Abstract
In this study, a field experiment was conducted to evaluate the growth and yield responses of Sri Lankan lowland rice (Oryza sativa L.) with the application of beneficial Arbuscular mycorrhizal fungi (AMF) inoculum and intercropping with highly mycorrhizal-dependent vetiver grass (Chrysopogon zizanioides L.) under two different soil nutrient management systems (NMSs): conventional/chemical (CNMS) and organic (ONMS). The experiment was designed as a split plot with three blocks. Each CNMS and ONMS experiment included an untreated control (T0) and three treatments—AMF inoculation (T1), vetiver intercropping (T2), and the combination of AMF and vetiver (T3). According to the results, the colonization of rice roots with AMF was not affected significantly by the treatments and ranged from 0–15.8%. The effect was very low or absent in the early stage and then higher in the later stages of the rice plant. Furthermore, plant growth was not significantly different between the two NMSs, although grain yield was significantly higher (p < 0.05) in ONMS than for the respective controls (T0), with the order T1 (0.45 kg/m2) > T2 (0.42 kg/m2) > T3 (0.41 kg/m2) in CNMS and T2 (0.44 kg/m2) > T1 (0.41 kg/m2) > T3 (0.40 kg/m2), thus suggesting the utilization of AMF and vetiver in a lowland rice farming system is beneficial.
1. Introduction
1.1. The Current Scenario of Rice Production
Rice (Oryza sativa L.) is considered a staple food in many countries in the world, including Sri Lanka. Rice is known to supply 45% of the total calories and 40% of the total protein requirements for an average Sri Lankan [1]. The demand for rice in Sri Lanka is estimated to grow at a rate of 1.1% per year [1]. Consequently, rice production needs to increase at a rate of 2.9% per year to meet this demand [1]. This has led to the adoption of high-yielding cultivars, machinery, pesticides, and inorganic fertilizer in Sri Lankan rice cultivation, resulting in a significant increase in agricultural productivity. Nevertheless, recent policy changes banning and restricting the use of agrochemicals, including fertilizers, has led to multiple constraints on rice production, resulting in huge yield losses and an increase in market value [2]. On top of that, on one hand, it is true that minimizing the use of synthesized agrochemicals in rice cultivation has always been a better option to mitigate their negative impact on the environment and human health [3]. On the other hand, sustainable agriculture is a growing concept in today’s world, where public acceptance and demand for more eco-friendly and organic farming products are increasing daily [4]. In this regard, the use of bio-fertilizers such as nitrogen fixers, potassium solubilizers, phosphorus solubilizers, plant-growth-promoting rhizobacteria, cyanobacteria, and algae is a desirable alternative to synthetic fertilizers [5,6]. Nevertheless, each of the aforesaid biofertilizers has its own pros and cons. Effectiveness is one of the most common problems with these biofertilizers; therefore, many studies have been carried out to address this scenario.
1.2. Arbuscular Mycorrhizal Fungi and Their Importance
It has long been known that Arbuscular mycorrhizal fungi (AMF) are valuable candidates for use as bio-fertilizers [7]. They have been found to be associated with about 80% of studied terrestrial plants. AMF belong to the phylum Glomeromycota and show an obligate symbiotic interaction with their host [8]. In this association, AMF provide several benefits to plants, e.g., increasing seed germination and plant growth [7,9] and protecting against unfamiliar environmental conditions, insect pests [10], and pathogens [11]. It is interesting to discuss AMF and their rice hosts specifically, as these are the main considerations of this study. Like their benefits on many other plants, AMF enhance rice plant growth and development by improving photosynthesis and other metabolite activities [12], such as the uptake of several macro- and micronutrients, including nitrogen (N), phosphorus (P), potassium (K), copper (Cu), and zinc (Zn) [13]. In addition, they modify soil properties and increase the ability of rice plants to withstand various abiotic stresses, while improving their productivity [13]. Moreover, it has been recorded that AMF enhance the resistance of rice plants to pests and diseases [14,15].
In Sri Lanka, rice is traditionally cultivated under lowland conditions [16] by sowing pregerminated rice seeds or transplanting seedlings on puddled and leveled soil [17]. However, upland/aerobic rice cultivation can also be found, but very rarely, known as “Kekulan” or “manawari”, which involves the cultivation of dry rice seeds on dry soil [17]. Although lowland rice is practiced prominently, mycorrhizal colonization is found to be very low in lowland rice due to the anoxic environment caused by soil flooding [18]. Nonetheless, AMF readily form associations with upland rice plants [19,20]. It has been demonstrated that rice root colonization by AMF is reduced under flooding conditions, decreasing from 43% to 27% [21], leading to a decline in AMF species diversity and abundance in the soil [19]. In certain cases, they have adapted and thrived in flooded conditions and have been reported to colonize lowland rice roots [22,23,24,25]. Further, colonization and abundance of AMF in lowland rice can be improved through the direct inoculation of AMF into the field [26,27]. In addition, several attempts have been made to promote the AMF symbioses of lowland rice through farming practices such as water management [28,29], the addition of organic amendments (e.g., compost and biochar) [30], crop rotation [20,31], and different rice farming systems (e.g., the system of rice intensification (SRI) method) [32]. Intercropping, which involves cultivating two or more crop species simultaneously in the same field area [33], is also a potential field practice to increase AMF abundance in soil. Regarding this aspect, a plethora of studies have been conducted on the intercropping of mycorrhizal plants along with upland rice; however, so far, no such studies have been reported for lowland rice cultivation [34,35,36,37,38]. From our point of view, this might be due to the limited availability of mycorrhizal plants adapted to lowland conditions or their limited compatibility with agronomic and cultural practices of rice cultivation.
1.3. The Aim of This Study
In the present study, we aimed to intercrop a selected efficient mycorrhizal symbiont, both with and without field inoculation of farm-produced AMF, to increase the abundance of AMF in lowland rice soil. For this, we selected vetiver grass (Chrysopogon zizanioides L.), which has high economic value [39], to intercrop with lowland rice due to its highly mycorrhizal potential [40] and ability to withstand water-logged conditions [41]. We expect vetiver grass to be compatible with the agronomic practices of rice farming since both plants belong to the same family, the Poaceae [42]. We further evaluate their effects on lowland rice growth and yield under both conventional and organic nutrient management to assess their potential to meet future rice demands sustainably.
2. Materials and Methods
2.1. Experimental Site and Design
The field experiment was carried out in the research unit of the Faculty of Agriculture, Rajarata University of Sri Lanka at Puliyankulama, Anuradhapura, North Central Province of the island during the 2019/2020 Maha season (wet season, November–March), where the mean seasonal temperature is 29.2 °C and rainfall is 962 mm. A conventional rice field that has clay loam, Low Humic Glay (LHG) soil, and has been followed over three years was selected as the experimental site.
The experiment used the split-plot randomized complete block design (RCBD) with three blocks. Two nutrient management systems (conventional and organic) were distributed randomly in the main plots. The subplots were allocated to an untreated control (T0) and the other three treatments—soil inoculation of AMF (T1), intercropping of vetiver (T2), and a combination of AMF and vetiver (T3). The main plots were surrounded by bunds with a height of 0.3 m and a width of 0.45 m. Drainage canals were prepared between the main plots’ bunds to prevent cross-contamination of added organic and inorganic fertilizers. The subplot area was approximately 34 m2, and each plot was separated by a 0.3 m high and 0.3 m wide bund.
