Impact of Adding Anaerobic Digestate to Soil and Consequences on Crop Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Anaerobic Digestate
2.2.1. Experiment 1—Impact of Adding Anaerobic Digestate to Soil on Barley Yield and Elemental Composition
2.2.2. Experiment 2—Impact of Digestate on Barley Production over Six Weeks
2.2.3. Experiment 3—Soil Nitrogen Dynamics
2.3. pH, EC, Nitrogen and Carbon Analysis
2.3.1. Measurement of Total Nitrogen and Phosphorus in Soil and Plant
2.3.2. Determination of Ammonium, Nitrate and Nitrite in Soil Pore Water Samples
2.3.3. Measurement of Calcium, Magnesium and Potassium Content of Soils and Plants
2.4. Statistical Analysis
3. Results
3.1. Application of Powerhouse Digestate to Soil—Experiment 1
3.1.1. Plant Growth
3.1.2. Soil Properties
3.1.3. Concentration of Elements in Plant Material
3.2. Application of Gaskfarm Digestate to Soil—Experiment 1
3.2.1. Plant Growth
3.2.2. Soil Properties
3.2.3. Concentration of Elements in Plant Material
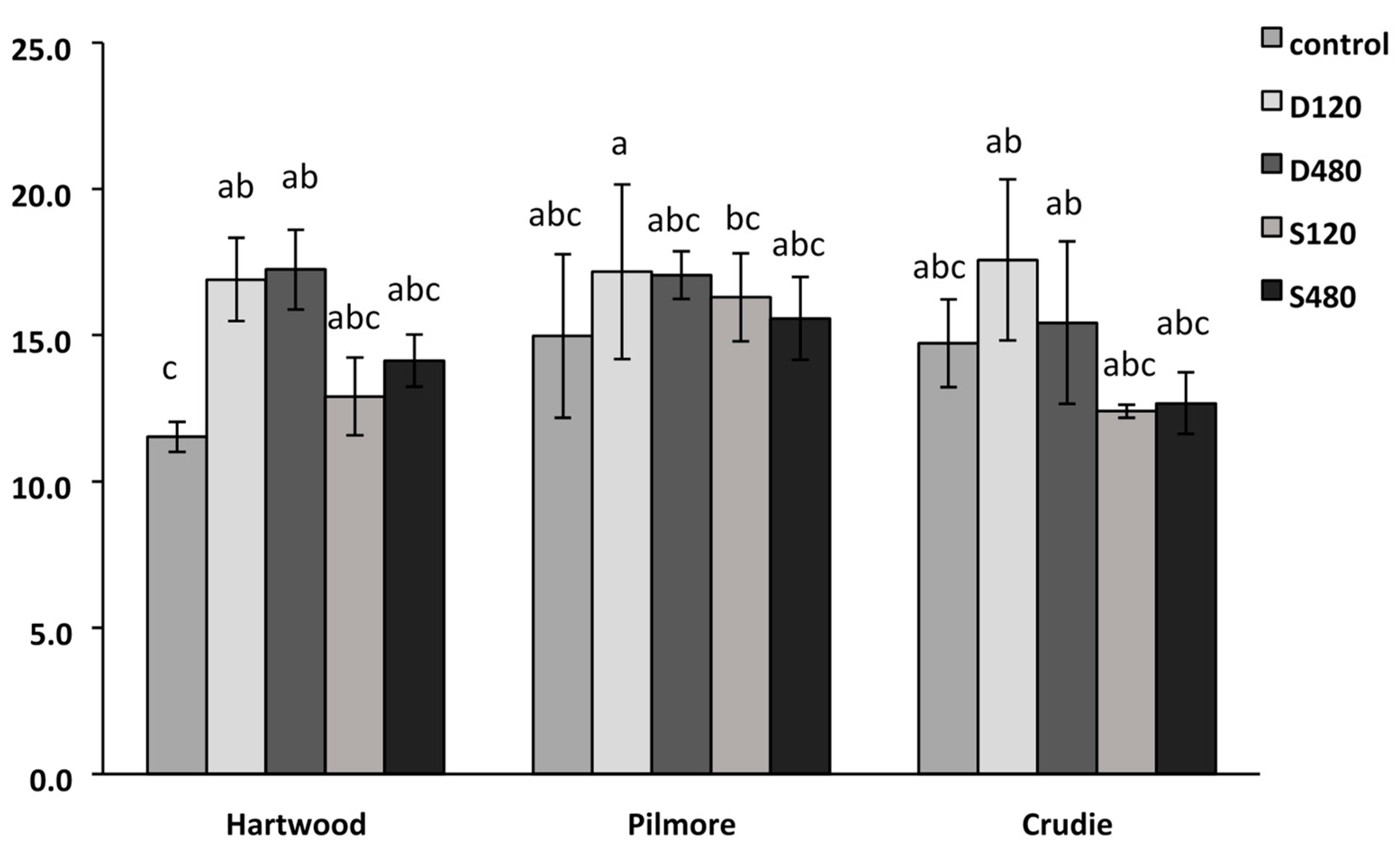
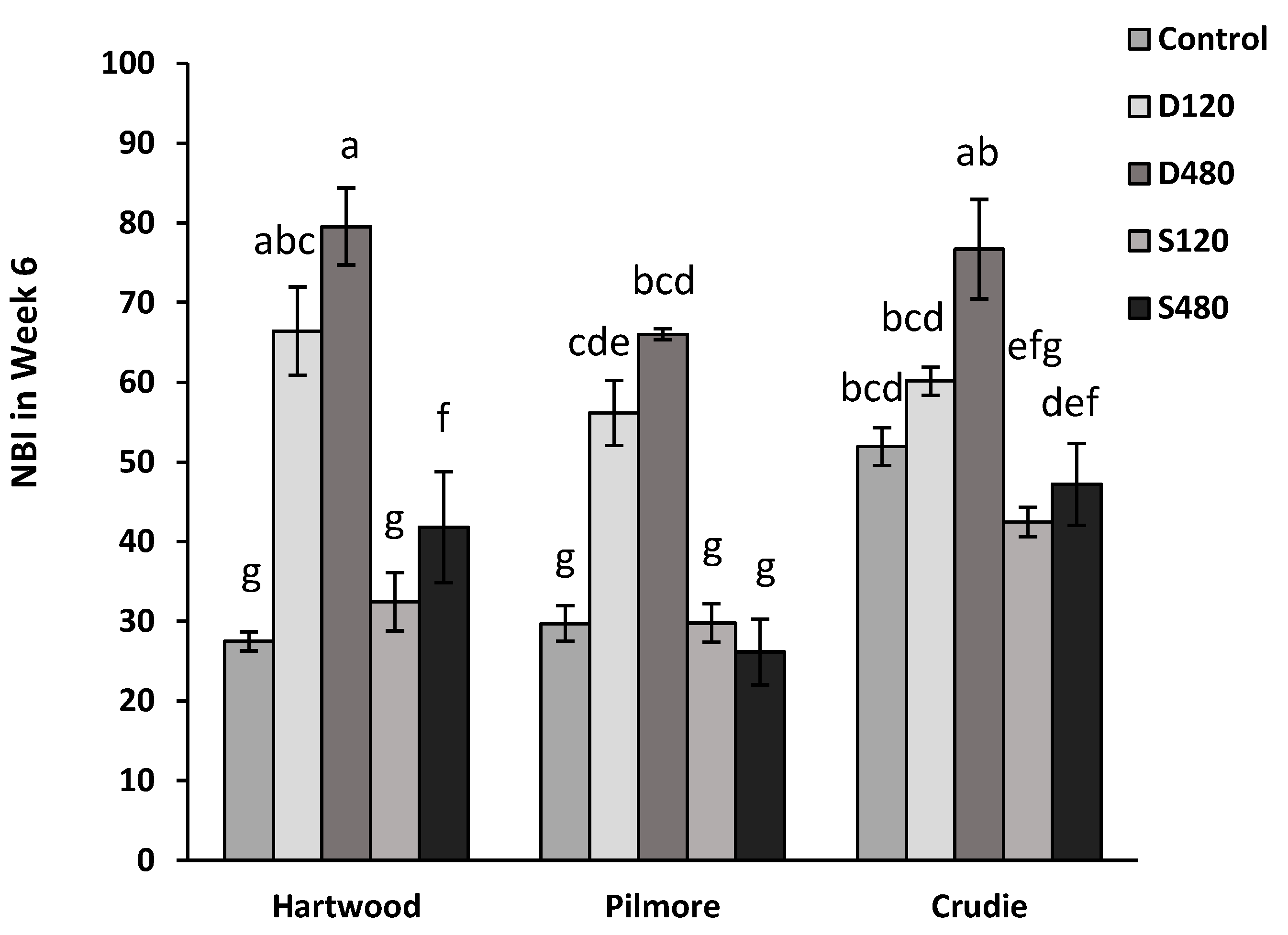
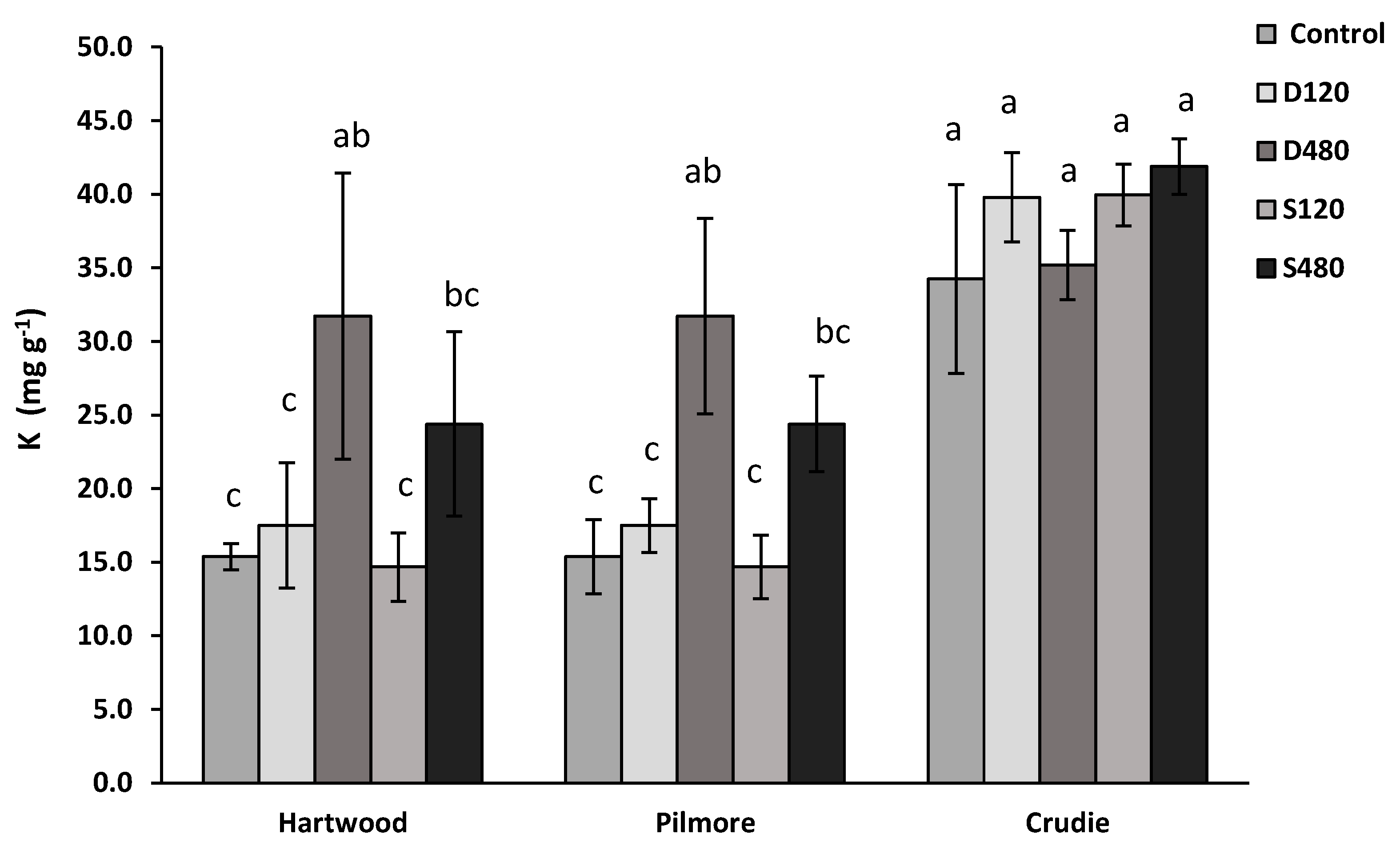
3.3. Impact of Anaerobic Digestate (Using Gaskfarm Digestate) in a Range of Soils—Experiment 2
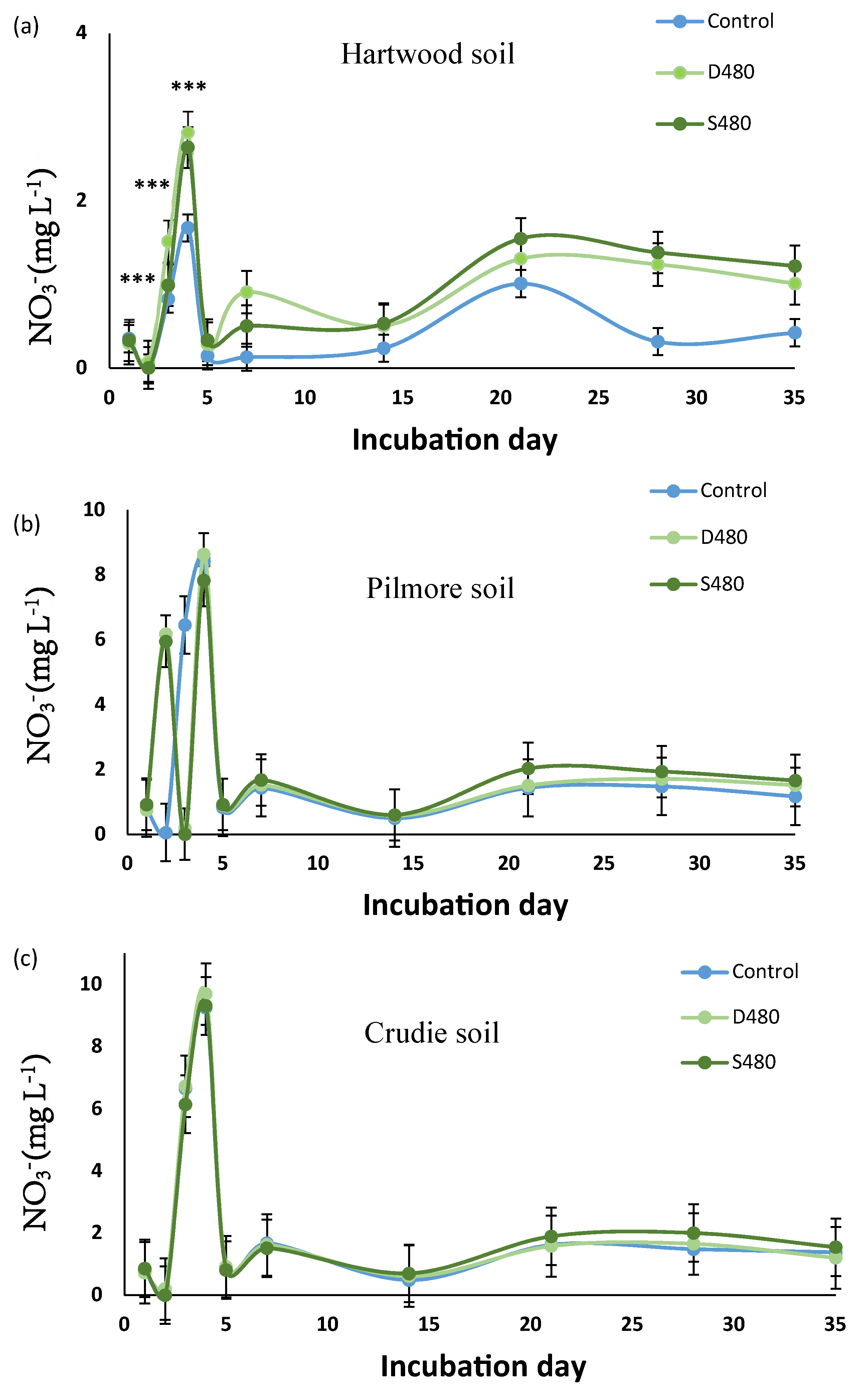
3.4. Soil Nitrogen Dynamics—Experiment 3
3.4.1. Ammonium
3.4.2. Nitrite
3.4.3. Nitrate
4. Discussion
4.1. Impacts of High Applications of Digestate
4.2. Impact of Lower Applications of Digestate
4.3. The Interaction between Treatment and Soil Characteristics on Crop Growth
4.4. Nitrate Leaching
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SEPA. Scottish Environment Protection Agency. The Waste (Scotland) Regulations 2012. 2012. Available online: https://www.legislation.gov.uk/ssi/2012/148 (accessed on 23 June 2022).
