Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Soil Incubation Study (Exp. 1)
2.1.1. Experimental Conditions
2.1.2. Experimental Design and Analysis
2.2. Greenhouse Trial (Exp. 2)
2.2.1. Experimental Conditions
2.2.2. Experimental Design, Measurements, and Analysis
2.3. Statistical Analysis
3. Results
3.1. Nitrogen Mineralized from Organic Fertilizers during Laboratory Incubation Assay
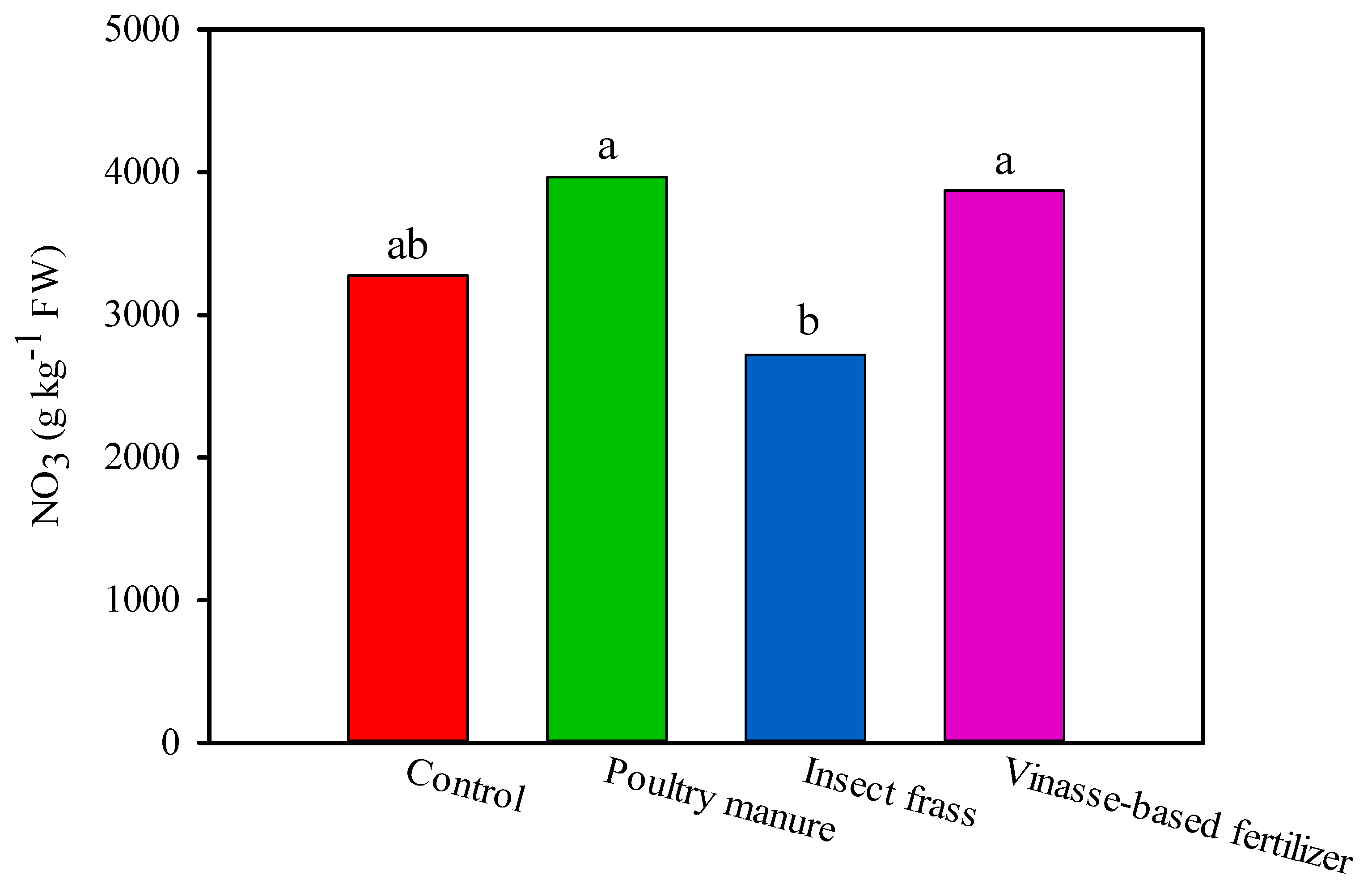
3.2. Agronomic Response of Lettuce and Soil Enzymatic Activity under Greenhouse Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamidov, A.; Helming, K.; Bellocchi, G.; Bojar, W.; Dalgaard, T.; Ghaley, B.B.; Hoffmann, C.; Holman, I.; Holzkämper, A.; Krzeminska, D.; et al. Impacts of climate change adaptation options on soil functions: A review of European case-studies. Land Degrad. Dev. 2018, 29, 2378–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonneveld, C. Effects of Salinity on Substrate Grown Vegetables and Ornamentals in Greenhouse Horticulture. Ph.D. Thesis, University of Wageningen, Wageningen, The Netherlands, 2000. [Google Scholar]
- European Commission. Farm to Fork: New Rules to Reduce the Risk and Use of Pesticides in the EU.; European Commission: Brussels, Belgium, 2022; pp. 1–3. Available online: https://ec.europa.eu/commission/presscorner/detail/en/qanda_22_3694 (accessed on 14 October 2022).