Fifteen-day-old seedlings of rice (Bg 300 variety—three-month duration) were selected for this experiment. The seedlings were raised in a dapog nursery and transplanted with two plants per hill, at a 0.2 × 0.2 m spacing, on the puddled and leveled plots. On the same day, uniformly aged vetiver grass (C. zizanioides L.) slips that were trimmed at 15 cm lengths from the root system were intercropped without changing the rice plant density in the desired subplots (in two rows; four plants per one row at 1 × 1.8 m spacing). Inorganic fertilizers (Urea 0.0225 kg/m2, Triple Super Phosphate (TSP) 0.0055 kg/m2, muriate of potash (MOP) 0.006 kg/m2, and zinc sulfate (ZnSO4) 0.0005 kg/m2) and commercially available compost (0.25 kg/m2) were applied to the main plots, according to the manufacturers recommended dosage and guidelines. Applications of mycorrhizal inoculum to the desired subplots were performed with 2 kg of prepared inoculum before transplantation and 1 kg of inoculum at the 3rd, 5th, and 7th weeks after transplanting.
2.2. Preparation of Native AMF Inoculum
AMF inoculum was prepared by establishing trap cultures. Soil samples from the upper layer (0–15 cm) with fine root fragments of herbaceous plants [43] were collected from undisturbed water-logged sites in a dry zone environment. AMF spores were then thoroughly homogenized with sterilized fine sand and decomposed cow dung in a ratio of 1:3:1 (v/v) and then added to sterilized plastic pots. Untreated sorghum (Sorghum bicolor) seeds were surface sterilized with 70% ethanol for two minutes and 1% sodium hypochlorite (NaOCl) for three minutes, followed by washing with sterile distilled water (7–10 times) and soaking in sterile distilled water for 12 h [44]. Then, the seeds were sown in pots (60–80 seeds per pot) and kept in the plant house for six weeks. Pots were irrigated (manual watering of filtered tap water) regularly as per the requirement of the plants, and no synthetic fertilizers were added. A rhizospheric sand–soil mixture containing colonized root fragments that were separated from the trap plant culture was used as the AMF inoculum. Before soil application, representative root samples were stained and checked for their AMF colonization potential, as described below.
2.3. Sampling
After transplanting, nine randomly selected healthy hills were carefully uprooted from a 1 m2 area of each subplot at the tillering stage (3 weeks after transplanting; WAT), panicle initiation stage (6 WAT), heading stage (9 WAT), and harvesting stage (12 WAT). Plants were thoroughly washed (without damaging the roots) to remove any adherent soil particles before determining the growth parameters of plant height and the dry weights of shoots and roots. At the harvesting stage, yield components (e.g., panicles/m2, spikelet/panicle, filled-grain percent, and weight of 1000 grains) were measured to assess the grain yield (kg/m2). At each sampling, 5–10 fine rice roots per plant were separated, washed with tap water, and stored in 70% ethanol solution until the percentage of mycorrhizal colonization was determined.
2.4. Mycorrhizal Colonization
The root colonization percentage of AMF was determined after staining with trypan blue, adopting the procedure described by Phillips and Hayman [45]. The rice plant roots stored in 70% ethanol were washed with distilled water, cut into 1 cm lengths, transferred into test tubes containing 10% (w/v) potassium hydroxide (KOH), and incubated at 90 °C for 15–30 min. Thereafter, roots were washed with running tap water and acidified with 1% hydrochloric acid (HCl) for another 15–20 min. The root segments were stained with trypan blue (0.05% of trypan blue in lactoglycerol) at 90 °C for five minutes. Then, the stained root fragments were washed with running tap water and destained by lactoglycerol.
