Adsorptive Analysis of Azo Dyes on Activated Carbon Prepared from Phyllanthus emblica Fruit Stone Sequentially via Hydrothermal Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbents
2.3. Adsorption Studies
3. Results and Discussion
3.1. Characterization of the Adsorbents
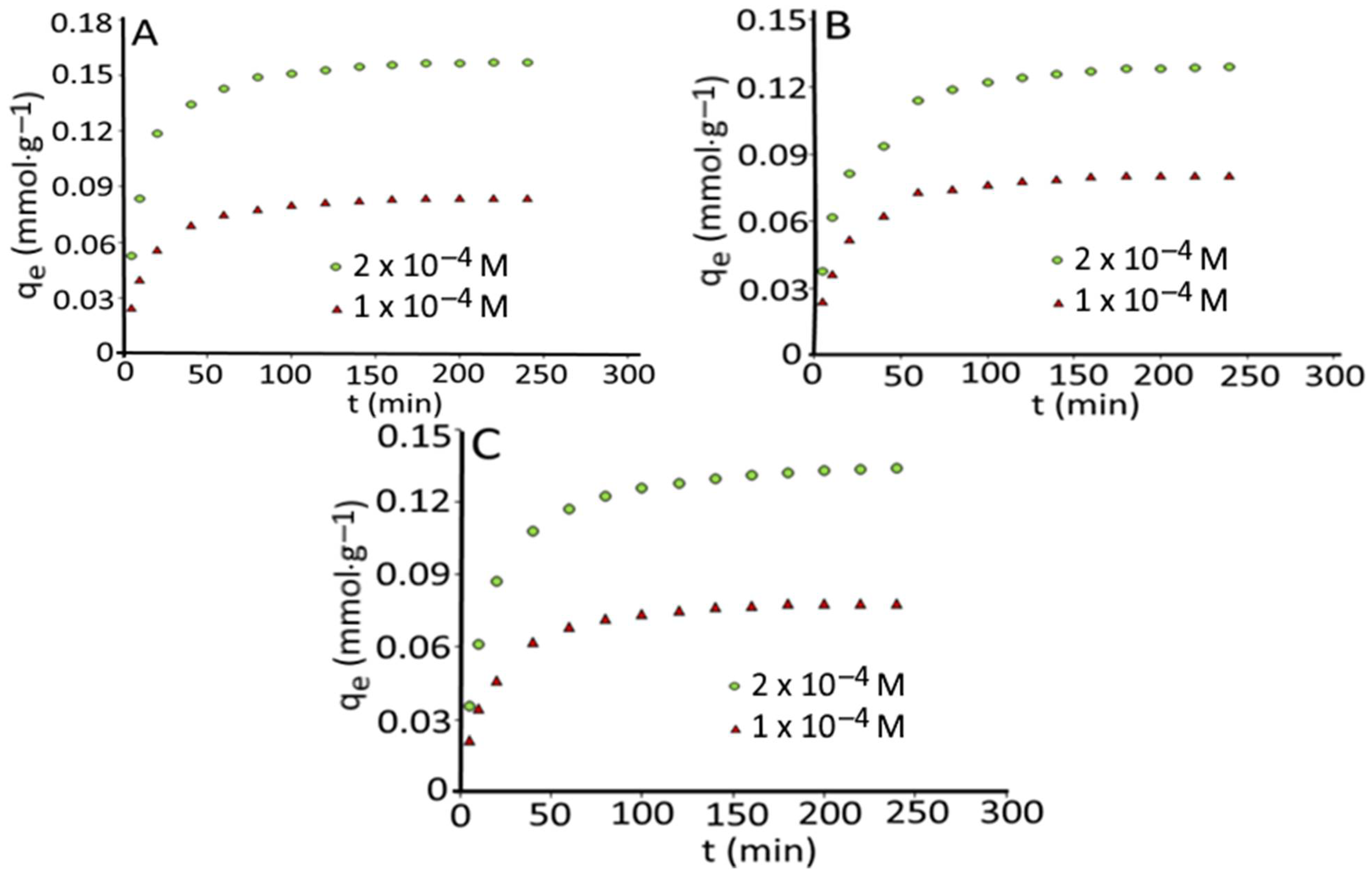
3.2. Effect of Initial Concentration and Contact Time
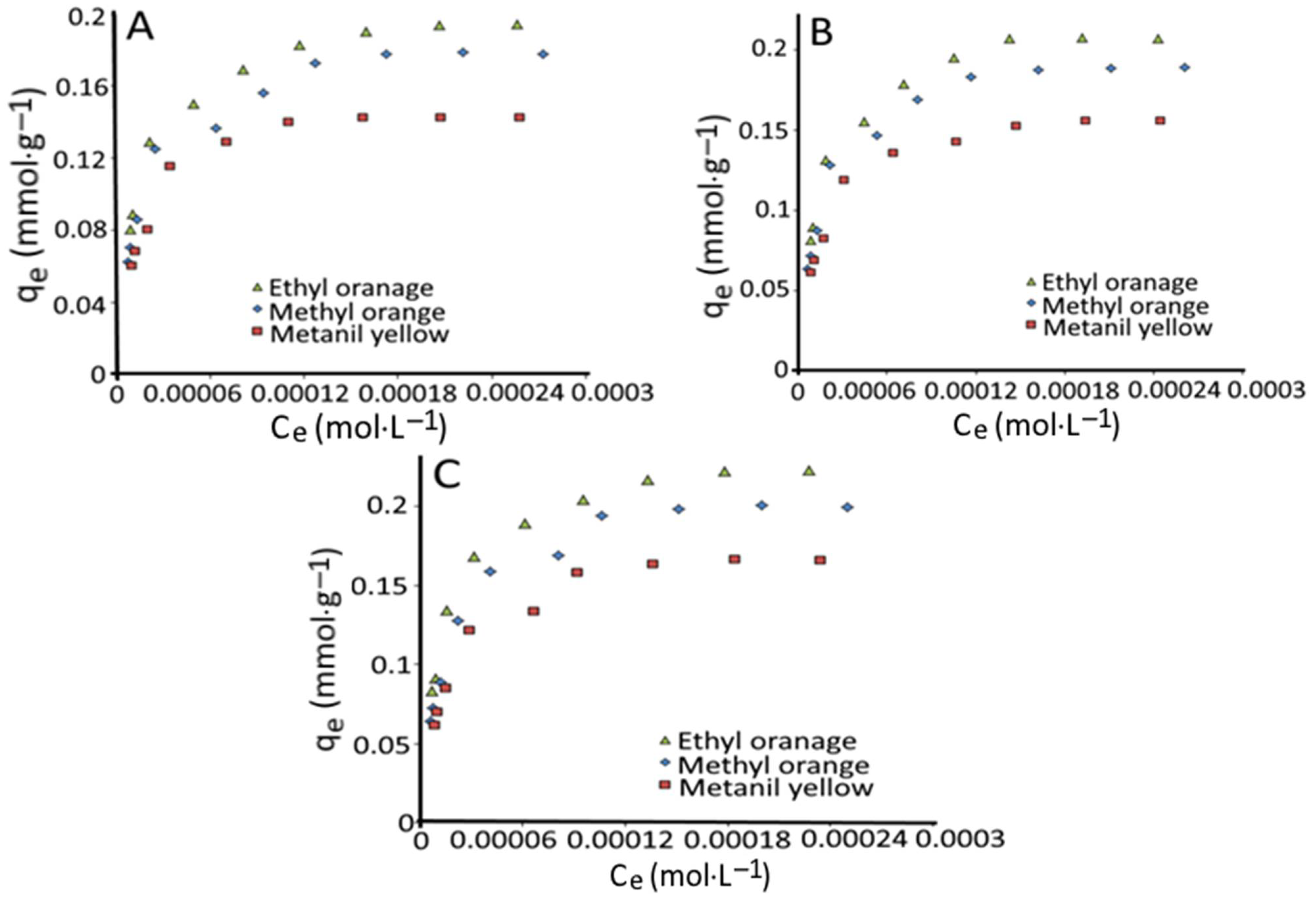
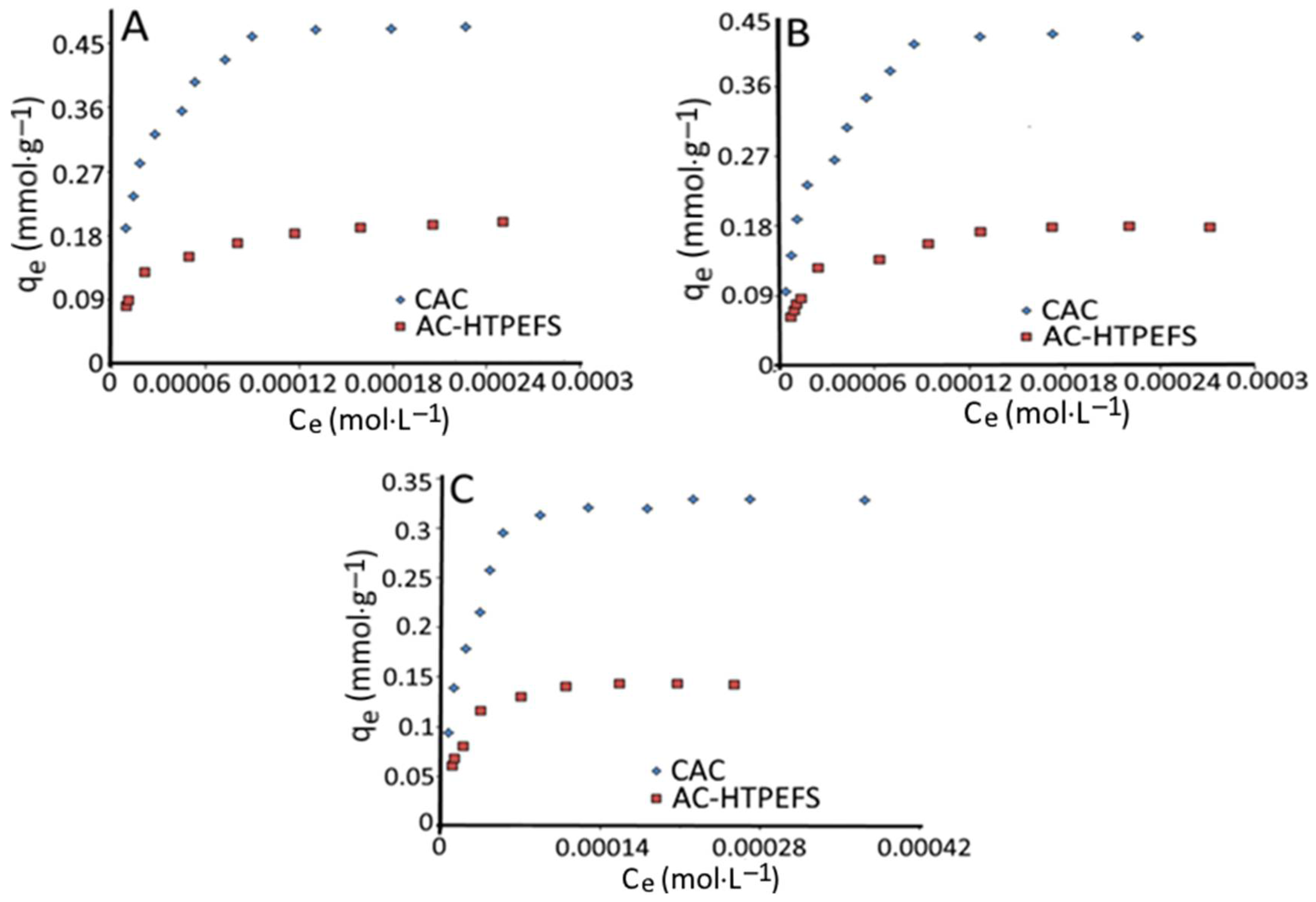
3.3. Adsorption Isotherms
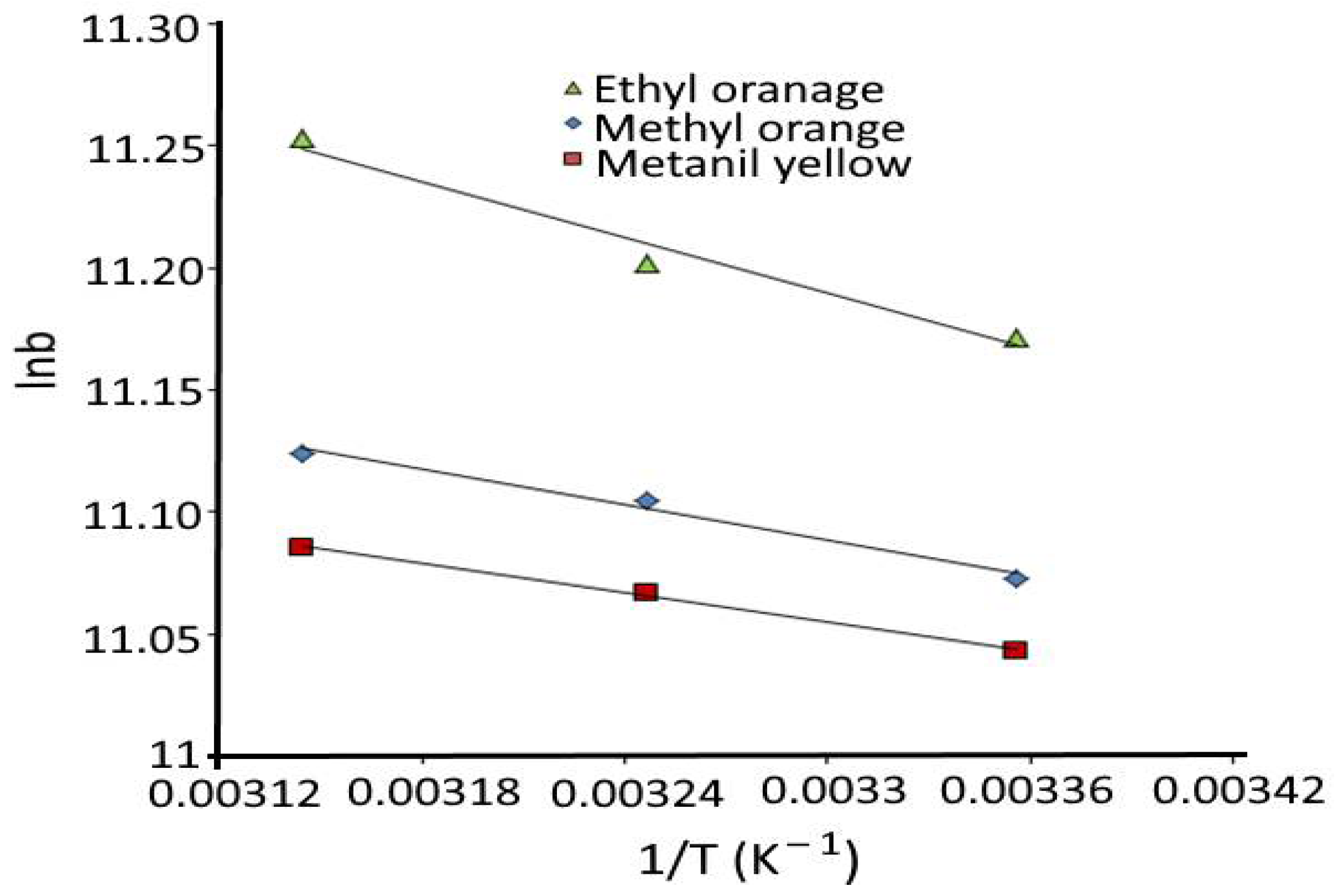
3.4. Thermodynamics of Adsorption
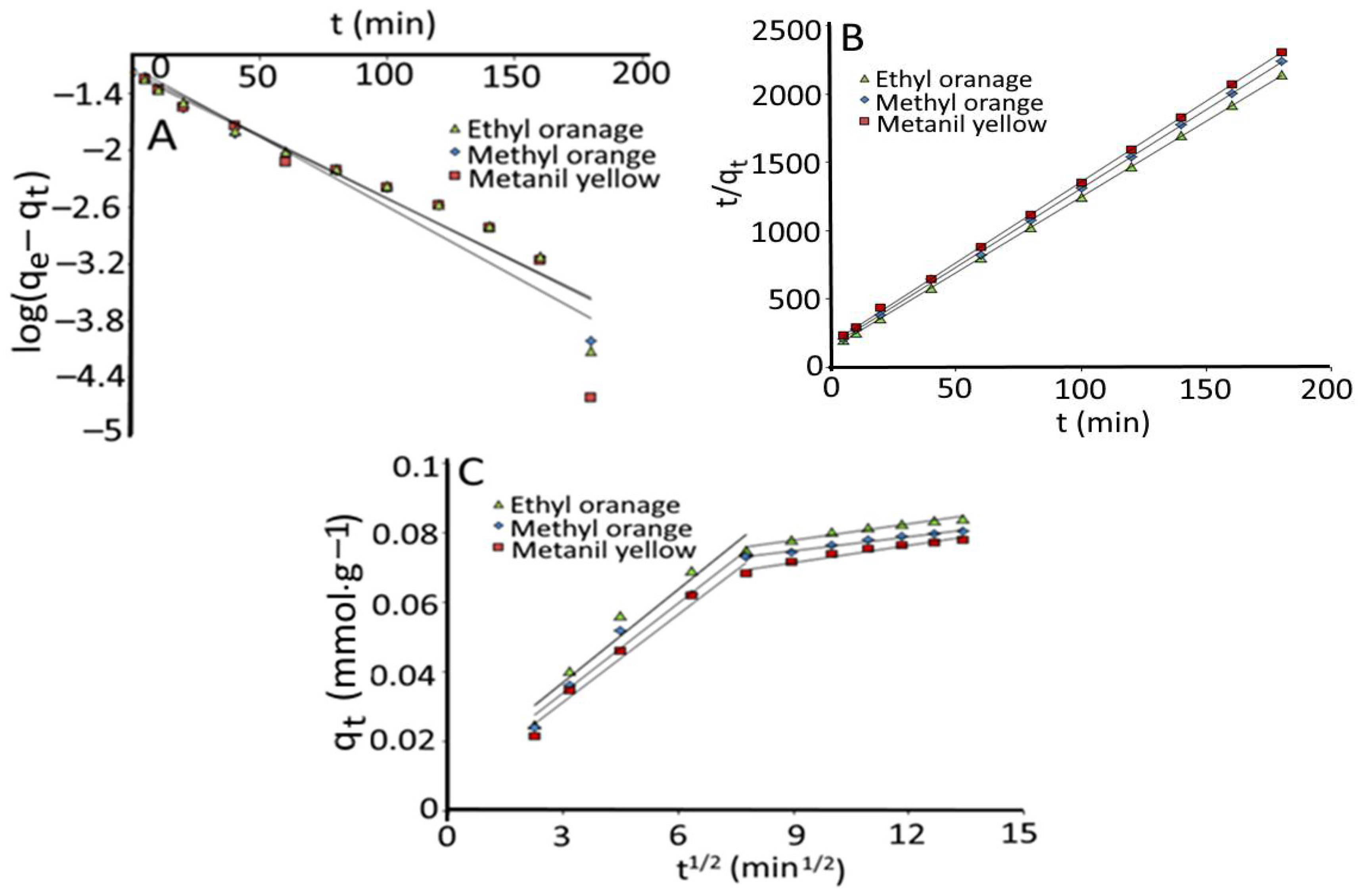
3.5. Kinetics Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–371. [Google Scholar] [CrossRef]
- Hefford, R. Colourants and dyes for the cosmetics industry. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 175–203. [Google Scholar]
- Allen, R. The chemistry of azo dyes. In Colour Chemistry; Springer: Berlin/Heidelberg, Germany, 1971; pp. 21–36. [Google Scholar]
- Gordon, P.F.; Gregory, P. Azo Dyes. In Organic Chemistry in Colour; Springer: Berlin/Heidelberg, Germany, 1987; pp. 95–162. [Google Scholar]
- Saratale, R.G.; Saratale, G.D.; Chang, J.-S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Chaudhary, M.; Suhas; Singh, R.; Tyagi, I.; Ahmed, J.; Chaudhary, S.; Kushwaha, S. Microporous activated carbon as adsorbent for the removal of noxious anthraquinone acid dyes: Role of adsorbate functionalization. J. Environ. Chem. Eng. 2021, 9, 106308. [Google Scholar] [CrossRef]