- Walsh, J.J.; Rousk, J.; Edwards-Jones, G.; Jones, D.L.; Williams, A.P. Fungal and bacterial growth following the application of slurry and anaerobic digestate of livestock manure to temperate pasture soils. Biol. Fertil. Soils 2012, 48, 889–897. [Google Scholar] [CrossRef]
- Tampio, E.; Tapio, S.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; De la Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertiliser performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maise cultivation for three consecutive years. Sci. Total Environ. 2017, 599, 1885–1894. [Google Scholar] [CrossRef]
- Stinner, W.; Deuker, A.; Leithold, G. Effects of different manuring systems with and without biogas digestion on nitrogecycle and crop yield in mixed organic dairy farming systems. Nutr. Cycl. Agroecosyst. 2008, 82, 209–232. [Google Scholar]
- Gunnarsson, A.; Bengtsson, F.; Caspersen, S. Use efficiency of nitrogen from biodigested plant material by ryegrass. J. Plant Nutr. Soil Sci. 2010, 173, 113–119. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Insam, H. Residues from renewable energy production: Their value for fertilising pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Coelho, J.J.; Hennssy, A.; Casey, I.; Woodcock, T.; Kennedy, N. Responses of ryegrass, white clover, soil plant primary macronutrients and microbial abundance to application of anaerobic digestates, cattle slurry and inorganic N-fertiliser. Appl. Soil Ecol. 2019, 144, 112–122. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Bhogal, A.; Rollett, A.; Taylor, M.; Williams, J.R. Precision application techniques reduce ammonia emissions following food-based digestate applications to grassland. Nutr. Cycl. Agroecosyst. 2018, 110, 151–159. [Google Scholar] [CrossRef]
- Van der Eerben, L.J.M.; de Visser, P.H.B.; van Dijk, C.J. Risk of damage to crops in the direct neighborhood of ammonia sources. Environ. Pollut. 1998, 102, 49–53. [Google Scholar] [CrossRef]
- Beretta, A.N.; Silbermann, A.V.; Paladino, L.; Torres, D.; Bassahun, D.; Musselli, R.; García-Lamohte, A. Soil texture analyses using a hydrometer: Modification of the Bouyoucos method. Cienc. Investig. Agrar. 2014, 41, 263–271. [Google Scholar] [CrossRef]
- Analytical Services Unit, Departament of Plant and Soil Science, School of Biological Sciences, University of Aberdeen: Aberdeen, UK, 2021. Available online: https://www.abdn.ac.uk/study/undergraduate/degree-programmes/828/CD27/plant-and-soil-science/ (accessed on 23 June 2022).
- Mortola, N.; Romaniuk, R.; Consenstino, V.; Eiza, M.; Carfagno, P.; Rizzo, P.; Bres, P.; Riera, N.; Roba, M.; Dutti, M.; et al. Potential Use of a Poultry Manure Digestate as a Biofertiliser: Evaluation of Soil Properties and Lactuca sativa Growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Cartelat, A.; Goulas, Y.; Meyer, S.; Stafford, J. In-the-Field Assessment of Wheat-Leaf Polyphenolics Using the New Optical Leaf-Clip Dualex; Stafford, J.V., Ed.; Precision Agriculture ‘05, 43-250; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005. [Google Scholar]
- ISO 5663; Water Quality-Determination of Kjeldahl Nitrogen—Method after Mineralisation with Selenium. ISO: Geneva, Switzerland, 1984.