The modified grid transect method [46] was used for AMF quantification. Ten root segments were randomly selected from each stained sample and mounted in glycerin on a microscopic slide. The roots were then gently squeezed under a cover slip and viewed using a compound microscope (Labomed-Sigma, Feasterville, PA, USA). The presence of AMF structures such as arbuscules, vesicles, or intercellular aseptic hyphae was scored for 100 intersections of roots per sample. Then, the percentage of AMF-colonized roots intersecting over the total number of intersections was calculated.
2.5. Statistical Analysis
Statistical analyses were performed using the SAS (version 9.0) statistical tool. Analysis of variance (two-way ANOVA) was carried out using the repeated-measure mixed model. The growth stage was considered as the repeated factor. Before the analysis, AMF colonization percentages were tested for normality and log-transformed. The mean separation was performed using the Least Significant Difference (LSD) method at a 5% probability level.
3. Results
3.1. AMF Colonization of Rice Roots under Different Treatments
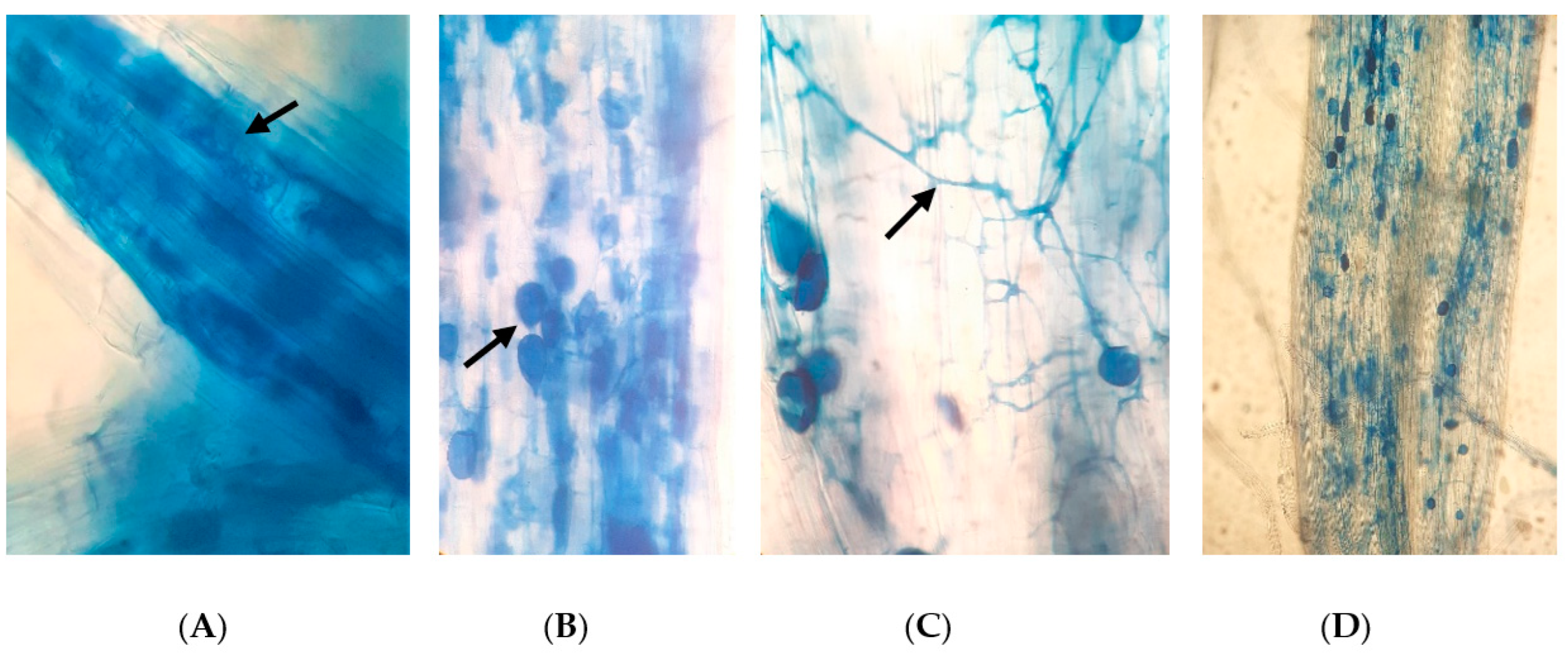
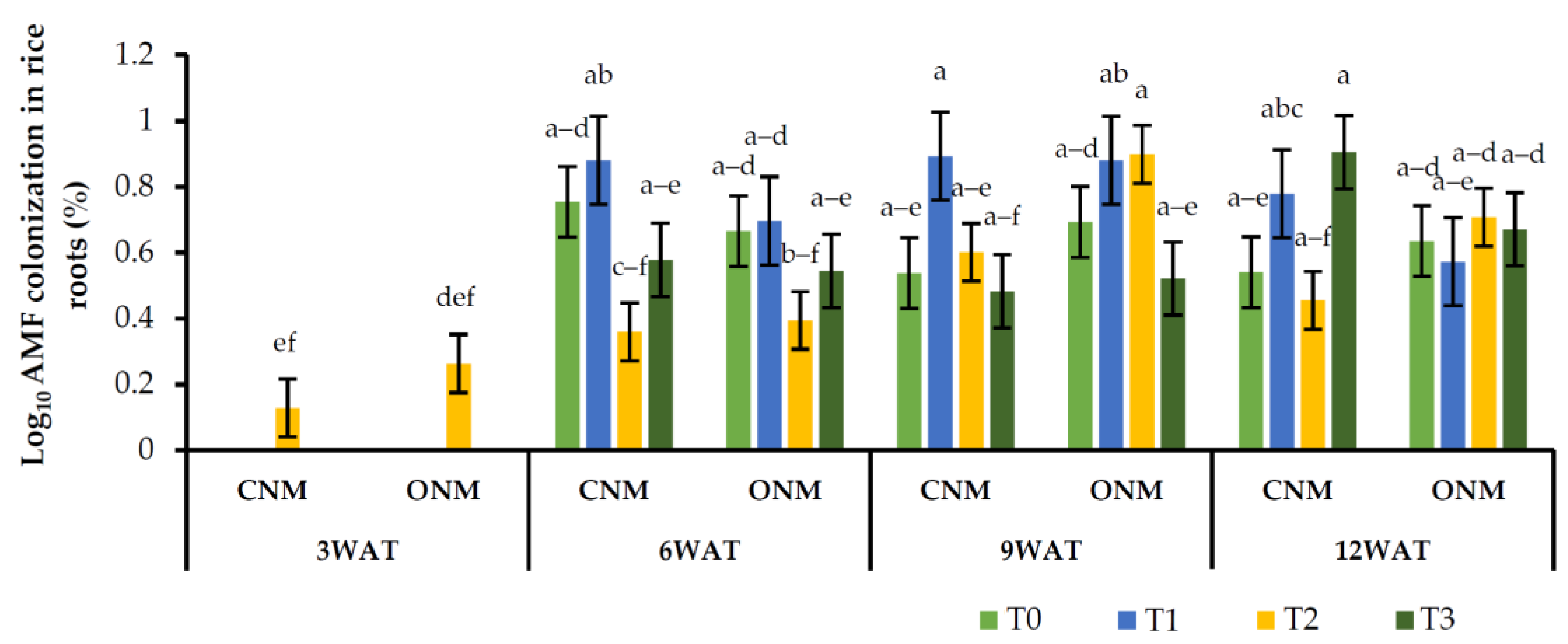
Under the trypan-blue-stained rice roots, we observed arbuscules, vesicles, and intraradical hyphae of AMF (Figure 1). Among them, fungal hyphae and vesicles were observed to be prominent compared with arbuscules. The AMF colonization in the various treatments was initially notified as lower or appeared to be absent during the early stage of rice plant growth (3 WAT). In turn, higher colonization rates were observed in the later stages, from panicle initiation (6 WAT) to the harvesting stage (12 WAT) (Figure 2).
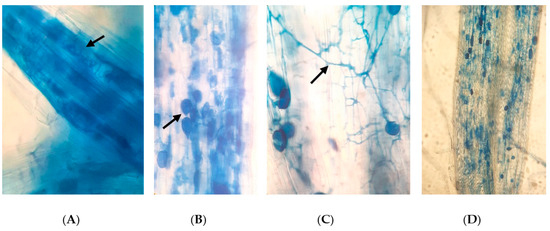
Figure 1.
Different AMF structures observed in rice roots (blue in color, also shown in arrows) (×400): (A) arbuscules, (B) vesicles, (C) fungal hyphae; and (D) a highly colonized rice root (×100).
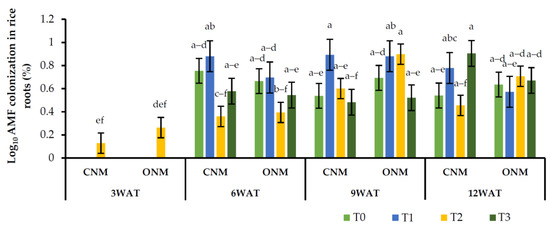
Figure 2.
Effects of different treatments on rice root colonization. Means followed by the same letters are not significantly different (p < 0.05). T0: control; T1: AMF inoculation; T2: vetiver intercropping; T3: AMF + vetiver; CNM: conventional nutrient management; ONM: organic nutrient management; WAT: weeks after transplanting.
The observed mean root colonization percentages of AMF at the different growth stages of rice plants are shown in Figure 2, where it can be seen that root colonization ranges between 0% and 15.8%. The rice root colonization rates were not consistently increased in AMF-inoculated treatments (T1 and T3) compared with noninoculated treatments (T0 and T2); in some cases, there was no increase, or they remained unchanged. Furthermore, we observed a considerable amount of AMF colonization (0–4.98%) in the noninoculated controls (Figure 2). As previously mentioned, our experiment produced inconsistent observations in the mycorrhizal colonization of rice roots against intercropping vetiver grass with or without AMF inoculation (T3 and T2, respectively). Further, AMF colonization in vetiver was very low under waterlogged conditions (data are not presented). Similarly, AMF colonization did not differ between the rice plants grown under both nutrient management systems.