- Ooi, J.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Lim, S.S.; Gan, S. Assessment of fish scales waste as a low cost and eco-friendly adsorbent for removal of an azo dye: Equilibrium, kinetic and thermodynamic studies. Bioresour. Technol. 2017, 245, 656–664. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.; Carrott, M.R.; Singh, R.; Singh, L.; Chaudhary, M. An innovative approach to develop microporous activated carbons in oxidising atmosphere. J. Clean. Produc. 2017, 156, 549–555. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin—From natural adsorbent to activated carbon: A review. Biores. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef]
- Sadia, M.; Ahmad, I.; Ali, F.; Zahoor, M.; Ullah, R.; Khan, F.A.; Ali, E.A.; Sohail, A. Selective Removal of the emerging dye basic blue 3 via molecularly imprinting technique. Molecules 2022, 27, 3276. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Rehan, K.; Rehan, I.; Ali, F.; Waris, S.; Zahoor, M.; Salman, S.M.; Khan, S.; Rehan, M.S. Physicochemical and instrumental characterization of rice husk and its potential use as a low cost adsorbent for mutagenic dye bromophenol blue. Z. Für Phys. Chem. 2021, 235, 1263–1277. [Google Scholar] [CrossRef]
- Jabeen, S.; Alam, S.; Shah, L.A.; Zahoor, M.; Rahman, N.U.; Khan, F.A.; Ullah, R.; Ali, E.A.; Murthy, H.C.A.; Sohail, A. Removal of safranin-T and toluidine from water through gum Arabic/acrylamide hydrogel. Ads. Sci. Technol. 2022, 2022, 6100791. [Google Scholar] [CrossRef]