- Willer, H.; Trávní, J.; Meier, C.; Schlatter, B. (Eds.) The World of Organic Agriculture Statistics and Emerging Trends 2022; Research Institute of Organic Agriculture FiBL, Frick, and IFOAM—Organics International: Bonn, Germany, 2022; pp. 1–345. Available online: https://www.fibl.org/fileadmin/documents/shop/1344-organic-world-2022.pdf (accessed on 14 October 2022).
- Sun, X.; Ye, Y.; Ma, Q.; Guan, Q.; Jones, D.L. Variation in enzyme activities involved in carbon and nitrogen cycling in rhizosphere and bulk soil after organic mulching. Rhizosphere 2021, 19, 100376. [Google Scholar] [CrossRef]
- Antonious, G.F.; Turley, E.T.; Dawood, M.H. Monitoring Soil Enzymes Activity before and after Animal Manure Application. Agriculture 2020, 10, 166. [Google Scholar] [CrossRef]
- Hu, W.; Jiao, Z.; Wu, F.; Liu, Y.; Dong, M.; Ma, X.; Fan, T.; An, L.; Feng, H. Long-term effects of fertilizer on soil enzymatic activity of wheat field soil in Loess Plateau, China. Ecotoxicology 2014, 23, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.-H.; Chung, R.-S.; Wang, F.-N. Effect of different types of organic fertilizers on the chemical properties and enzymatic activities of an Oxisol under intensive cultivation of vegetables for 4 years. Soil Sci. Plant Nutr. 2008, 54, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Ciriminna, R.; Scurria, A.; Tizza, G.; Pagliaro, M. Volcanic ash as multi-nutrient mineral fertilizer: Science and early applications. JSFA Rep. 2022, 2, 528–534. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Li, F. Soil Enzyme Activities and Soil Fertility Dynamics. In Advances in Citrus Nutrition; Springer: Dordrecht, The Netherlands, 2012; pp. 143–156. [Google Scholar] [CrossRef]
- Tittarelli, F.; Båth, B.; Ceglie, F.; García, M.; Möller, K.; Reents, H.; Védie, H.; Voogt, W. Soil fertility management in organic greenhouse: An analysis of the European context. Acta Hortic. 2017, 1164, 113–126. [Google Scholar] [CrossRef]
- Loecke, T.D.; Cambardella, C.A.; Liebman, M. Synchrony of net nitrogen mineralization and maize nitrogen uptake following applications of composted and fresh swine manure in the Midwest U.S. Nutr. Cycl. Agroecosyst. 2012, 93, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, M.; Salerno, A.; Rea, E.; Colla, G. Nitrogen availability from organic and organic-mineral fertilizers. In Proceedings of the 18th International Symposium of CIEC ‘More Sustainability in Agriculture: New Fertilizers and Fertilization Management’, Rome, Italy, 8–12 November 2009. [Google Scholar]
- Carassay, L.R.; Bustos, D.A.; Golberg, A.D.; Taleisnik, E. Tipburn in salt-affected lettuce (Lactuca sativa L.) plants results from local oxidative stress. J. Plant Physiol. 2012, 169, 285–293. [Google Scholar] [CrossRef]
- Sago, Y. Effects of Light Intensity and Growth Rate on Tipburn Development and Leaf Calcium Concentration in Butterhead Lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Pavlou, G.C.; Ehaliotis, C.D.; Kavvadias, V.A. Effect of organic and inorganic fertilizers applied during successive crop seasons on growth and nitrate accumulation in lettuce. Sci. Hortic. 2007, 111, 319–325. [Google Scholar] [CrossRef]
- Gunes, A.; Post, W.N.K.; Kirkby, E.A.; Aktas, M. Influence of partial replacement of nitrate by amino acid nitrogen or urea in the nutrient medium on nitrate accumulation in NFT grown winter lettuce. J. Plant Nutr. 1994, 17, 1929–1938. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Craddock, V.M. Toxicology: Nitrosamines and human cancer: Proof of an association? Nature 1983, 306, 638. [Google Scholar] [CrossRef]