3.2. Rice Plant Growth and Yield under Different Treatments
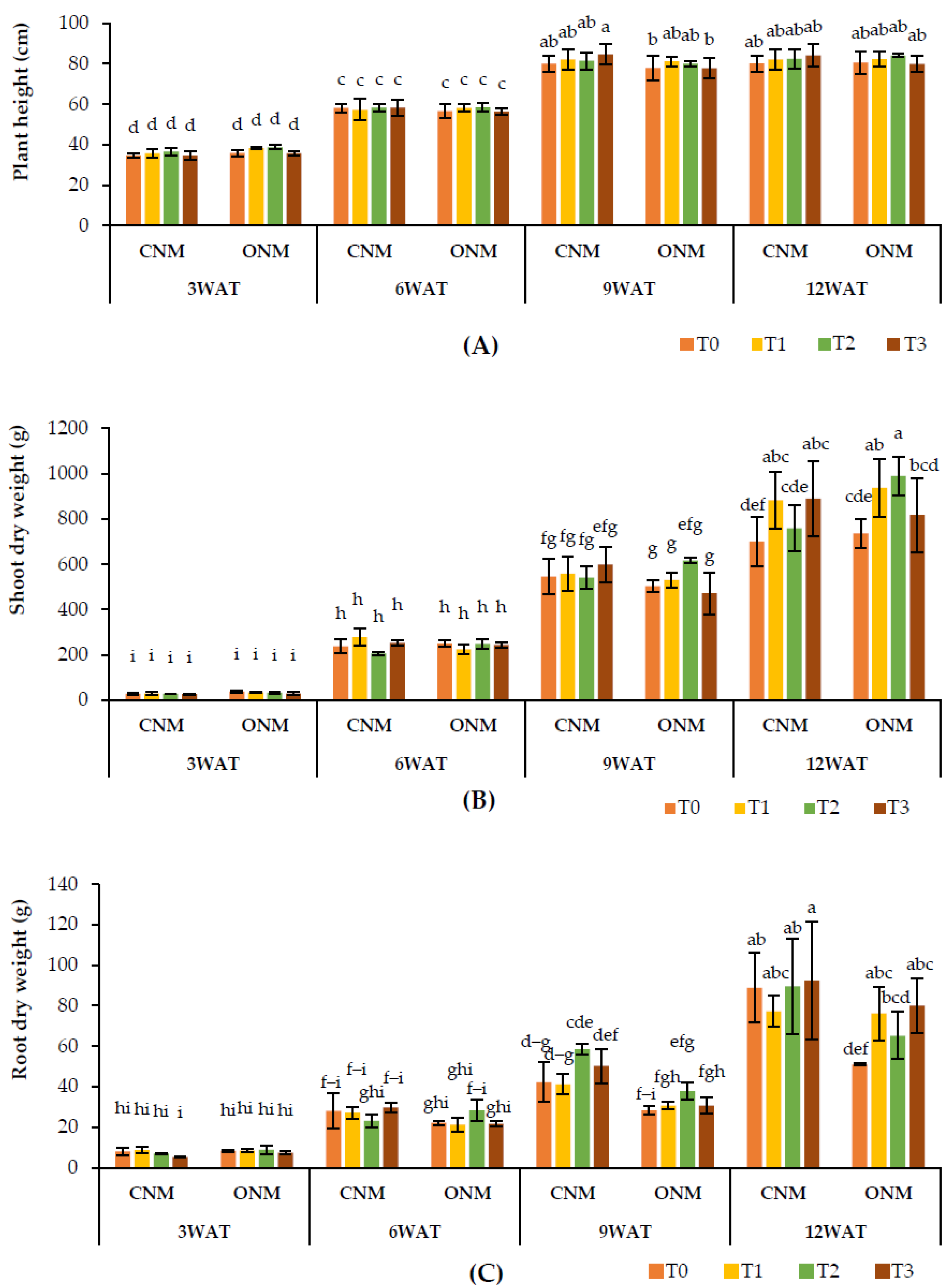
The effect of AMF inoculation on rice plant heights remained nonsignificant, except at the heading stage (9 WAT), which showed significantly higher (p < 0.05) plant heights in T1 with the ONMS and T3 with the CNMS compared with the respective controls (Figure 3A). Shoot dry weights were significantly higher at 12 WAT when AMF were applied alone (T1) and together with vetiver (T3) under both NMSs (Figure 3B). However, root dry weight was significantly lower in T1 with the CNMS, while it was significantly higher in T3 at 12 WAT. Also, it was significantly higher in both T1 and T3 with the ONMS (Figure 3C).
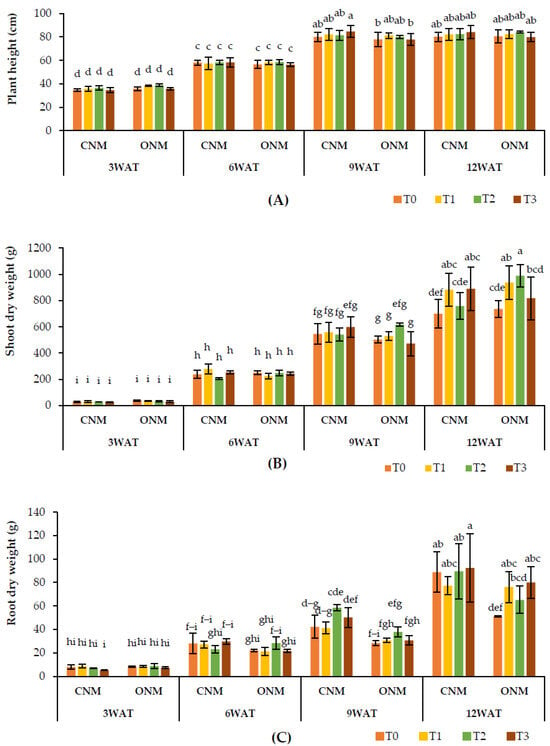
Figure 3.
Effect of treatments on (A) plant height, (B) shoot dry weight, and (C) shoot dry weight at different growth stages of the rice plant. Means followed by the same letters are not significantly different (p < 0.05). T0: control; T1: AMF inoculation; T2: vetiver intercropping; T3: AMF + vetiver; CNM: conventional nutrient management; ONM: organic nutrient management; WAT: weeks after transplanting.
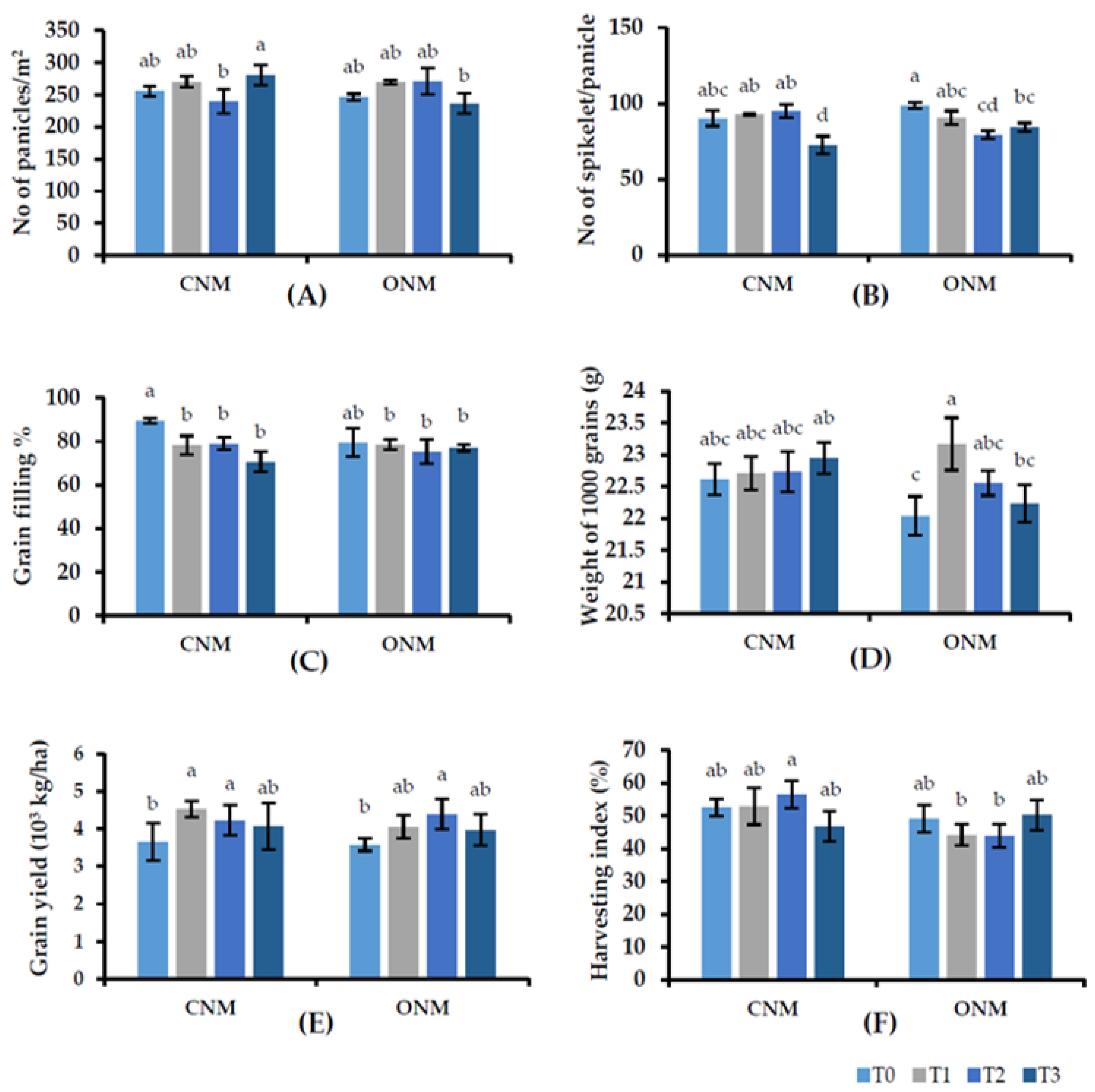
Results on rice yield components also show inconsistent variations against the application of AMF. The grain yields were significantly higher in each AMF-added treatment (T1 or T3) than in their respective controls (Figure 4E). Intercropping vetiver (T2 and T3) had no significant negative effect on rice plant growth. Vetiver intercropping was significantly better or showed no significant difference compared with the control, except for the shoot dry weight at 6 WAT in the CNMS. Interestingly, rice yield significantly increased in the vetiver-intercropped plots, with or without AMF inoculation, despite no significant increase in rice root colonization by vetiver grass. Furthermore, we observed no negative effects caused by the field management practices of rice cultivation (e.g., irrigation, nutrient management, and pest control) on the growth of vetiver grass.