- Kushwaha, S.; Suhas; Chaudhary, M.; Tyagi, I.; Bhutiani, R.; Goscianska, J.; Ahmed, J.; Manila; Chaudhary, S. Utilization of Phyllanthus emblica fruit stone as a potential biomaterial for sustainable remediation of lead and cadmium ions from aqueous solutions. Molecules 2022, 27, 3355. [Google Scholar] [CrossRef] [PubMed]
- Suhas; Gupta, V.K.; Singh, L.P.; Chaudhary, M.; Kushwaha, S. A novel approach to develop activated carbon by an ingenious hydrothermal treatment methodology using Phyllanthus emblica fruit stone. J. Clean. Prod. 2021, 288, 125643. [Google Scholar] [CrossRef]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef]
- Din, A.T.M.; Hameed, B.; Ahmad, A.L. Batch adsorption of phenol onto physiochemical-activated coconut shell. J. Hazard. Mater. 2009, 161, 1522–1529. [Google Scholar]
- Dursun, G.; Çiçek, H.; Dursun, A.Y. Adsorption of phenol from aqueous solution by using carbonised beet pulp. J. Hazard. Mater. 2005, 125, 175–182. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 111, 3973–3993. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Temkin, M. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Muthaiyan, K.; Tamilarasan, R. Modeling of experimental data for the adsorption of methyl orange from aqueous solution using a low cost activated carbon prepared from Prosopis juliflora. Pol. J. Chem. Technol. 2013, 15, 29–39. [Google Scholar]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- Ghosh, G.; Chakraborty, T.; Zaman, S.; Nahar, M.; Kabir, A.H.M.E. Removal of methyl orange dye from aqueous solution by a low-cost activated carbon prepared from mahagoni (Swietenia mahagoni) bark. Environ. Pollut. 2020, 6, 171–184. [Google Scholar]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]
