Effects of Temperature and Light on the Germination-Promoting Activity by Melatonin in Almond Seeds without Stratification
Abstract
:1. Introduction
2. Material and Methods
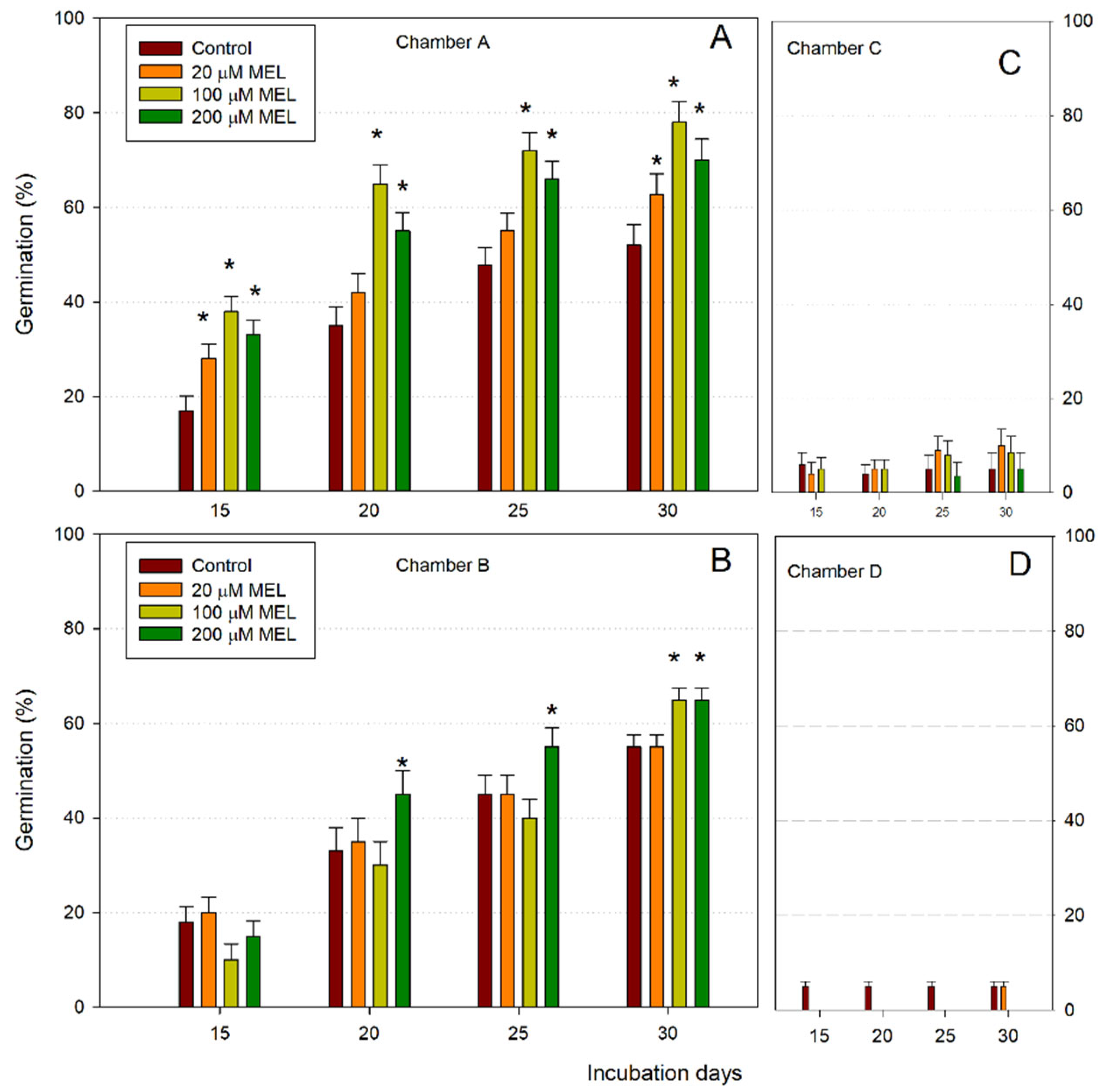
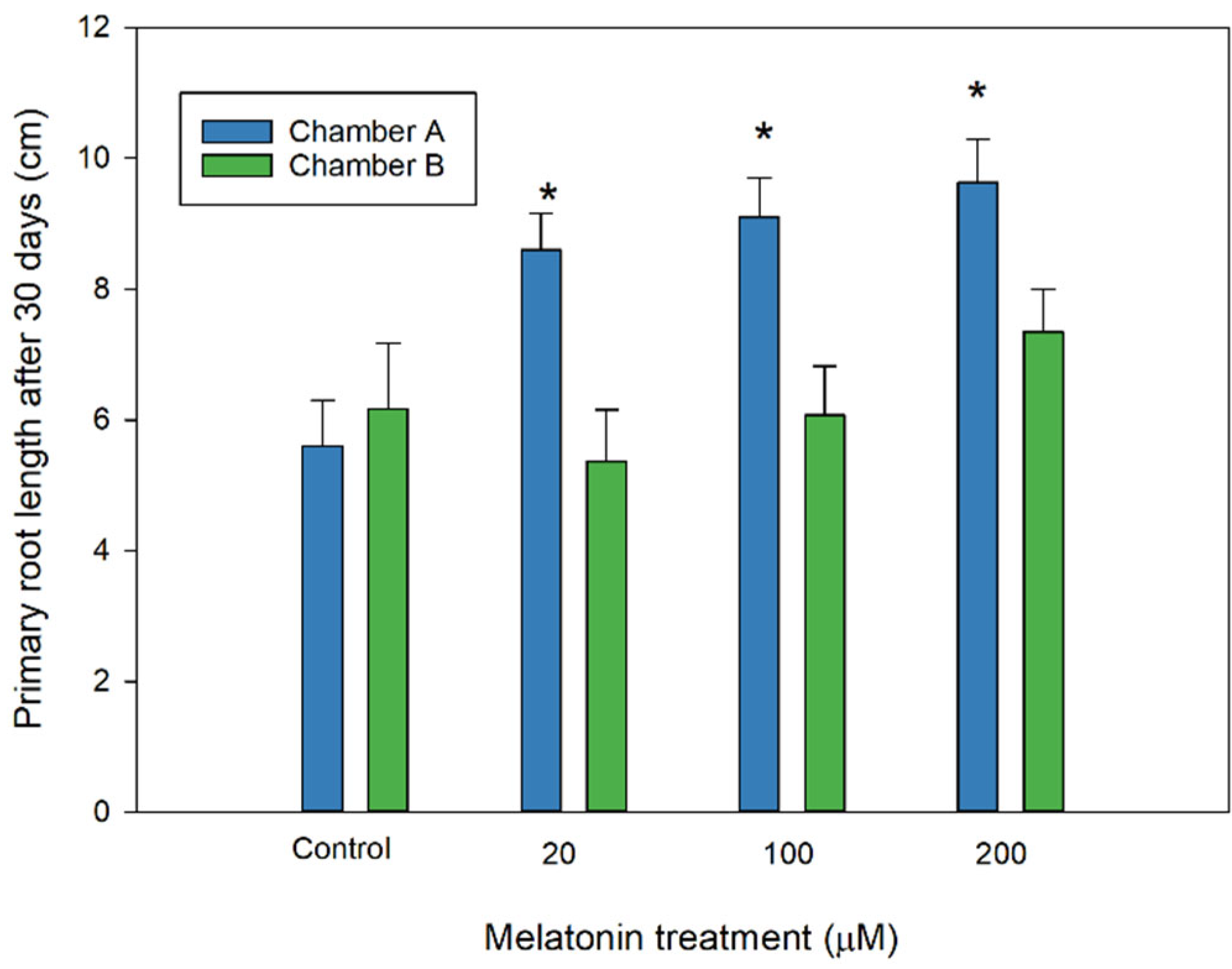
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halstead, T. Tree Nuts: World Markets and Trade. USDA Foreign Agric. Serv. 2022. Available online: https://www.fas.usda.gov/data/tree-nuts-world-markets-and-trade (accessed on 17 August 2022).
- Raouf, A.; Salih, K.; Mohammed, A. Examination of Some Nut Traits and Release from Dormancy Along with Germination Capacity in Some Bitter Almond Genotypes. Tikrit J. Agric. Sci. 2022, 21, 25–32. [Google Scholar]
- García-Gusano, M.; Martínez-García, P.J.; Dicenta, F. Seed Germination Time as a Criterion for the Early Selection of Late-Flowering Almonds. Plant Breed. 2010, 129, 578–580. [Google Scholar] [CrossRef]
- Zigas, R.P.; Coombe, B.G. Seedling Development in Peach, Prunus persica (L.) Batsch. I. Effects of Testas and Temperature. Funct. Plant Biol. 1977, 4, 349–358. [Google Scholar] [CrossRef]
- García-Gusano, M.; Martínez-Gómez, P.; Dicenta, F. Breaking Seed Dormancy in Almond (Prunus dulcis (Mill.) D.A. Webb). Sci. Hort. 2004, 99, 363–370. [Google Scholar] [CrossRef]
- García-Gusano, M.; Martínez-Gómez, P.; Dicenta, F. Influence of Stratification, Heat and Removal of Teguments on Breaking of Seed Dormancy in Almond. In Proceedings of the XIII GREMPA Meeting on Almonds and Pistachios; Options Méditerranéennes: Série A. Séminaires Méditerranéens; n. 63; Oliveira, M.M., Cordeiro, V., Eds.; Centre International de Hautes Études Agronomiques Méditerranéennes (CIHEAM): Zaragoza, Spain, 2005; Volume 63, pp. 373–377. [Google Scholar]
- Zigas, R.P.; Coombe, B.G. Seedling Development in Peach, Prunus persica (L.) Batsch. II. Effects of Plant Growth Regulators and Their Possible Role. Funct. Plant Biol. 1977, 4, 359–369. [Google Scholar] [CrossRef]
- Rahemi, A.; Taghavi, T.; Fatahi, R.; Ebadi, A.; Hassani, D.; Chaparro, J.; Gradziel, T. Seed Germination and Seedling Establishment of Some Wild Almond Species. Afr. J. Biotech. 2011, 10, 7780–7786. [Google Scholar] [CrossRef]