- Esteves, C.; Fareleira, P.; Castelo-Branco, M.; Lopes, I.; Mota, M.; Murta, D.; Menino, R. Black soldier fly larvae frass increases the soil’s residual nutrient content and enzymatic activity—A lettuce production trial. J. Insects Food Feed 2022, 8, 1431–1440. [Google Scholar] [CrossRef]
- Cavalheiro, T.R.T.; Alcoforado, R.D.O.; Silva, V.S.D.A.; Coimbra, P.P.S.; Mendes, N.D.S.; Cavalcanti, E.D.C.; Jurelevicius, D.D.A.; Gonçalves, C.B.D.A. The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity. Sustainability 2020, 13, 128. [Google Scholar] [CrossRef]
- Trupiano, D.; Cocozza, C.; Baronti, S.; Amendola, C.; Vaccari, F.P.; Lustrato, G.; Di Lonardo, S.; Fantasma, F.; Tognetti, R.; Scippa, G.S. The Effects of Biochar and Its Combination with Compost on Lettuce (Lactuca sativa L.) Growth, Soil Properties, and Soil Microbial Activity and Abundance. Int. J. Agron. 2017, 2017, 3158207. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.M.; Gonçalves, E.R.; Endres, L.; Gomes, T.C.A.; Jadoski, C.J.; Nascimento, L.A.; Santos, E.D. Photo-synthetic measurements in lettuce submitted to different agroindustrial residue composting. Pesqui. Apl. Agrotecnol. 2010, 3, 103–112. [Google Scholar] [CrossRef]
- Ueno, C.R.J.; Da Costa, A.C.S.; Gimenes, M.L.; Zanin, G.M. Agricultural recycling of biodigested vinasse for lettuce production. Ambient. E Agua Interdiscip. J. Appl. Sci. 2014, 9, 593–601. [Google Scholar] [CrossRef]
- Trinchera, A.; Baratella, V.; Rinaldi, S.; Renzaglia, M.; Marcucci, A.; Rea, E. Greenhouse lettuce: Assessing nutrient use efficiency of digested livestock manure as organic N-fertiliser. Acta Hortic. 2014, 1041, 63–69. [Google Scholar] [CrossRef]
- Liu, C.-W.; Sung, Y.; Chen, B.-C.; Lai, H.-Y. Effects of Nitrogen Fertilizers on the Growth and Nitrate Content of Lettuce (Lactuca sativa L.). Int. J. Environ. Res. Public Health 2014, 11, 4427–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J. Effect of food waste compost on microbial population, soil enzyme activity and lettuce growth. Bioresour. Technol. 2004, 93, 21–28. [Google Scholar] [CrossRef]
- Akça, M.O.; Namlı, A. Effects of poultry litter biochar on soil enzyme activities and tomato, pepper and lettuce plants growth. Eurasian J. Soil Sci. 2015, 4, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Cheng, Z.; Meng, H. Growth of wheat and lettuce and enzyme activities of soils under garlic stalk decomposition for different durations: Function and utilization of garlic stalk decomposition for different durations. J. Sci. Food Agric. 2017, 97, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, C.; Cheng, J.; Wang, F.; Qiu, L. Effects of biogas residues containing antibiotics on soil enzyme activity and lettuce growth. Environ. Sci. Pollut. Res. 2019, 26, 6116–6122. [Google Scholar] [CrossRef] [PubMed]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A. Soil Enzyme Activity Response under the Amendment of Different Types of Biochar. Agronomy 2022, 12, 569. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Baillie, I.C.; Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods. J. Ecol. 1990, 78, 547. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties (Agronomy 9); Norman, A.G., Ed.; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar] [CrossRef]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; Soil and Plant Analysis Council, Inc.: Boca Raton, FL, USA; CRC Press: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Microbial activity in soils under mediterranean environmental conditions. Soil Biol. Biochem. 1994, 26, 1185–1191. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Factors affecting glucosidase and galactosidase activities in soils. Soil Biol. Biochem. 1990, 22, 891–897. [Google Scholar] [CrossRef]
- Biró, B.; Toscano, G.; Horváth, N.; Matics, H.; Domonkos, M.; Scotti, R.; Rao, M.A.; Wejden, B.; French, H.K. Vertical and horizontal distributions of microbial abundances and enzymatic activities in propylene-glycol-affected soils. Environ. Sci. Pollut. Res. 2014, 21, 9095–9108. [Google Scholar] [CrossRef] [PubMed]
- Parham, J.; Deng, S. Detection, quantification and characterization of β-glucosaminidase activity in soil. Soil Biol. Biochem. 2000, 32, 1183–1190. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing soil fertility in organic farming systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Uddin, S.; Islam, M.R.; Jahangir, M.M.R.; Rahman, M.M.; Hassan, S.; Hassan, M.M.; Abo-Shosha, A.A.; Ahmed, A.F.; Rahman, M.M. Nitrogen Release in Soils Amended with Different Organic and Inorganic Fertilizers under Contrasting Moisture Regimes: A Laboratory Incubation Study. Agronomy 2021, 11, 2163. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Da Silva, A.; Rossetto, R.; Bonnecine, J.; Piemonte, M.; Muraoka, T. Net and Potential Nitrogen Mineralization in Soil with Sugarcane Vinasse. Sugar Tech. 2013, 15, 159–164. [Google Scholar] [CrossRef]
- Argenta, G.; Da Silva, P.R.F.; Sangoi, L. Leaf relative chlorophyll content as an indicator parameter to predict nitrogen fertilization in maize. Ciênc Rural 2004, 34, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.W.; Mengel, K.; Kirkby, E.A. Principles of plant nutrition. Ann. Bot. 2004, 93, 479–480. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Jägerbrand, A.K.; Kudo, G. Short-Term Responses in Maximum Quantum Yield of PSII (Fv/Fm) to ex situ Temperature Treatment of Populations of Bryophytes Originating from Different Sites in Hokkaido, Northern Japan. Plants 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Bolhar-Nordenkampf, H.R.; Long, S.; Baker, N.R.; Oquist, G.; Schreiber, U.; Lechner, E.G. Chlorophyll Fluorescence as a Probe of the Photosynthetic Competence of Leaves in the Field: A Review of Current Instrumentation. Funct. Ecol. 1989, 3, 497. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop Salt Tolerance-Current Assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Wichern, J.; Wichern, F.; Joergensen, R.G. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 2006, 137, 100–108. [Google Scholar] [CrossRef]
- Iqbal, T.; Joergensen, R.G.; Knoblauch, C.; Lucassen, R.; Singh, Y.; Watson, C.; Wichern, F. Rice straw addition does not substantially alter microbial properties under hypersaline soil conditions. Biol. Fertil. Soils 2016, 52, 867–877. [Google Scholar] [CrossRef]
- Chahal, S.S.; Choudhary, O.P.; Mavi, M.S. Organic amendments decomposability influences microbial activity in saline soils. Arch. Agron. Soil Sci. 2017, 63, 1875–1888. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid Land Res. Manag. 2018, 33, 1–21. [Google Scholar] [CrossRef]