- Subbaiah, M.V.; Kim, D.-S. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef]
- Machrouhi, A.; Alilou, H.; Farnane, M.; El Hamidi, S.; Sadiq, M.; Abdennouri, M.; Tounsadi, H.; Barka, N. Statistical optimization of activated carbon from Thapsia transtagana stems and dyes removal efficiency using central composite design. J. Sci. Adv. Mater. Dev. 2019, 4, 544–553. [Google Scholar] [CrossRef]
- Guo, X.; Wei, Q.; Du, B.; Zhang, Y.; Xin, X.; Yan, L.; Yu, H. Removal of Metanil Yellow from water environment by amino functionalized graphenes (NH2-G)–Influence of surface chemistry of NH2-G. Appl. Surf. Sci. 2013, 284, 862–869. [Google Scholar] [CrossRef]
- Santra, A.K.; Pal, T.K.; Datta, S. Removal of metanil yellow from its aqueous solution by fly ash and activated carbon produced from different sources. Sep. Sci. Technol. 2008, 43, 1434–1458. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, V.K.; Bhatnagar, A.; Suhas. Utilization of industrial waste products as adsorbents for the removal of dyes. J. Hazard. Mater. 2003, 101, 31–42. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Chaudhary, M.; Suhas; Singh, R.; Yilmaz, M.; Chaudhary, S.; Kushwaha, S. Role of the similar molecular weight dyes on the adsorption by activated carbon. Desaliation Water Treat. 2021, 244, 343–354. [Google Scholar] [CrossRef]
