, isopetasol, protocatechuic acid, salicylic acid, sporogen, vanillic acid | [160,164] |
| Phoma sp. | chenopodolans, cyperin, cytochalasins, desoxaphomin, herbarumin, hydroxybenzaldehyde, hydroxymelein, macrocidins, nitrophthalic acid, phomachalasin, putaminoxin, spirostaphylostrychnine, tenuazonic acid | [164,165,166,167,168,171,172,173] |
| Phomopsis sp. | nonenolide, phomentrioloxin | [160] |
| Phyllosticta sp. | phyllostictine, scytolide | [160,164] |
| Preussia sp. | cyperine | [160] |
| Pyrenophora sp. | papyracillic acid, pyrenophorin, triticone | [160,164] |
| Pyricularia sp. | tenuazonic acid | [160] |
| Rutstroemia sp. | ethylfusarubin, terpestacin | [164] |
| Scytalidium sp. | scytolide | [160] |
| Stagonospora sp. | modiolide, stagonolide | [160] |
| Stemphylium sp. | pyrenophorin | [164] |
| Name of Product and Manufacturer | Place and Year of Registration | Microorganisms | Target Weeds | References |
|---|---|---|---|---|
| Albobacteryn manufacturer unknown | Ukraine year unknown | Achromobacter album | various species | [5,30] |
| Camperico Japan Tobacco | Japan 1997 | strain JTP482 Xanthomonas campestris pv. poae | Poa annua L. | [5,30,142,183] |
| Organo-Sol manufacturer unknown | Canada 2010 | strain LTP-111 Lactobacillus casei, strain LTP-21 Lactobacillus rhamnous, strain LL64/CSL Lactobacillus lactis ssp. Lactis, strain LL102/CSL Lactobacillus lactis ssp. Lactis, strain M11/CSL Lactobacillus lactis ssp. Cremoris | Trifolium repens L., Trifolium pretense L., Lotus corniculatus L., Medicago lupulina L., Oxalis acetosella L. | [5,30,142,183] |
| MBI-005 EP Marrone Bio Innovations | USA Japan 2012 | thaxtomin A strain RL-110 Streptomyces acidiscabies | cotyledonous species | [5,30,142] |
| Name of Product and Manufacturer | Place and Year of Registration | Microorganisms | Target Weeds | References |
|---|---|---|---|---|
| Acremonium diospyri manufacturer unknown | Canada 1960 | Acremonium diospyri | Diospyros virginiana L. | [30,183] |
| Lubao manufacturer unknown | China 1963 | Colletotrichum gloeosporioides f. sp. Cuscutae | Cuscuta chinensis Lam., Cuscuta australis R. Br. | [30,183] |
| DeVine Valent Bioscences Crop | USA 1981 | strain MVW Phytophthora palmivora, Phytophthora citrophthora | Morrenia odorata Hook. &Arn. | [5,30,142,183] |
| Collego (Lockdown) Encore Technologies (Natural Industries) | USA 1982 (2006) | strain ATCC 20,358 Colletotrichum gloeosporioides f. sp. aeschynomene | Aeschynomene virginica L. | [5,30,142,183] |
| Casst manufacturer unknown | USA 1983 | Alternaria cassiae | Cassia spp. | [30,183] |
| ABG-5003 manufacturer unknown | USA 1984 | Cercosporarodmanii | Echhornia crassipes Mart. Solms | [30,183] |
| Velgo manufacturer unknown | Canada 1987 | Colletotrichum coccodes | Abutilon theophrasti Medik. | [30,183] |
| Dr.BioSedge manufacturer unknown | USA 1987 | Puccinia canaliculata | Cyperus esculentus L. | [30,183] |
| BioMal Philom Bios (Novozymes) | Canada 1992 | strain ATCC 20,767 Colletotrichum gloeosporioides f. sp. malvae | Malva pussila Sm. | [5,30,142,183] |
| Stumpout PPRI Weed Pathology Unit, Stellenbosch | South Africa 1997 | Cylindrobasidium laeve | Poa annua L., Acacia sp. | [30,183] |
| BioChon manufacturer unknown | Netherlands Canada 1997 | Chondrostereum purpureum | Prunus serotina Ehrh. | [30,183] |
| Hakatak manufacturer unknown | South Africa 1999 | Colletotrichum acutatum | Hakea gummosis, Hakea sericea Schrad. and J.C.Wendl. | [30] |
| MycoTech Paste Mycoforestis Corp | Canada 2002 | strain HQ1 Chondrostereum purpureum | deciduous trees | [5,30,142,183] |
| Woad Warrior Greenville Farms | USA 2002 | Puccinia thlaspeos | Isastis tinctoria L. | [5,30,142,183] |
| Chontrol (Ecoclear) MycoLogic Inc. | Canada (USA) 2004 (2007) | strain PFC 2139 Chondrostereum purpureum | deciduous trees | [5,30,183] |
| Smoulder Loveland Products Inc. | USA 2005 | strain 059 Alternaria destruens | Cuscuta spp. | [5,30,142,183] |
| Sarritor Sarritor Inc. | Canada 2007 | strain IMI 344,141 Sclerotinia minor | Taraxacum officinale F.H.Wigg. | [5,30,142,183] |
| Striga manufacturer unknown | Africa 2008 | Fusarium oxysporum f sp. stigae | Striga hermonthica Delile Benth., Striga asiatica L. Kuntze | [30] |
| Name unknown The Scotts Company | Canada USA 2012 | strain 94-44B Phoma macrostoma | cotyledonous species | [5,30,183] |
| Gibbatrianth manufacturer unknown | India 2014 | Gibbago trianthemae | Trianthema portulacastrum L. | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. https://doi.org/10.3390/agronomy12081808
Kubiak A, Wolna-Maruwka A, Niewiadomska A, Pilarska AA. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy. 2022; 12(8):1808. https://doi.org/10.3390/agronomy12081808
Chicago/Turabian StyleKubiak, Adrianna, Agnieszka Wolna-Maruwka, Alicja Niewiadomska, and Agnieszka A. Pilarska. 2022. "The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy" Agronomy 12, no. 8: 1808. https://doi.org/10.3390/agronomy12081808
APA StyleKubiak, A., Wolna-Maruwka, A., Niewiadomska, A., & Pilarska, A. A. (2022). The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy, 12(8), 1808. https://doi.org/10.3390/agronomy12081808