- Piccolo, A.; Pietramellara, G.; Mbagwu, J. Use of humic substances as soil conditioners to increase aggregate stability. Geoderma 1997, 75, 267–277. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Santos, M.P.; Dobbss, L.B.; Olivares, F.L.; Canellas, L.P.; Binzel, M.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 2010, 231, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Havelcová, M.; Mizera, J.; Sýkorová, I.; Pekař, M. Sorption of metal ions on lignite and the derived humic substances. J. Hazard. Mater. 2009, 161, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.-B. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total. Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Kong, Q.; Qiu, Z.; Chen, L.; Shen, D. Patterns of heavy metal immobilization by MSW during the landfill process. Chem. Eng. J. 2019, 375, 122060. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B. Assessing soil quality in different agro-ecosystems through biochemical and chemico-structural properties of humic substances. Soil Tillage Res. 1999, 51, 129–137. [Google Scholar] [CrossRef]
- Gramss, G.; Ziegenhagen, D.; Sorge, S. Degradation of Soil Humic Extract by Wood- and Soil-Associated Fungi, Bacteria, and Commercial Enzymes. Microb. Ecol. 1999, 37, 140–151. [Google Scholar] [CrossRef]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- Killham, K.; Firestone, M.K. Proline transport increases growth efficiency in salt-stressed Streptomyces griseus. Appl. Environ. Microbiol. 1984, 48, 239–241. [Google Scholar] [CrossRef]
- Schimel, J.P.; Scott, W.J.; Killham, K. Changes in Cytoplasmic Carbon and Nitrogen Pools in a Soil Bacterium and a Fungus in Response to Salt Stress. Appl. Environ. Microbiol. 1989, 55, 1635–1637. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Commission Regulation (EU) No 1258/2011 of 2 December 2011 amending Regulation (EC) No. 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, L320, 15–17. [Google Scholar]
- Ayyobi, H.; Olfati, J.-A.; Peyvast, G.-A. The effects of cow manure vermicompost and municipal solid waste compost on peppermint (Mentha piperita L.) in Torbat-e-Jam and Rasht regions of Iran. Int. J. Recycl. Org. Waste Agric. 2014, 3, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Mills, H.A.; Jones, J.B., Jr. Plant Analysis Handbook II. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing: Athens, Greece, 1996. [Google Scholar]
- Zahidah, A.R.; Rosenani, A.B.; Hajar, A.S.; Nozulaidi, N.M. Effects of Different Types of Organic Fertilizer on Biomass Yield, Bioactive Compounds and Heavy Metals Contents of Phyllanthus Niruri. J. Phys. Conf. Ser. 2021, 2000, 012005. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Li, J.; Li, S. Effects of Several Organic Fertilizers on Heavy Metal Passivation in Cd-Contaminated Gray-Purple Soil. Front. Environ. Sci. 2022, 10, 884. [Google Scholar] [CrossRef]
- Wikar, J. The effect of fertilizing soils degraded by the metallurgical industry on the content of elements in Lactuca sativa L. Sci. Rep. 2021, 11, 4072. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wu, X.; Bolan, N.; Kirkham, M.; Yang, J.; Chen, Z. Inhibition of cadmium uptake by wheat with urease-producing bacteria combined with sheep manure under field conditions. Chemosphere 2022, 293, 133534. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Dalal, R. Urease activity in some Trinidad soils. Soil Biol. Biochem. 1975, 7, 5–8. [Google Scholar] [CrossRef]