- Clark, M. Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Hummert, J. Femtosecond XUV photoelectron spectroscopy of organic molecules in aqueous solution. J. Phys. Chem. Lett. 2018, 9, 6649–6655. [Google Scholar] [CrossRef]
- Guo, Y.; Kaplan, S.; Karanfil, T. The significance of physical factors on the adsorption of polyaromatic compounds by activated carbons. Carbon 2008, 46, 1885–1891. [Google Scholar] [CrossRef]
- Cornelissen, G.; Elmquist, M.; Groth, I.; Gustafsson, Ö. Effect of sorbate planarity on environmental black carbon sorption. Environ. Sci. Technol. 2004, 38, 3574–3580. [Google Scholar] [CrossRef]
- Boyd, B.J.; Drummond, C.J.; Krodkiewska, I.; Grieser, F. How chain length, headgroup polymerization, and anomeric configuration govern the thermotropic and lyotropic liquid crystalline phase behavior and the air− water interfacial adsorption of glucose-based surfactants. Langmuir 2000, 16, 7359–7367. [Google Scholar] [CrossRef]
- Liu, Q.-S.; Zheng, T.; Wang, P.; Jiang, J.-P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Weber, W.J., Jr. Evolution of a technology. J. Environ. Eng. 1984, 110, 899–917. [Google Scholar] [CrossRef]
- Duran, C.; Ozdes, D.; Gundogdu, A.; Senturk, H.B. Kinetics and isotherm analysis of basic dyes adsorption onto almond shell (Prunus dulcis) as a low cost adsorbent. J. Chem. Eng. Data 2011, 56, 2136–2147. [Google Scholar] [CrossRef]

| Name | Structure | Molecular Weight | Molecular Formula |
|---|---|---|---|
| Ethyl orange (EO) | 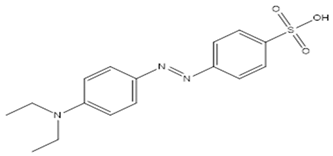 | 355.39 | C16H18N3NaO3S |
| Methyl orange (MO) |  | 327.33 | C14H14N3NaO3S |
| Metanil yellow (MY) |  | 375.38 | C18H14N3NaO3S |
