Subcellular Element Distribution in Shoots of Wheat Grown in an Acidic Soil with Native AMF Extraradical Mycelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Soil Fertility
2.2. Plants and Experimental Design
2.3. Subcellular Partitioning
2.4. Sample Digestion and Element Quantification
2.5. Statistical Treatment
3. Results
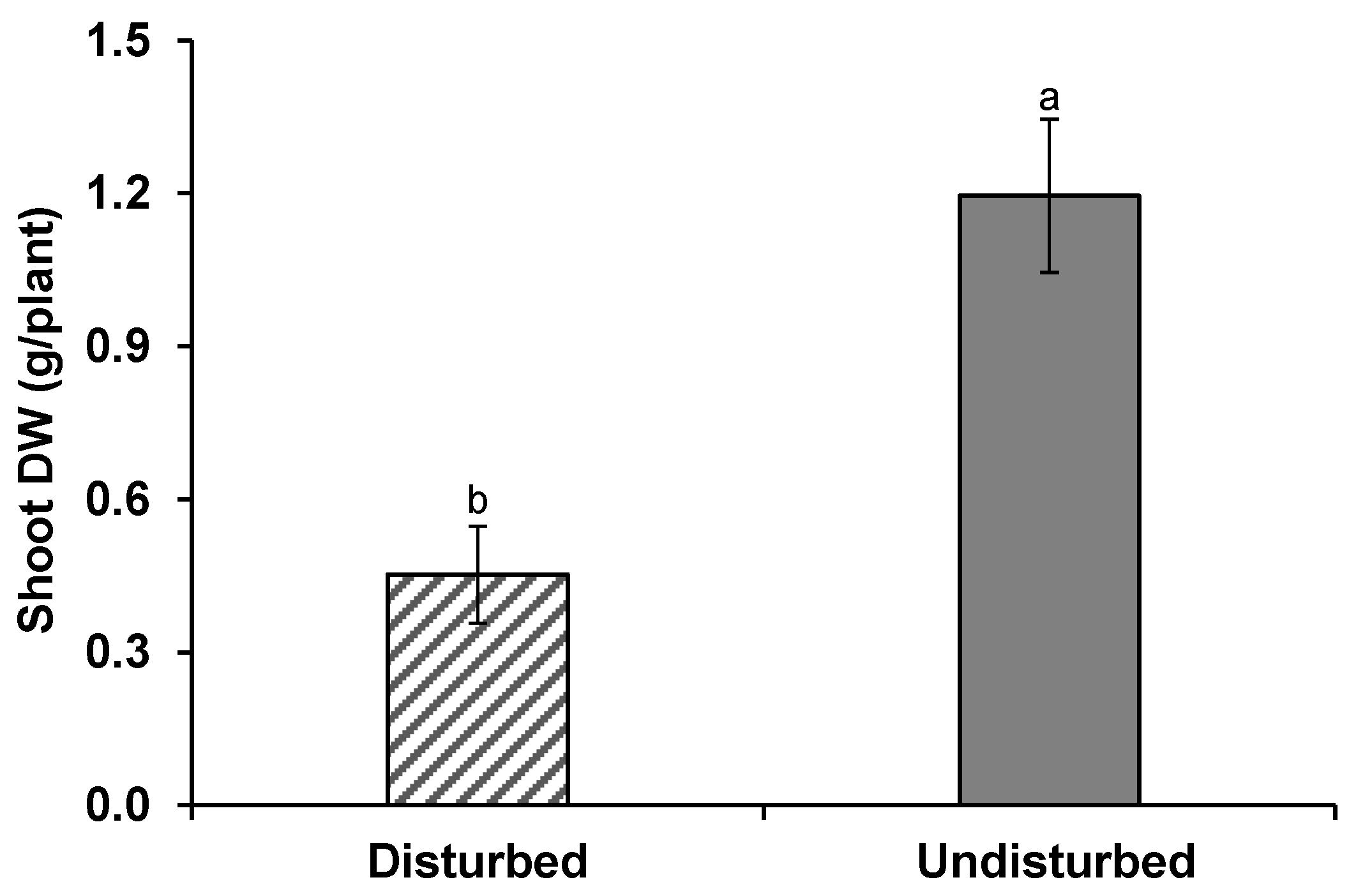
3.1. Wheat Growth
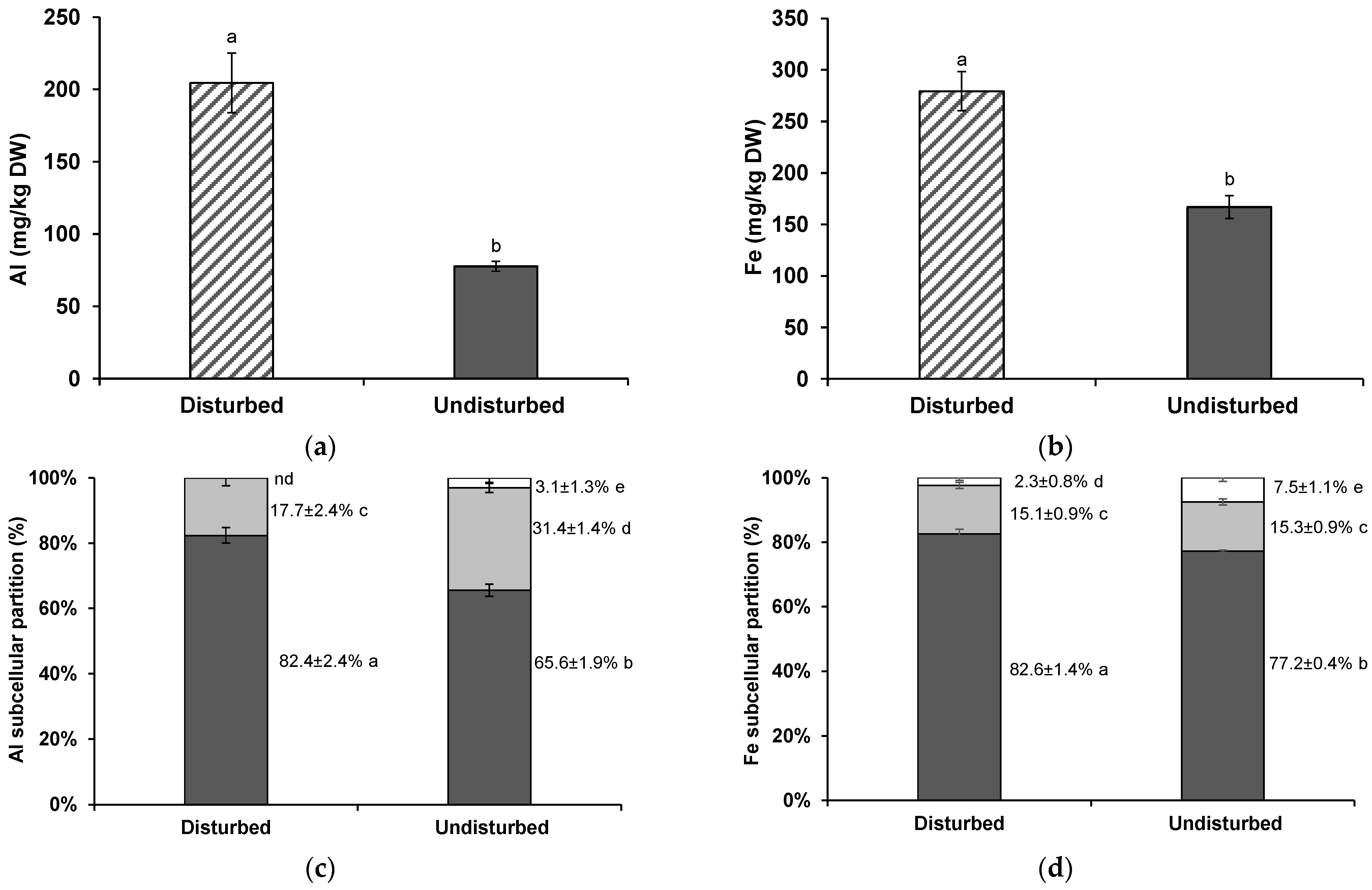
3.2. Al and Fe Shoot Concentrations and Subcellular Distribution
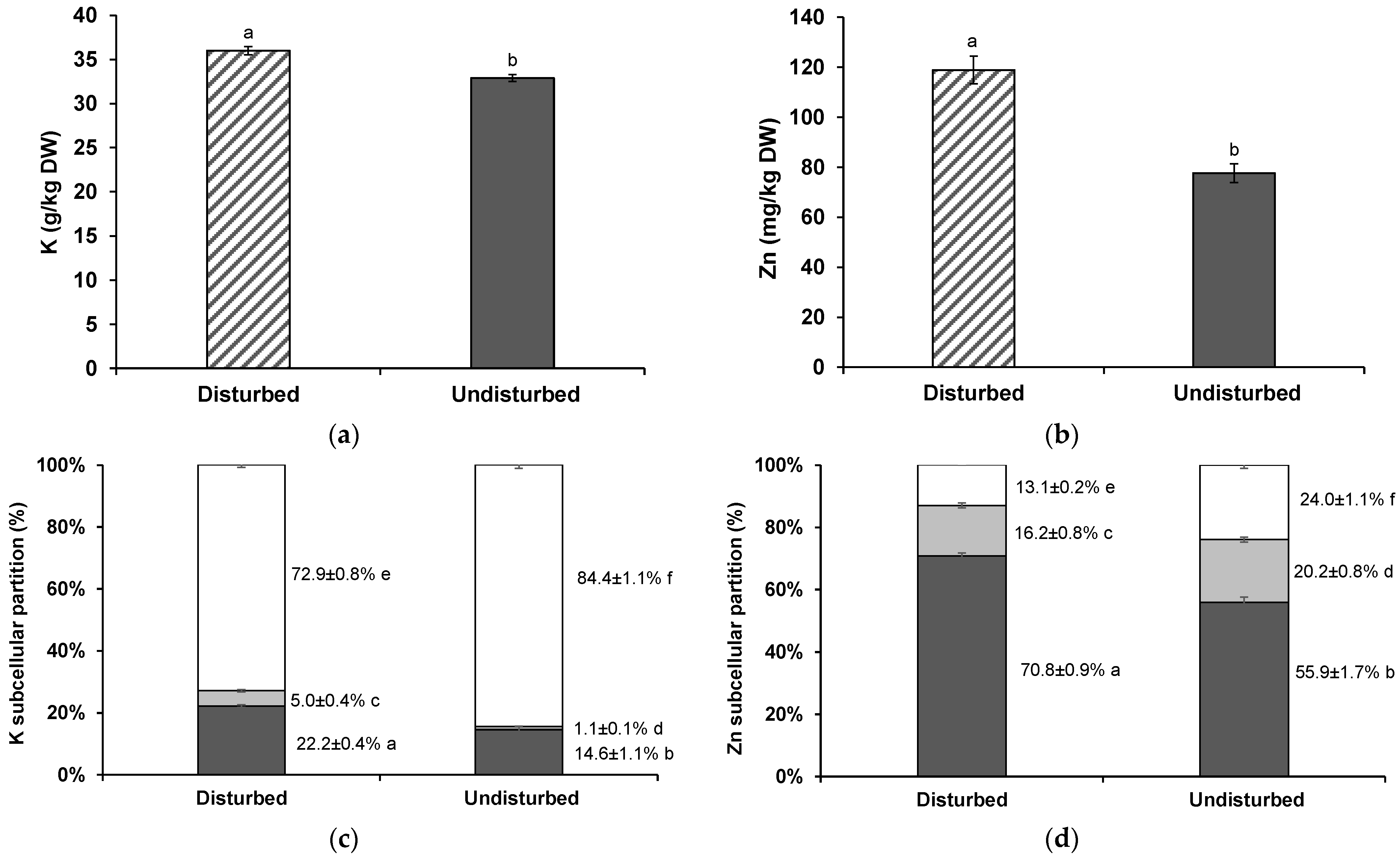
3.3. K and Zn Shoot Concentrations and Subcellular Distribution
3.4. Na and Si Shoot Concentrations and Subcellular Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khabaz-Saberi, H.; Barker, S.J.; Rengel, Z. Tolerance to Ion Toxicities Enhances Wheat Grain Yield in Acid Soils Prone to Drought and Transient Waterlogging. Crop Pasture Sci. 2014, 65, 862–867. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese Phytotoxicity: New Light on an Old Problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Millaleo, R.; Diaz, M.R.; Ivanov, A.; Mora, M.; Alberdi, M. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Setter, T.L.; Waters, I. Waterlogging Induces High to Toxic Concentrations of Iron, Aluminum, and Manganese in Wheat Varieties on Acidic Soil. J. Plant Nutr. 2006, 29, 899–911. [Google Scholar] [CrossRef]
- Carvalho, M.; Goss, M.J.; Teixeira, D.M. Manganese Toxicity in Portuguese Cambisols Derived from Granitic Rocks: Causes, Limitations of Soil Analyses and Possible Solutions. Rev. Cienc. Agrar. 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Alho, L.; Carvalho, M.; Brito, I.; Goss, M.J. The Effect of Arbuscular Mycorrhiza Fungal Propagules on the Growth of Subterranean Clover (Trifolium subterraneum L.) under Mn Toxicity in Ex Situ Experiments. Soil Use Manag. 2015, 31, 337–344. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.; Alho, L.; Brigido, C.; van Tuinen, D.; Felix, M.; Carvalho, M. Agronomic Management of AMF Functional Diversity to Overcome Biotic and Abiotic Stresses—The role of Plant Sequence and Intact Extraradical Mycelium. Fungal Ecol. 2019, 40, 72–81. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Ferreira, D.; Barrulas, P.; Brito, I.; Pinto, A.P.; Carvalho, M. Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil. Soil Syst. 2022, 6, 50. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Conceição, T.A.; Teixeira, D.M.; Brito, I.; Barrulas, P.; Pinto, A.P.; Vaz, M.; Carvalho, M. Arbuscular Mycorrhiza Extraradical Mycelium Promotes Si and Mn Subcellular Redistribution in Wheat Grown under Mn Toxicity. Int. J. Plant Biol. 2022, 13, 82–94. [Google Scholar] [CrossRef]
- Faria, J.M.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic Levels of Manganese in an Acidic Cambisol Alters Antioxidant Enzymes Activity, Element Uptake and Subcellular Distribution in Triticum Aestivum. Ecotoxicol. Environ. Saf. 2020, 193, 110355. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. Aluminium, Iron and Silicon Subcellular Redistribution in Wheat Induced by Manganese Toxicity. Appl. Sci. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Le Bot, J.; Goss, M.; Carvalho, M.J.G.P.R.; Van Beusichem, M.L.; Kirkby, E.A. The Significance of the Magnesium to Manganese Ratio in Plant Tissues for Growth and Alleviation of Manganese Toxicity in Tomato (Lycopersicon esculentum) and Wheat (Triticum aestivum) Plants. Plant Soil 1990, 124, 205–210. [Google Scholar] [CrossRef]
- de Vargas, J.P.; dos Santos, D.R.; Bastos, M.C.; Schaefer, G.; Parisi, P.B. Application Forms and Types of Soil Acidity Corrective: Changes in Depth Chemical Attributes in Long Term Period Experiment. Soil Tillage Res. 2019, 185, 47–60. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms Underlying Beneficial Plant–Fungus Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Rintoul, N.L.J. Arbuscular Mycorrhizal Associations in Plant Nutrition and Health. CAB Rev. Perspect. Agric. Veter Sci. Nutr. Nat. Resour. 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Alho, L.; Goss, M. Managing Arbuscular Mycorrhizal Fungi for Bioprotection: Mn Toxicity. Soil Biol. Biochem. 2014, 68, 78–84. [Google Scholar] [CrossRef]