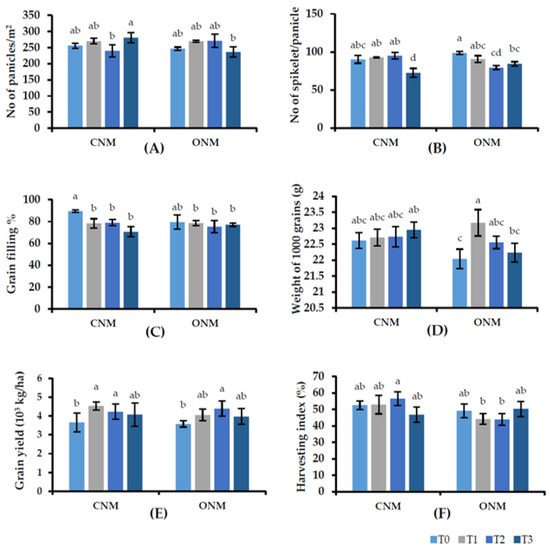
Figure 4.
Effect of treatments on (A) panicles/m2, (B) spikelet/panicle, (C) grain filling percent, (D) 1000 grain weight, (E) grain yield (kg/ha), and (F) harvesting index with different nutrient management systems. Means followed by the same letters are not significantly different (p < 0.05). T0: control; T1: AMF inoculation; T2: vetiver intercropping; T3: AMF + vetiver; CNM: conventional nutrient management; ONM: organic nutrient management.
In the two NMSs, all plant growth parameters were statistically non-significant, except for plant heights at 3 WAT and 9 WAT, as well as root dry weight at 9 WAT. Plant height was significantly higher in the ONMS than the CNMS at 3 WAT, and both plant height and root dry weight were significantly higher in the CNMS at 9 WAT. Also, none of the yield parameters significantly differed between the two NMSs.
4. Discussion
AMF are typically characterized by finely branched hyphal structures (arbuscules) within the cortical cells of plant roots [47]. In addition, AMF consist of intraradical hyphae, extraradical mycelia, large spores, and terminal globular structures, referred to as vesicles in some species [48]. Among the structures we observed in rice roots, the presence of arbuscules was notably lower compared with intraradical hyphae and vesicles. Bernaola et al. [25] also reported similar observations in naturally colonized rice roots and suggested that the lack of arbuscules was due to their tendency to degrade quickly.
In this field experiment, initial AMF colonization rates were very low but increased during the later stages of rice plant growth. This could be attributed to the initial anoxic conditions in the soil caused by continuous inundation used for weed management at the beginning of the rice cultivation. Aerenchyma tissues, which supply oxygen to rice roots under flooded conditions, are developed in the rice root cortical cells as a response to flooding, limiting the space for AMF to colonize [22]. Later, once the field is drained, AMF can colonize within cortical cells with the disappearance of these tissues. The authors in [23] also reported similar results to our study, that is, no or rare mycorrhizal colonization at the initial stage bur occurring at the heading and ripening stage of the rice plant, suggesting that this is due to the less-developed aerenchyma tissues during the seedling stage, and as the plant matures, these tissues become more developed, providing AMF with sufficient oxygen. Rice root colonization can persist even under initial submergence conditions by inoculating AMF at the nursery stage [49]. Furthermore, we observed a considerable amount of AMF colonization in the noninoculated controls, which is in agreement with Purakayastha and Chhonkar [50], Wangiyana et al. [20], and Chareesri et al. [27], who reported 2.6%, 3–5%, and 7% of indigenous AMF colonization in noninoculated lowland rice plants, respectively. Similarly, Kalamulla et al. [30] and Chen et al. [24] have reported 36.40% and 19.5 ± 7.2% natural AMF colonization rates from the lowland rice farmer fields in Sri Lanka and China, respectively.
In our experiment, field inoculation of AMF did not show a consistent increase in rice root colonization. However, previous studies have shown that significantly higher root colonization rates can be achieved by adding AMF inoculum. For instance, Secilia and Bagyaraj [26] and Zhang et al. [51] reported significantly higher root colonization (12–19% and 31–33%, respectively) by AMF inoculation in lowland rice. In the present study, we followed a farmer-adaptable method for AMF inoculum production, establishing the trap cultures by obtaining AMF from undisturbed natural soils. However, AMF in natural habitats might be less compatible and less adaptable to agricultural conditions, as only a few AMF are better adapted to agricultural settings than natural habitats [52]. Furthermore, when the indigenous AMF community in the field has reached its carrying capacity, it can also be challenging to establish a foreign AMF species [53]. These factors could be possible reasons for the inconsistent colonization rates observed in our study. Verbruggen et al. [53] suggested selecting an adaptable species that is lacking in the existing field or inoculating a mixture of species to increase the success rate of AMF field application. The authors also described that the timing and method of distributing AMF also influence the establishment of added inoculum. Therefore, inoculating AMF at the nursery stage and applying AMF unevenly or in a patchy manner after crop establishment would enhance the establishment of added AMF by reducing competition with indigenous AMF.
Studies found that AMF can form a common fungal network in intercropping systems by connecting plants arranged in rows [54,55]. Although we used vetiver grass to establish a common mycorrhizal network in the lowland soil, the results of mycorrhizal colonization in rice roots are inconsistent. AMF colonization of vetiver roots is also reported to be very low under waterlogged conditions, although it is known to be a highly mycorrhizal plant. One possible reason for this lower colonization rate in our intercropping system might be the incompatibility of AMF species used, as vetiver grass has shown a stronger response to native AMF than to other sources [56]. Therefore, it is better to select AMF species that are compatible with both plants in the intercropping system to maximize benefits. Further, the low porosity in the lowland soil might also prevent the interconnection of the AMF network. The rice plants grown under both nutrient management systems also resulted in nonsignificant AMF colonization rates, suggesting that AMF can survive even in the presence of agrochemicals. Our findings also produced contrasting results compared with the work conducted by Dhillion and Ampornpan [57], who reported a significantly higher colonization level in the nonchemical treatments than all other chemical fertilizer treatments. Further, Watanarojanaporn et al. [32] reported higher AMF colonization in lowland rice roots in compost-applied treatments rather than mineral fertilizers, while our study resulted in statistically nonsignificant colonization rates between compost and mineral-fertilizer-applied treatments.