| Dyes | Temperature (°C) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qmax (mmol·g−1) | b (L·mol−1) | R2 | Kf (mmol·g−1) | n | R2 | bT (KJ·mol−1) | AT (L·mg−1) | R2 | ||
| EO | 25 | 0.202 | 7.11 × 104 | 0.991 | 3.33 | 3.17 | 0.948 | 0.176 | 2.58 | 0.980 |
| 35 | 0.215 | 7.33 × 104 | 0.991 | 4.46 | 2.97 | 0.958 | 0.159 | 2.33 | 0.985 | |
| 45 | 0.233 | 7.71 × 104 | 0.984 | 5.77 | 2.84 | 0.939 | 0.146 | 2.45 | 0.976 | |
| MO | 25 | 0.187 | 6.43 × 104 | 0.990 | 3.23 | 3.07 | 0.945 | 0.206 | 2.38 | 0.956 |
| 35 | 0.199 | 6.77 × 104 | 0.989 | 4.18 | 2.91 | 0.950 | 0.187 | 2.32 | 0.982 | |
| 45 | 0.210 | 6.84 × 104 | 0.994 | 5.13 | 2.79 | 0.955 | 0.146 | 2.66 | 0.976 | |
| MY | 25 | 0.158 | 6.25 × 104 | 0.984 | 2.58 | 3.13 | 0.938 | 0.194 | 1.700 | 0.973 |
| 35 | 0.167 | 6.40 × 104 | 0.988 | 2.95 | 3.08 | 0.939 | 0.187 | 2.02 | 0.982 | |
| 45 | 0.178 | 6.52 × 104 | 0.993 | 3.72 | 2.92 | 0.955 | 0.181 | 1.91 | 0.978 | |
| Adsorbent | Dyes | Maximum amount Adsorbed | References |
|---|---|---|---|
| Activated carbon prepared from Prosopis juliflora bark | Methyl orange | 10.29 mg·g−1 | [27] |
| Activated carbon prepared from apricot stones | Methyl orange | 32.25 mg·g−1 | [28] |
| Activated carbon derived from Mahagoni bark | Methyl orange | 6.071 mg·g−1 | [29] |
| Activated carbon prepared from waste orange and lemon peels | Methyl orange | 33 mg·g−1 | [30] |
| Aminated pumpkin seed powder | Methyl orange | 143.7 mg·g−1 | [31] |
| Activated carbon from Thapsia transtagana stems | Methyl orange | 118.10 mg·g−1 | [32] |