- Faria, J.; Teixeira, D.; Pinto, A.; Brito, I.; Barrulas, P.; Carvalho, M. The Protective Biochemical Properties of Arbuscular Mycorrhiza Extraradical Mycelium in Acidic Soils Are Maintained throughout the Mediterranean Summer Conditions. Agronomy 2021, 11, 748. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. Arbuscular Mycorrhiza Inoculum Type Influences Phosphorus Subcellular Distribution in Shoots of Wheat Grown in Acidic Soil under Sustainable Agricultural Practices. Biol. Life Sci. Forum 2020, 4, 62. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.; Brito, I.; Carvalho, M. Diversity of Native Arbuscular Mycorrhiza Extraradical Mycelium Influences Antioxidant Enzyme Activity in Wheat Grown Under Mn Toxicity. Bull. Environ. Contam. Toxicol. 2021, 108, 451–456. [Google Scholar] [CrossRef]
- Santos, E.F.; Santini, J.M.K.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.; Kamei, S.; Kawai, S. Amelioration of Manganese Toxicity in Young Rice Seedlings with Potassium. J. Plant Nutr. 2003, 26, 1301–1314. [Google Scholar] [CrossRef]
- Alam, S.; Akiha, F.; Kamei, S.; Huq, S.M.I.; Kawai, S. Mechanism of Potassium Alleviation of Manganese Phytotoxicity in Barley. J. Plant Nutr. 2005, 28, 889–901. [Google Scholar] [CrossRef]
- Doncheva, S.; Poschenrieder, C.; Stoyanova, Z.; Georgieva, K.; Velichkova, M.; Barceló, J. Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ. Exp. Bot. 2009, 65, 189–197. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of Silicon-Mediated Alleviation of Heavy Metal Toxicity in Plants: A Review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Cherghvareh, L.; Dashtebani, F. Effect of Silicon Supplementation on Wheat Plants under Salt Stress. J. Plant Process Funct. 2016, 5, 1–11. [Google Scholar]
- Campos, C.; Carvalho, M.; Brígido, C.; Goss, M.J.; Nobre, T. Symbiosis Specificity of the Preceding Host Plant Can Dominate but Not Obliterate the Association between Wheat and Its Arbuscular Mycorrhizal Fungal Partners. Front. Microbiol. 2018, 9, 2920. [Google Scholar] [CrossRef]