- Koukourikou-Petridou, M. Paclobutrazol Affects Growth of Almond Fruits and Germination of Almond Seeds. Plant Growth Regul. 1996, 20, 267–269. [Google Scholar] [CrossRef]
- San, B.; Yildirim, A.N. Seed and in Vitro Embryo Germination in Aged Almond. Seed Sci. Technol. 2009, 37, 365–371. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- van Tassel, D.L.; Roberts, N.; O’Neill, S.D. Melatonin from Higher Plants: Isolation and Identification of N-Acetyl-5-Methoxytryptamine. Plant Physiol. 1995, 108, 101. [Google Scholar]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022; in press. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An Overview of the Importance and Mediating Functions of Melatonin against Environmental Stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Regulatory Role of Melatonin in the Redox Network of Plants and Plant Hormone Relationship in Stress. In Hormones and Plant Response; Plant in Challenging Environments; Gupta, D.K., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–272. ISBN 978-3-030-77477-6. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Prot. Pept. Sci. 2022, 22, 413–429. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin Promotes Seed Germination under High Salinity by Regulating Antioxidant Systems, ABA and GA4 Interaction in Cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin Improves the Germination Rate of Cotton Seeds under Drought Stress by Opening Pores in the Seed Coat. PeerJ. 2020, 8, e9450. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous Melatonin Accelerates Seed Germination in Cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial Effects of Abscisic Acid and Melatonin in Overcoming Drought Stress in Cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Pan, J.; Wang, H.; Reiter, R.; Li, X.; Zongmin, M.; Zhang, J.; Yao, Z.; Zhao, D.; Yu, D. Melatonin Inhibits Seed Germination by Crosstalk with Abscisic Acid, Gibberellin, and Auxin in Arabidopsis. J. Pineal Res. 2021, 70, e12736. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Bai, Y.