| AC-HTPEFS | Methyl orange | 61.2 mg·g−1/0.187 mmol·g−1 | This study |
| Amino functionalized graphenes | Metanil yellow | 71.62 mg·g−1 | [33] |
| Rice Husk activated carbon | Metanil yellow | 52.83 mg·g−1 | [34] |
| Cocunut shell derived activated carbon | Metanil yellow | 79.69 mg·g−1 | [34] |
| Activated carbon from tomato processing solid waste | Metanil yellow | 385 mg·g−1 | [35] |
| AC-HTPEFS | Metanil yellow | 59.3 mg·g−1/0.158 mmol·g−1 | This study |
| Adsorbents from steel and fertilizer industries wastes | Ethyl orange | 198 mg·g−1 | [36] |
| AC-HTPEFS | Ethyl orange | 71.8 mg·g−1/0.202 mmol·g−1 | This study |
| Dyes | Temperature (°C) | −ΔG° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔH° (kJ·mol−1) |
|---|---|---|---|---|
| EO | 25 35 45 | 27.7 28.7 29.8 | 104 | 3.18 |
| MO | 25 35 45 | 27.4 28.5 29.4 | 100 | 2.45 |
| MY | 25 35 45 | 27.4 28.3 29.3 | 97.4 | 1.67 |
| Dyes | Co (mol·L−1) | qe (exp) (mmol·g−1) | Pseudo-First Order | Pseudo-Second Order | Intraparticle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (cal) (mmol·g−1) | K1 (min−1) | R2 | qe (cal) (mmol·g−1) | K2 (g.mmol−1 ·min−1) | R2 | Kp1 | C1 | R2 | Kp2 | C2 | R2 | |||
| EO | 1 × 10−4 | 0.085 | 0.0637 | 3.08 × 10−2 | 0.948 | 0.090 | 0.900 | 0.999 | 0.0089 | 0.0103 | 0.944 | 0.0016 | 0.0637 | 0.884 |
| MO | 1 × 10−4 | 0.0804 | 0.0755 | 3.39 × 10−2 | 0.880 | 0.087 | 0.862 | 0.999 | 0.0086 | 0.0083 | 0.971 | 0.0014 | 0.0625 | 0.986 |