- Brígido, C.; van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the Biological Diversity of AM Fungi by Combination of Host Plant Succession and Integrity of Extraradical Mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus Acquisition Efficiency Related to Root Traits: Is Mycorrhizal Symbiosis a Key Factor to Wheat and Barley Cropping? Front. Plant Sci. 2018, 9, 752. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; de Carvalho, M.; Chatagnier, O.; van Tuinen, D. Impact of Tillage System on Arbuscular Mycorrhiza Fungal Communities in the Soil under Mediterranean Conditions. Soil Tillage Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- de Novais, C.B.; Avio, L.; Giovannetti, M.; de Faria, S.M.; Siqueira, J.O.; Sbrana, C. Interconnectedness, Length and Viability of Arbuscular Mycorrhizal Mycelium as Affected by Selected Herbicides and Fungicides. Appl. Soil Ecol. 2019, 143, 144–152. [Google Scholar] [CrossRef]
- Zhu, C.; Ling, N.; Guo, J.; Wang, M.; Guo, S.; Shen, Q. Impacts of Fertilization Regimes on Arbuscular Mycorrhizal Fungal (AMF) Community Composition Were Correlated with Organic Matter Composition in Maize Rhizosphere Soil. Front. Microbiol. 2016, 7, 1840. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A Meta-Analysis of Context-Dependency in Plant Response to Inoculation with Mycorrhizal Fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Pinto, A.P.; Teixeira, D.M.; Brito, I.; Dias, L.; Barrulas, P.; Alho, L.; Carvalho, M. Elemental Composition and Antioxidant Enzyme Activity of Roots and Shoots of Wheat Grown in Manganese Spiked Montado Alentejano Soil. Free Radic. Biol. Med. 2018, 120, S151. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; Da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Muhammad, N.; Zvobgo, G.; Zhang, G.-P. A Review: The Beneficial Effects and Possible Mechanisms of Aluminum on Plant Growth in Acidic Soil. J. Integr. Agric. 2019, 18, 1518–1528. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. Chapter 17—Adaptation of Plants to Adverse Chemical Soil Conditions. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 409–472. ISBN 978-0-12-384905-2. [Google Scholar]