Generally, it is expected that the effects of AMF on plant growth will be enhanced when fungal colonization rates are high [27]. However, Ruíz-Sánchez et al. [12] highlighted that plant growth stimulation by AMF symbiosis does not always depend upon the degree of root colonization; instead, it mainly relies on the fungal and plant species involved, along with environmental conditions. Additionally, there is no reported threshold value of root colonization necessary to enhance plant growth [12], and thus variable results could be recorded. In our study, rice plant growth was not markedly affected by the added AMF inoculum; however, it did not produce an overall negative impact. However, previous studies reported contrary results; for instance, Ruíz-Sánchez et al. [58] and Gewaily [28] reported significantly higher plant heights with the application of AMF inoculation in lowland rice. Secilia and Bagyaraj [26] reported that the field application of AMF significantly increased shoot biomass but not root biomass. Bao et al. [59] reported that there is no effect of AMF on leaf, stem, or root biomass in lowland rice. Although the growth remained nonsignificant, grain yield was significantly higher in each AMF-added treatment. In line with this, Chareesri et al. [27] also observed significantly higher rice yield with no such effect on rice growth due to AMF and suggested that this could have happened due to the increased N and P allocation to the panicles during the grain-filling stage in the AMF-colonized plants. However, Gewaily et al. [28] observed that considerable growth and yield of lowland rice were once associated with AMF.
In an intercropping system, two or more different crops are grown simultaneously in the same field [33]. In such a condition, selecting crops that offer mutual benefits and reducing competition are crucial. Overall, in our intercropping system, vetiver grass did not negatively affect the rice plants’ growth. Interestingly, it significantly increased rice yield, with or without AMF inoculation, despite no significant increase in rice root colonization by vetiver grass. These results imply that vetiver does not compete detrimentally with rice plants for space and nutrients throughout the growing season of rice. Note that the underlying mechanisms are largely unclear and need further investigations. Digging more, Praveen et al. [60] reported that vetiver intercropping did not affect the growth and yield of (upland) rice during the first growing season. According to them, negative impacts on plant growth are possible when rice is cultivated again with the same vetiver plants due to interspecific competition for space and soil nutrition. Furthermore, the agronomic practices of rice cultivation may not adversely affect the growth of vetiver grass, suggesting vetiver can be successfully intercropped with lowland rice. In contrast, Ahadiyat and Ranamukhaarachchi [61] reported enhanced growth and yield in maize when intercropped with vetiver. In the current study, we implemented T3 to obtain a synergistic effect due to the combined benefits of AMF and vetiver; however, no such effect was observed in most of the measured parameters. The two NMSs showed statistically nonsignificant results for most of the growth parameters and all the yield parameters. This observation suggests that ONM is as effective as CNM in lowland rice cultivation, because chemical fertilizers provide readily available nutrients to plants, while compost releases nutrients slowly through microbial activity and, in addition, improves the physical soil conditions that are favorable for healthy plant growth [62]. Therefore, organic manure could be a better alternative to synthetic chemical fertilizers, as it is environmentally friendly.
5. Conclusions
This study showed that field inoculation with native AMF, intercropping of vetiver, and AMF plus vetiver could improve plant productivity, particularly resulting in higher grain yields that might contribute to the sustainable attainment of future demands. However, there was no noticeable growth improvement with the addition of the treatments. Moreover, rice plant growth, yield, and AMF colonization did not differ significantly between the organic and conventional nutrient management systems. Interestingly, no negative impact was observed for the colonization of AMF under the CNMS. Therefore, overall, it is suggested that AMF inoculums can be utilized to obtain a higher yield (as resulted; 0.45 kg/m2) in CNMSs. Vetiver alone showed the best results (over the AMF inoculum) in the ONMS (0.44 kg/m2); thus, using this method in such a farming system could be better than the other methods. Further, regarding the aspect of on-farm production of AMF inoculum through the trap culture method, we recommend obtaining AMF starter inoculums from the same field that will be inoculated in the future. Additionally, if it is for an intercropping system, rhizospheric soil from both plants is suggested to be used for trap culturing.
Author Contributions
Conceptualization, K.W.A.M. and P.N.Y.; data curation, D.M.D.D., T.D.C.P. and X.W.; formal analysis, K.W.A.M., D.M.D.D., T.D.C.P. and P.N.Y.; funding acquisition, A.M.E., D.D. and T.M.D.; investigation, S.C.K., S.L.S., A.M.E., T.M.D., A.K.H.P., P.N.Y. and X.W.; methodology, K.W.A.M., D.M.D.D., A.K.H.P. and X.W.; project administration, S.C.K.; resources, A.M.E. and S.C.K.; software, T.D.C.P.; supervision, S.C.K.; validation, S.L.S. and P.N.Y.; visualization, S.L.S., A.K.H.P. and X.W.; writing—original draft, K.W.A.M., S.C.K. and P.N.Y.; writing—review and editing, S.C.K., T.D.C.P., S.L.S., A.M.E., T.M.D., A.K.H.P., S.C.K., D.D., P.N.Y. and X.W. All authors have read and agreed to the published version of the manuscript.
Funding
Financial assistance given by the World Bank Group through the project Accelerating Higher Education Expansion and Development Operation (AHEAD), DOR, Grant No. 79, Rajarata University of Sri Lanka, is highly appreciated. This research was partially supported by the National Natural Science Foundation of China (NSFC 32260004) and the Researchers Supporting Project number RSP2023R197, King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
SCK and DQD thanks the “Yunnan Revitalization Talents Support Plan” (“Young Talents” Program and “High-End Foreign Experts” Program) for support. Xiao-Yan Wang thanks the Academician (Expert) Workstation of Yunnan Province Program (No. 202105AF150014) for financial support. The authors extend their appreciation to the Researchers Supporting Project number RSP2023R197, King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Senanayake, S.M.P.; Premaratne, S.P. An Analysis of the Paddy/Rice Value Chains in Sri Lanka. Asia. Pac. J. Rural Dev. 2016, 26, 105–126. [Google Scholar] [CrossRef]