-L.; Gong, C.; Song, W.; Wu, Y.; Ye, T.; Feng, Y.-Q. The Phytomelatonin Receptor PMTR1 Regulates Seed Development and Germination by Modulating Abscisic Acid Homeostasis in Arabidopsis thaliana. J. Pineal Res. 2022, 72, e12797. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X.; et al. Daily Rhythms of Phytomelatonin Signaling Modulate Diurnal Stomatal Closure via Regulating Reactive Oxygen Species Dynamics in Arabidopsis. J. Pineal Res. 2020, 68, e12640. [Google Scholar] [CrossRef]
- Shi, H.; Wei, Y.; Wang, Q.; Reiter, R.J.; He, C. Melatonin Mediates the Stabilization of DELLA Proteins to Repress the Floral Transition in Arabidopsis. J. Pineal Res. 2016, 60, 373–379. [Google Scholar] [CrossRef]



| Chamber | Temperature | Light:Dark | Photoperiod | Intensity (µE/m2·s) |
|---|---|---|---|---|
| A | 10 ± 2 °C | L:D | 16:8 h | 400–500 |
| B | 10 ± 2 °C | D | -- | -- |
| C | 22 ± 1 °C | L:D | 16:8 h | 400–500 |
| D | 22 ± 1 °C | D | -- | -- |
| E * | 24 ± 1 °C 15 ± 1 °C | L D | 16 h 8 h | 1200–1300 -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sánchez, S.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Effects of Temperature and Light on the Germination-Promoting Activity by Melatonin in Almond Seeds without Stratification. Agronomy 2022, 12, 2070. https://doi.org/10.3390/agronomy12092070
García-Sánchez S, Cano A, Hernández-Ruiz J, Arnao MB. Effects of Temperature and Light on the Germination-Promoting Activity by Melatonin in Almond Seeds without Stratification. Agronomy. 2022; 12(9):2070. https://doi.org/10.3390/agronomy12092070
Chicago/Turabian StyleGarcía-Sánchez, Sara, Antonio Cano, Josefa Hernández-Ruiz, and Marino B. Arnao. 2022. "Effects of Temperature and Light on the Germination-Promoting Activity by Melatonin in Almond Seeds without Stratification" Agronomy 12, no. 9: 2070. https://doi.org/10.3390/agronomy12092070
APA StyleGarcía-Sánchez, S., Cano, A., Hernández-Ruiz, J., & Arnao, M. B. (2022). Effects of Temperature and Light on the Germination-Promoting Activity by Melatonin in Almond Seeds without Stratification. Agronomy, 12(9), 2070. https://doi.org/10.3390/agronomy12092070