- Lemes, E.M.; Rodrigues, G.I.; De Paula, A.D.M.; De Lima, D.T.; Torres, J.L.R. Mycorrhizal Associations in Cerrado Soils: A Review of Benefits and Management. Aust. J. Crop Sci. 2016, 10, 1504–1510. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Shi, S.; Luo, X.; Dong, X.; Qiu, Y.; Xu, C.; He, X. Arbuscular Mycorrhization Enhances Nitrogen, Phosphorus and Potassium Accumulation in Vicia faba by Modulating Soil Nutrient Balance under Elevated CO2. J. Fungi 2021, 7, 361. [Google Scholar] [CrossRef]
- Lone, R.; Shuab, R.; Khan, S.; Ahmad, J.; Koul, K.K. Influence of Mycorrhizal Inoculation on Carrot Growth, Metabolites and Nutrition. J. Plant Nutr. 2018, 41, 432–444. [Google Scholar] [CrossRef]
- Xu, F.-J.; Zhang, A.-Y.; Yu, Y.-Y.; Sun, K.; Tang, M.-J.; Zhang, W.; Xie, X.-G.; Dai, C.-C. Soil Legacy of Arbuscular Mycorrhizal Fungus Gigaspora margarita: The Potassium-Sequestering Glomalin Improves Peanut (Arachis hypogaea) Drought Resistance and Pod Yield. Microbiol. Res. 2021, 249, 126774. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The Potassium Transporter SlHAK10 Is Involved in Mycorrhizal Potassium Uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Alam Bhat, M.; et al. Arbuscular Mycorrhizal Fungi and Its Major Role in Plant Growth, Zinc Nutrition, Phosphorous Regulation and Phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.-X.; Liu, Y.-M.; Liu, D.-Y.; Chen, X.-P.; Zou, C.-Q. Zinc Uptake by Roots and Accumulation in Maize Plants as Affected by Phosphorus Application and Arbuscular Mycorrhizal Colonization. Plant Soil 2017, 413, 59–71. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Tyerman, S.; Cavagnaro, T. The Dual Benefit of Arbuscular Mycorrhizal Fungi under Soil Zinc Deficiency and Toxicity: Linking Plant Physiology and Gene Expression. Plant Soil 2017, 420, 375–388. [Google Scholar] [CrossRef]
- Ercoli, L.; Schüßler, A.; Arduini, I.; Pellegrino, E. Strong Increase of Durum Wheat Iron and Zinc Content by Field-Inoculation with Arbuscular Mycorrhizal Fungi at Different Soil Nitrogen Availabilities. Plant Soil 2017, 419, 153–167. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in Plants: Perception, Signalling, and Regulation of Sodium Fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Krishnasamy, K.; Bell, R.; Ma, Q. Wheat Responses to Sodium Vary with Potassium Use Efficiency of Cultivars. Front. Plant Sci. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Horst, W.J.; Marschner, H. Effect of Silicon on Manganese Tolerance of Bean Plants (Phaseolus vulgaris L.). Plant Soil 1978, 50, 287–303. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Li, Z.; Fan, F.; Liang, Y. Silicon Ameliorates Manganese Toxicity by Regulating Manganese Transport and Antioxidant Reactions in Rice (Oryza sativa L.). Plant Soil 2012, 354, 407–419. [Google Scholar] [CrossRef]
- Rogalla, H.; Römheld, V. Role of Leaf Apoplast in Silicon-Mediated Manganese Tolerance of Cucumis sativus L. Plant Cell Environ. 2002, 25, 549–555. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Bogdanović, J.; Maksimović, V.; Nikolic, M. Silicon Modulates the Metabolism and Utilization of Phenolic Compounds in Cucumber (Cucumis sativus L.) Grown at Excess Manganese. J. Plant Nutr. Soil Sci. 2007, 170, 739–744. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Pinto, A.P.; Teixeira, D.M.; Barrulas, P.; Brito, I.; Carvalho, M. Subcellular Element Distribution in Shoots of Wheat Grown in an Acidic Soil with Native AMF Extraradical Mycelium. Agronomy 2022, 12, 2173. https://doi.org/10.3390/agronomy12092173
Faria JMS, Pinto AP, Teixeira DM, Barrulas P, Brito I, Carvalho M. Subcellular Element Distribution in Shoots of Wheat Grown in an Acidic Soil with Native AMF Extraradical Mycelium. Agronomy. 2022; 12(9):2173. https://doi.org/10.3390/agronomy12092173
Chicago/Turabian StyleFaria, Jorge M. S., Ana Paula Pinto, Dora Martins Teixeira, Pedro Barrulas, Isabel Brito, and Mário Carvalho. 2022. "Subcellular Element Distribution in Shoots of Wheat Grown in an Acidic Soil with Native AMF Extraradical Mycelium" Agronomy 12, no. 9: 2173. https://doi.org/10.3390/agronomy12092173
APA StyleFaria, J. M. S., Pinto, A. P., Teixeira, D. M., Barrulas, P., Brito, I., & Carvalho, M. (2022). Subcellular Element Distribution in Shoots of Wheat Grown in an Acidic Soil with Native AMF Extraradical Mycelium. Agronomy, 12(9), 2173. https://doi.org/10.3390/agronomy12092173