- Connor, M.; Malabayabas, A.J.B.; de Guia, A.H.; Wehmeyer, H.; Pame, A.R.P.; Htwe, N.M.; Zhong, X.; Fu, Y.; Liang, K.; Pan, J.; et al. Environmental, Social, and Economic Challenges in Lowland Rice Production. In Closing Rice Yield Gaps in Asia: Innovations, Scaling, and Policies for Environmentally Sustainable Lowland Rice Production; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 27–92. ISBN 9783031379468. [Google Scholar] [CrossRef]
- Yapa, N.; Dub, W.; Madhushan, A.; Yan, K.; Asad, S.; Karunarathna, S.C.; Bamunuarachchige, C. Potential of Biofertilizers and Natural Soil Amendments to Mitigate Heavy Metal Contents of Soil in Lowland Rice (Oryza sativa L.) Farming. Sci. Asia 2022, 48, 326–334. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of Organic Farming for Achieving Sustainability in Agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Yapa, N.; Lakmali, D.; Zoysa, D.; Silva, K.S.; Manawadu, C.; Herath, B.M.; Madhushan, A.; Perera, G.; Ratnayakae, O.; Kapilan, R.; et al. Biofertilizers: An Emerging Trend in Agricultural Sustainability. Chiang Mai J. Sci. 2022, 49, 608–640. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, A Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Madhushan, K.W.A.; Herath, B.M.M.D.; Karunarathna, S.C.; Yapa, P.N. Role of Arbuscular Mycorrhizal Fungi in Sustainable Crop Production and Forestry in Sri Lanka—A review. Stud. Fungi 2021, 6, 437–449. [Google Scholar] [CrossRef]
- Schüßler, A.; Martin, H.; Cohen, D.; Fitz, M.; Wipf, D. Arbuscular Mycorrhiza: Studies on the Geosiphon Symbiosis Lead to the Characterization of the First Glomeromycotan Sugar Transporter. Plant Signal. Behav. 2007, 2, 431–434. [Google Scholar] [CrossRef]
- Jayakody, N.; Madhushan, A.; Pelawatta, A.; Yapa, N. Effect of Biofertilizers and Organic Amendments on Germination and Seedling Growth of Common Dry Zone Forest Species in Sri Lanka: Sustainable Reforestation Practices in Sri Lanka. Turk. J. Agric.-Food Sci. Technol. 2023, 11, 287–291. [Google Scholar] [CrossRef]
- Gehring, C.; Bennett, A. Mycorrhizal Fungal–Plant–Insect Interactions: The Importance of a Community Approach. Environ. Entomol. 2009, 38, 93–102. [Google Scholar] [CrossRef]
- Weng, W.; Yan, J.; Zhou, M.; Yao, X.; Gao, A.; Ma, C.; Cheng, J.; Ruan, J. Roles of Arbuscular mycorrhizal Fungi as a Biocontrol Agent in the Control of Plant Diseases. Microorganisms 2022, 10, 1266. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Aroca, R.; Muñoz, Y.; Polón, R.; Ruiz-Lozano, J.M. The Arbuscular Mycorrhizal Symbiosis Enhances the Photosynthetic Efficiency and the Antioxidative Response of Rice Plants Subjected to Drought Stress. J. Plant Physiol. 2010, 167, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Kumar, U.; Sugitha, T.C.K.; Parameswaran, C.; Sahoo, S.; Binodh, A.K.; Jahan, A.; Anandan, A. Arbuscular Mycorrhizal Fungi (AMF) for Sustainable Rice Production. In Advances in Soil Microbiology: Recent Trends and Future Prospects; Springer: Singapore, 2017; pp. 99–126. ISBN 9789811073793. [Google Scholar] [CrossRef]
- Bernaola, L.; Cosme, M.; Schneider, R.W.; Stout, M. Belowground Inoculation with Arbuscular Mycorrhizal Fungi Increases Local and Systemic Susceptibility of Rice Plants to Different Pest Organisms. Front. Plant Sci. 2018, 9, 747. [Google Scholar] [CrossRef]
- Campos-soriano, L.; García-martínez, J.; Segundo, B.S. The Arbuscular Mycorrhizal Symbiosis Promotes the Systemic Induction of Regulatory Defence-related Genes in Rice Leaves and Confers Resistance to Pathogen Infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Gunda, T.; Hornberger, G.M. Minimizing irrigation water demand: An evaluation of shifting planting dates in Sri Lanka. Ambio 2018, 47, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, W.M.W.; Mutunayake, M.M.P.; Bandara, C.; Rao, A.N.; Bhandari, D.C.; Ladha, J.K. Direct-Seeded Rice Culture in Sri Lanka: Lessons from Farmers. Field Crops Res. 2011, 121, 53–63. [Google Scholar] [CrossRef]
- Ilag, L.L.; Rosales, A.M.; Elazegui, F.A.; Mew, T.W. Changes in the Population of Infective Endomycorrhizal Fungi in A Rice-Based Cropping System. Plant Soil 1987, 103, 67–73. [Google Scholar] [CrossRef]
- Chialva, M.; Ghignone, S.; Cozzi, P.; Lazzari, B.; Bonfante, P.; Abbruscato, P.; Lumini, E. Water Management and Phenology Influence the Root-Associated Rice Field Microbiota. FEMS Microbiol. Ecol. 2020, 96, fiaa146. [Google Scholar] [CrossRef]
- Wangiyana, W.; Cornish, P.; Morris, E. Arbuscular Mycorrhizal Fungi Dynamics in Contrasting Cropping Systems on Vertisol and Regosol Soils of Lombok, Indonesia. Exp. Agric. 2006, 2, 427–439. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzad, N.; Barzeghar, R. Phosphorus Mobilization and Uptake in Mycorrhizal Rice (Oryza sativa L.) Plants under Flooded and Non-Flooded Conditions. Acta Agric. Slov. 2009, 93, 153. [Google Scholar] [CrossRef]
- Vallino, M.; Fiorilli, V.; Bonfante, P. Rice Flooding Negatively Impacts Root Branching and Arbuscular Mycorrhizal Colonization, But Not Fungal Viability. Plant Cell Environ. 2014, 37, 557–572. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, Y.; Björn, L.O.; Rosendahl, S.; Olsson, P.A.; Li, S.; Fu, X. Community Dynamics of Arbuscular Mycorrhizal Fungi in High-Input and Intensively Irrigated Rice Cultivation Systems. Appl. Environ. Microbiol. 2015, 81, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Wu, F.-Y.; Li, H.; Chan, W.-F.; Wu, S.-C.; Wong, M.-H. Mycorrhizal Colonization Status of Lowland Rice (Oryza sativa L.) in the Southeastern Region of China. Environ. Sci. Pollut. Res. Int. 2017, 24, 5268–5276. [Google Scholar] [CrossRef]
- Bernaola, L.; Cange, G.; Way, M.O.; Gore, J.; Hardke, J.; Stout, M. Natural colonization of Rice by Arbuscular Mycorrhizal Fungi in Different Production Areas. Rice Sci. 2018, 25, 169–174. [Google Scholar] [CrossRef]
- Secilia, J.; Bagyaraj, D.J. Evaluation and First-Year Field Testing of Efficient Vesicular Arbuscular Mycorrhizal Fungi for Inoculation of Wetland Rice Seedlings. World J. Microbiol. Biotechnol. 1994, 10, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased Arbuscular Mycorrhizal Fungal Colonization Reduces Yield Loss of Rice (Oryza sativa L.) Under Drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- Gewaily, S. Influence of Arbuscular Mycorrhizal (AMF) Inoculation on the Performance of Sakha 107 Rice Cultivar under Different Irrigation Intervals. Environ. Biodivers. Soil Secur. 2019, 3, 119–130. [Google Scholar] [CrossRef]
- Klinnawee, L.; Noirungsee, N.; Nopphakat, K.; Runsaeng, P.; Chantarachot, T. Flooding Overshadows Phosphorus Availability in Controlling the Intensity of Arbuscular Mycorrhizal Colonization in Sangyod Muang Phatthalung Lowland Indica Rice. Sci. Asia 2021, 47, 202. [Google Scholar] [CrossRef]
- Kalamulla, R.; Sandaruwan, D.; Karunarathna, S.C.; Stephenson, S.L.; Tibpromma, S.; Elgorban, A.M.; Al-Rejaie, S.; Yapa, P.N.; Suwannarach, N. Assessment of Community Dynamics of Arbuscular Mycorrhizal Fungi in the Rice (Oryza sativa L.) Rhizosphere and Potential Application as Biofertilizer. Sustainability 2022, 14, 16537. [Google Scholar] [CrossRef]