- Jha, D.K.; Sharma, G.D.; Mishra, R.R. Soil microbial population numbers and enzyme activities in relation to altitude and forest degradation. Soil Biol. Biochem. 1992, 24, 761–767. [Google Scholar] [CrossRef]
- Amador, J.A.; Glucksman, A.M.; Lyons, J.B.; Gorres, J.H. Spatial distribution of soil phosphatase activity within a riparian forest. Soil Sci. 1997, 162, 808–825. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis, Part 2 Microbiological and Biochemical Properties; Mickelson, S.H., Bighan, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–826. [Google Scholar]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Kızılkaya, R.; Bayraklı, B. Effects of N-enriched sewage sludge on soil enzyme activities. Appl. Soil Ecol. 2005, 30, 192–202. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Kong, C. Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 2009, 45, 436–441. [Google Scholar] [CrossRef]
- Ajwa, H.A.; Tabatabai, M.A. Decomposition of different organic materials in soils. Biol. Fertil. Soils 1994, 18, 175–182. [Google Scholar] [CrossRef]
- Turner, B.L.; Hopkins, D.W.; Haygarth, P.M.; Ostle, N. β-Glucosidase activity in pasture soils. Appl. Soil Ecol. 2002, 20, 157–162. [Google Scholar] [CrossRef]
- Filipek-Mazur, B.; Pużyńska, K.; Tabak, M.; Pużyński, S. Enzymatic Activity of Soil under Spelt Grown in an Organic Farming System in Poland’s Temperate Climate. Agronomy 2020, 10, 930. [Google Scholar] [CrossRef]
- Wang, H.; Boutton, T.; Xu, W.; Hu, G.; Jiang, P.; Bai, E. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef] [Green Version]
- Chathurika, J.A.S.; Kumaragamage, D.; Indraratne, S.P.; Dandeniya, W.S. Improving soil carbon pool, soil fertility and yield of maize (Zea mays L.) in low-fertile tropical Alfisols by combining fertilizers with slow-decomposing organic amendments. J. Agric. Sci. 2019, 157, 45–54. [Google Scholar] [CrossRef]


| Fertilizer Treatment | SPAD Index | ||||
|---|---|---|---|---|---|
| 4 DAT | 9 DAT | 15 DAT | 23 DAT | 28 DAT | |
| Control | 14.93 | 19.52 c | 24.67 c | 24.90 | 24.65 |
| Poultry manure | 14.53 | 23.30 b | 26.27 b | 24.31 | 24.67 |
| Insect frass | 15.46 | 23.13 b | 26.33 b | 24.38 | 24.88 |
| Vinasse-based fertilizer | 15.25 | 24.63 a | 28.29 a | 25.20 | 25.24 |
| MSE | 0.35 | 0.39 | 0.41 | 0.64 | 1.13 |
| CV (%) | 4.27 | 8.99 | 5.47 | 3.28 | 3.26 |
| Tukey (5%) | 1.25 | 1.32 | 1.34 | 1.68 | 2.24 |
| Fertilizer Treatment | Fv/Fm | ||
|---|---|---|---|
| 20 DAT | 25 DAT | 30 DAT | |
| Control | 0.837 | 0.831 a | 0.852 |
| Poultry manure | 0.836 | 0.822 b | 0.845 |
| Insect frass | 0.842 | 0.834 a | 0.855 |
| Vinasse-based fertilizer | 0.838 | 0.830 ab | 0.849 |
| MSE | 0.000279 | 0.000014 | 0.000465 |
| CV (%) | 1.804 | 0.577 | 2.313 |
| Tukey (5%) | 0.035 | 0.008 | 0.045 |
| Fertilizer Treatment | Shoot Fresh Weight (g Plant−1) | Shoot Dry Weight (g Plant−1) | Root Dry Weight (g Plant−1) | Root to Shoot Ratio |
|---|---|---|---|---|
| Control | 132.31 c | 5.13 b | 1.91 | 0.39 |
| Poultry manure | 172.99 b | 7.49 ab | 2.46 | 0.34 |
| Insect frass | 230.99 a | 9.97 a | 2.41 | 0.24 |
| Vinasse-based fertilizer | 233.15 a | 9.33 a | 2.70 | 0.29 |
| MSE | 331.5 | 2.01 | 0.22 | 0.01 |
| CV (%) | 24.21 | 29.05 | 21.80 | 31.42 |
| Tukey (5%) | 38.24 | 2.98 | 1.00 | 0.19 |