| MY | 1 × 10−4 | 0.0781 | 0.074 | 3.09 × 10−2 | 0.936 | 0.085 | 0.796 | 0.999 | 0.0084 | 0.0061 | 0.975 | 0.0016 | 0.0567 | 0.962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhas; Kushwaha, S.; Tyagi, I.; Ahmed, J.; Chaudhary, S.; Chaudhary, M.; Stephen Inbaraj, B.; Goscianska, J.; Karri, R.R.; Sridhar, K. Adsorptive Analysis of Azo Dyes on Activated Carbon Prepared from Phyllanthus emblica Fruit Stone Sequentially via Hydrothermal Treatment. Agronomy 2022, 12, 2134. https://doi.org/10.3390/agronomy12092134
Suhas, Kushwaha S, Tyagi I, Ahmed J, Chaudhary S, Chaudhary M, Stephen Inbaraj B, Goscianska J, Karri RR, Sridhar K. Adsorptive Analysis of Azo Dyes on Activated Carbon Prepared from Phyllanthus emblica Fruit Stone Sequentially via Hydrothermal Treatment. Agronomy. 2022; 12(9):2134. https://doi.org/10.3390/agronomy12092134
Chicago/Turabian StyleSuhas, Sarita Kushwaha, Inderjeet Tyagi, Jahangeer Ahmed, Shubham Chaudhary, Monika Chaudhary, Baskaran Stephen Inbaraj, Joanna Goscianska, Rama Rao Karri, and Kandi Sridhar. 2022. "Adsorptive Analysis of Azo Dyes on Activated Carbon Prepared from Phyllanthus emblica Fruit Stone Sequentially via Hydrothermal Treatment" Agronomy 12, no. 9: 2134. https://doi.org/10.3390/agronomy12092134
APA StyleSuhas, Kushwaha, S., Tyagi, I., Ahmed, J., Chaudhary, S., Chaudhary, M., Stephen Inbaraj, B., Goscianska, J., Karri, R. R., & Sridhar, K. (2022). Adsorptive Analysis of Azo Dyes on Activated Carbon Prepared from Phyllanthus emblica Fruit Stone Sequentially via Hydrothermal Treatment. Agronomy, 12(9), 2134. https://doi.org/10.3390/agronomy12092134