- Paranavithana, T.M.; Marasinghe, S.; Perera, G.A.D.; Ratnayake, R.R. Effects of Crop Rotation on Enhanced Occurrence of Arbuscular Mycorrhizal Fungi and Soil Carbon Stocks of Lowland Paddy Fields in Seasonaly Dry Tropics. Paddy Water Environ. 2021, 19, 217–226. [Google Scholar] [CrossRef]
- Watanarojanaporn, N.; Boonkerd, N.; Tittabutr, P.; Longtonglang, A.; Young, J.P.W.; Teaumroong, N. Effect of Rice Cultivation Systems on Indigenous Arbuscular Mycorrhizal Fungal Community Structure. Environ. Microbiol. 2013, 28, 316–324. [Google Scholar] [CrossRef]
- Li, C.; Stomph, T.J.; Makowski, D.; Li, H.; Zhang, C.; Zhang, F.; van der Werf, W. The Productive Performance of Intercropping. Proc. Natl. Acad. Sci. USA 2023, 120, e2201886120. [Google Scholar] [CrossRef] [PubMed]
- Kusnarta, I.G.M.; Mawaddah, F.A.; Silawibawa, I.P.; Wangiyana, W.; Dulur, N.W.D.; Mahardika, I.B.K. Intercropping with Peanuts and Long-Term Application of Organic Wastes Improve Mycorrhizal Development and Growth of Red Rice under Aerobic Irrigation Systems. IOP Conf. Ser. Earth Environ. Sci. 2022, 1107, 012018. [Google Scholar] [CrossRef]
- Yang, X.; Qin, J.; Li, J.; Lai, Z.; Li, H. Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J. Hazard. Mater. 2021, 406, 124325. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.L.; Zhu, Q.Y.; Xu, P.X.; Jing, Y.X. The Intercropping and Arbuscular Mycorrhizal Fungus Decrease Cd Accumulation in Upland Rice and Improve Phytoremediation of Cd-Contaminated Soil by Sphagneticola calendulacea (L.) Pruski. J. Environ. Manag. 2021, 15, 113516. [Google Scholar] [CrossRef]
- Li, Y.; Ran, W.; Zhang, R.; Sun, S.; Xu, G. Facilitated Legume Nodulation, Phosphate Uptake and Nitrogen Transfer by Arbuscular Inoculation in an Upland Rice and Mung Bean Intercropping System. Plant Soil 2009, 315, 285–296. [Google Scholar] [CrossRef]
- Ren, L.; Lou, Y.; Zhang, N.; Zhu, X.; Hao, W.; Sun, S.; Shen, Q.; Xu, G. Role of Arbuscular Mycorrhizal Network in Carbon and Phosphorus Transfer between Plants. Biol. Fertil. Soils 2013, 49, 3–11. [Google Scholar] [CrossRef]
- Jindapunnapat, K.; Reetz, N.D.; MacDonald, M.H.; Bhagavathy, G.; Chinnasri, B.; Soonthornchareonnon, N.; Sasnarukkit, A.; Chauhan, K.R.; Chitwood, D.J.; Meyer, S.L.F. Activity of Vetiver Extracts and Essential Oil against Meloidogyne incognita. J. Nematol. 2018, 50, 147–162. [Google Scholar] [CrossRef]
- Lakmali, D.; Karunarathna, S.C.; Dauner, L.A.P.; Yapa, N. Potential of Vetiver (Chrysopogon zizanioides L.), Inoculated with Arbuscular Mycorrhizal Fungi, To Improve Soil Quality in Degraded Soil. Chiang Mai J. Sci. 2021, 48, 1247–1258. [Google Scholar]
- Zhang, F.; Peng, J.; Rong, Y.; Sun, S.; Zheng, Y. Removal of Atrazine from Submerged Soil using Vetiver grass (Chrysopogon zizanioides L.). Int. J. Phytoremediat. 2023, 25, 670–678. [Google Scholar] [CrossRef]
- Gupta, P.; Roy, S.; Mahindrakar, A.B. Treatment of Water Using Water Hyacinth, Water Lettuce and Vetiver Grass—A Review. Resour. Environ. 2012, 2, 202–215. [Google Scholar] [CrossRef]
- Chaurasia, B.; Pandey, A.; Palni, L. Distribution, Colonization and Diversity of Arbuscular Mycorrhizal Fungi Associated with Central Himalayan Rhododendrons. For. Ecol. Manag. 2005, 207, 315–324. [Google Scholar] [CrossRef]
- Selvakumar, G.; Kim, K.; Walitang, D.; Chanratana, M.; Kang, Y.; Chung, B.; Sa, T. Trap Culture Technique for Propagation of Arbuscular Mycorrhizal Fungi Using Different Host Plants. Hanguk Toyang Piryo Hakhoe Chi 2016, 49, 608–613. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A New Method Which Gives an Objective Measure of Colonization of Roots by Vesicular-Arbuscular Mycorrhizal Fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Millner, P.D. Biodiversity of Arbuscular Mycorrhizal Fungi in Agroecosystems. Agric. Ecosys. Environ. 1999, 74, 77–93. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, M.Z.; Hirata, H. Effect of Arbuscular Mycorrhizal Fungi Inoculation of Rice Seedlings at the Nursery Stage Upon Performance in the Paddy Field and Greenhouse. Plant Soil 1997, 191, 1–12. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Chhonkar, P.K. Influence of Vesicular-Arbuscular Mycorrhizal Fungi (Glomus etunicatum L.) on Mobilization of Zinc in Wetland Rice (Oryza sativa L.). Biol. Fertil. Soils 2001, 33, 323–327. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Ma, F.; Bloomfield, K.J.; Yang, J.; Atkin, O.K. Is Resource Allocation and Grain Yield of Rice Altered by Inoculation with Arbuscular Mycorrhizal Fungi? J. Plant Ecol. 2014, 8, 436–448. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil Type and Land Use Intensity Determine the Composition of Arbuscular Mycorrhizal Fungal Communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.A.; Rillig, M.C.; Kiers, E.T. Mycorrhizal Fungal Establishment in Agricultural Soils: Factors Determining Inoculation Success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Bei, S.; Li, H.; Christie, P.; Zhang, F.; Zhang, J. Arbuscular Mycorrhizal Fungi Contribute to Over Yielding by Enhancing Crop Biomass While Suppressing Weed Biomass in Intercropping Systems. Plant Soil 2016, 406, 173–185. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Lin, X. The Roles and Performance of Arbuscular Mycorrhizal Fungi in Intercropping Systems. Soil Ecol. Lett. 2022, 4, 319–327. [Google Scholar] [CrossRef]
- Santos, J.S.; Santos, J.F.S.; Lopes, L.J.D.E.O.; Mendonça, J.D.E.J.; Holanda, F.S.R.; Marino, R.H. Arbuscular Mycorrhizal Fungi and Dark Septate Endophytic Fungi on the Biomass Development of Vetiver Grass. Rev. Caatinga 2018, 31, 602–611. [Google Scholar] [CrossRef]
- Dhillion, S.S.; Ampornpan, L.-A. The Influence of Inorganic Nutrient Fertilization on the Growth, Nutrient Composition and Vesicular-Arbuscular Mycorrhizal Colonization of Pretransplant Rice (Oryza sativa L.) Plants. Biol. Fertil. Soils 1992, 13, 85–91. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; de Salamone, I.E.G.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and Arbuscular Mycorrhizal Colonization Enhance Rice Growth and Physiological Traits Under Well-Watered and Drought Conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Y.; Olsson, P.A. Arbuscular Mycorrhiza under Water—Carbon–phosphorus Exchange between Rice and Arbuscular Mycorrhizal Fungi under Different Flooding Regimes. Soil Biol. Biochem. 2019, 129, 169–177. [Google Scholar] [CrossRef]
- Praveen, A.; Mehrotra, S.; Singh, N. Rice Planted along with Accumulators in Arsenic Amended Plots Reduced Arsenic Uptake in Grains and Shoots. Chemosphere 2017, 184, 1327–1333. [Google Scholar] [CrossRef]
- Ahadiyat, Y.R.; Ranamukhaarachchi, S.L. Different Tillage and Maize Grass Intercropping on Root Systems, Growth and Yield of Rainfed Maize (Zea mays L.). Adv. Agric. Bot. 2011, 3, 33–38. [Google Scholar]
- Sarker, M.A.R.; Pramanik, M.Y.A.; Faruk, G.M.; Ali, M.Y. Effect of Green Manures and Levels of Nitrogen on Some Growth Attributes of Transplant Aman Rice. Pak. J. Biol. Sci. 2004, 7, 739–742. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).