| Fertilizer Treatment | Macronutrients (g/kg DW) | ||||
|---|---|---|---|---|---|
| N | P | K | Ca | Mg | |
| Control | 44.16 | 3.33 b | 82.72 | 14.09 | 5.63 |
| Poultry manure | 43.10 | 4.05 b | 90.30 | 12.93 | 5.95 |
| Insect frass | 41.05 | 4.07 b | 84.36 | 12.51 | 5.78 |
| Vinasse-based fertilizer | 42.55 | 5.09 a | 85.52 | 11.80 | 5.51 |
| MSE | 3.09 | 0,20 | 40.67 | 1.78 | 1.29 |
| CV (%) | 4.57 | 18.51 | 7.47 | 11.45 | 18.02 |
| Tukey (5%) | 3.69 | 0.95 | 13.39 | 2.80 | 2.39 |
| Fertilizer Treatment | Essential Trace Elements (mg/kg DW) | ||||||
|---|---|---|---|---|---|---|---|
| B | Fe | Mn | Zn | Cu | Mo | Ni | |
| Control | 21.72 | 190.89 | 285.36 | 52.40 | 12.78 | 0.37 a | 0.29 |
| Poultry manure | 26.70 | 185.91 | 314.19 | 65.55 | 19.77 | 0.39 a | 0.20 |
| Insect frass | 25.18 | 196.72 | 297.24 | 55.85 | 17.98 | 0.36 a | 0.27 |
| Vinasse-based fertilizer | 25.14 | 128.06 | 254.83 | 51.59 | 14.52 | 0.25 b | 0.18 |
| MSE | 16.44 | 9511.57 | 4866.77 | 177.74 | 17.32 | 0.002 | 0.011 |
| CV (%) | 16.55 | 52.32 | 23.02 | 23.48 | 28.81 | 19.31 | 44.78 |
| Tukey (5%) | 8.51 | 204.81 | 146.50 | 28.00 | 8.74 | 0.08 | 0.22 |
| Fertilizer Treatments | Non-Essential Trace Elements (mg/kg DW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Al | Ba | Cd | Co | Cr | Pb | Se | Sn | |
| Control | 3.62 | 440.17 | 19.04 | 0.24 | 0.23 a | 0.28 | 1.29 a | 0.92 | 0.89 |
| Poultry manure | 3.83 | 402.93 | 22.28 | 0.36 | 0.17 ab | 0.32 | 0.81 b | 0.84 | 0.83 |
| Insect frass | 4.66 | 338.47 | 25.35 | 0.26 | 0.17 ab | 0.40 | 0.77 b | 0.98 | 0.83 |
| Vinasse-based fertilizer | 3.61 | 254.33 | 17.63 | 0.28 | 0.09 b | 0.25 | 0.52 b | 1.02 | 0.83 |
| MSE | 1.127 | 32,475.66 | 42.56 | 0.004 | 0.001 | 0.027 | 0.044 | 0.034 | 0.004 |
| CV (%) | 26.68 | 49.27 | 31.32 | 25.99 | 37.12 | 50.66 | 40.59 | 19.04 | 7.48 |
| Tukey (5%) | 2.23 | 378.44 | 13.70 | 0.13 | 0.08 | 0.35 | 0.44 | 0.39 | 0.14 |
| Fertilizer Treatment | AcP (µg p-Nitrophenol/g h) | AlkP (µg p-Nitrophenol/g h) | ArS (µg p-Nitrophenol/g h) | DHA (µg Triphenyl formazan/g h) | NAGase—Chitinase (µg p-Nitrophenol/g h) | THA (µg Hydrolyzed FDA/g h) |
|---|---|---|---|---|---|---|
| Control | 48.74 b | 362.25 b | 4.83 c | 0.42 b | 8.84 c | 14.26 c |
| Poultry manure | 91.61 a | 619.94 a | 8.29 b | 1.87 a | 47.81 ab | 63.93 a |
| Insect frass | 99.35 a | 561.58 a | 11.43 a | 1.88 a | 57. 61 a | 61.31 a |
| Vinasse-based fertilizer | 65.35 ab | 336.50 b | 8.21 b | 0.84 b | 30.85 b | 35.56 b |
| MSE | 298.53 | 1766.50 | 1.81 | 0.19 | 71.26 | 26.31 |
| CV (%) | 34.13 | 28.14 | 32.90 | 61.52 | 56.66 | 49.10 |
| Tukey (5%) | 36.28 | 88.26 | 2.83 | 0.93 | 17.73 | 10.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy 2023, 13, 194. https://doi.org/10.3390/agronomy13010194
Cardarelli M, El Chami A, Iovieno P, Rouphael Y, Bonini P, Colla G. Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy. 2023; 13(1):194. https://doi.org/10.3390/agronomy13010194
Chicago/Turabian StyleCardarelli, Mariateresa, Antonio El Chami, Paola Iovieno, Youssef Rouphael, Paolo Bonini, and Giuseppe Colla. 2023. "Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil" Agronomy 13, no. 1: 194. https://doi.org/10.3390/agronomy13010194
APA StyleCardarelli, M., El Chami, A., Iovieno, P., Rouphael, Y., Bonini, P., & Colla, G. (2023). Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy, 13(1), 194. https://doi.org/10.3390/agronomy13010194