- Maddonni, G.A.; Otegui, M.E. Intra-specific competition in maize: Early establishment of hierarchies among plants affects final kernel set. Field Crops Res. 2004, 85, 1–13. [Google Scholar] [CrossRef]
- Ross, D.J.; Tate, K.R.; Speir, T.W.; Stewart, D.J.; Hewitt, A.E. Influence of biogas-digester effluent on crop growth and soil biochemical properties under rotational cropping. N. Z. J. Crop Hortic. Sci. 1989, 17, 77–87. [Google Scholar] [CrossRef]
- Bath, B.; Elfstrand, S. Use of Red Clover-Based Green Manure in Leek Cultivation. Biol. Agric. Hortic. 2008, 25, 269–286. [Google Scholar] [CrossRef]
- De Jager, M.; Giani, L. An investigation of the efects of hydrochar application rate on soil amelioration and plant growth in three diverse soils. Biochar 2021, 3, 349–365. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Haraldsen, T.K.; Andersen, U.; Krogstad, T.; Sørheim, R. Liquid digestate from anaerobic treatment of source separated household waste as fertiliser for barley. Waste Manag. Res. 2011, 29, 1271–1276. [Google Scholar] [CrossRef]
- Rigby, H.; Smith, S.R. The nitrogen fertiliser value and other agronomic benefits of industrial biowastes. Nutr. Cycl. Agroecosyst. 2014, 98, 137–154. [Google Scholar] [CrossRef]
- Hansen, D.J.; Blackmer, A.M.; Mallarino, A.P.; Wuebker, M.A. Performance-based evaluations of guidelines for nitrogen fertilizer application after animal manure. Agron. J. 2004, 96, 34–41. [Google Scholar]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Yield, content and nutrient uptake by winter wheat and spring barley in response to applications of digestate, cattle slurry and NPK mineral fertilizers. Arch. Agron. Soil Sci. 2019, 66, 1481–1496. [Google Scholar] [CrossRef]
- George, J.R.; Obermann, D. Spring defoliation to improve summer supply and quality of switchgrass. Agron. J. 1989, 81, 47–52. [Google Scholar] [CrossRef]
- Calderon, M.P.; Alburquerque, J.A.; Bustamante, M.A.; Carrillo, R.C. Guide for Agricultural Use of Digested Materials by Biomethanization; PSE: Madrid, Spain, 2011. (In Spanish) [Google Scholar]
- Rowell, D.M.; Prescott, C.E.; Preston, C.M. Decomposition and nitrogen mineralisation from biosolids and other organic materials: Relationship with initial chemistry. J. Environ. Qual. 2001, 30, 1401–1410. [Google Scholar] [CrossRef]
- Cookson, W.R.; Cornforth, I.S.; Rowarth, J.S. Winter soil temperature (2–15 C) effects on nitrogen transformations in clover green manure amended or unamended soils; a laboratory and field study. Soil Biol. Biochem. 2002, 34, 1401–1415. [Google Scholar] [CrossRef]
- Odlare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef]


| Treatments | D120 | D480 | S120 | S480 |
|---|---|---|---|---|
| Digestate (mL) | 91.3 | 360 | - | - |
| Urea (mL of 50 g L−1) | - | - | 28.4 | 112.3 |
| (MAP) (mL of 50 g L−1) | - | - | 6.17 | 24.3 |
| Treatments | D120 | D480 | S120 | S480 |
|---|---|---|---|---|
| Digestate (mL) | 6.125 | 24.54 | - | - |
| Urea (mL of 50 g L−1) | - | - | 0.3 | 1.22 |
| monoammonium phosphate MAP (mL of 50 g L−1) | - | - | 0.3 | 0.3 |
| Treatments | Craibstone | Insch | Hartwood | Pilmore | Crudie |
|---|---|---|---|---|---|
| pH | 5.2 ± 0.01 | 5 ± 0.01 | 5.7 ± 0.01 | 5.3 ± 0.01 | 6.1 ± 0.01 |
| EC μS cm−1 | 0.53 ± 0.2 | 0.35 ± 0.2 | 0.51 ± 0.2 | 0.18 ± 0.1 | 0.12 ± 0.1 |
| Total N (% by weight) | 0.39 ± 0.05 | 0.32 ± 0.05 | 0.20 ± 0.02 | 0.35 ± 0.05 | 0.33 ± 0.01 |
| Total C (% by weight) | 4.14 ± 0.1 | 3.64 ± 0.1 | 3.15 ± 0.6 | 5.73 ± 0.1 | 4.98 ± 0.25 |
| Clay (% by weight) | - | 14 ± 0.01 | 25 ± 0.01 | 20 ± 0.01 | 15 ± 0.01 |
| Silt (% by weight) | - | 30 ± 0.01 | 29 ± 0.01 | 14 ± 0.01 | 23 ± 0.01 |
| Sand (% by weight) | - | 56 ± 0.01 | 46 ± 0.01 | 66 ± 0.01 | 62 ± 0.01 |
| Ca (mg g−1) | 6.14 ± 1.15 | 6.87 ± 0.42 | 3.37 ± 0.2 | 6.70 ± 0.6 | 2.76 ± 0.8 |
| K (mg g−1) | 2.44 ± 1.15 | 0.95 ± 0.78 | 1.29 ± 0.9 | 2.86 ± 0.5 | 2.81 ± 0.6 |
| Mg (mg g−1) | 1.41 ± 0.27 | 6.46 ± 0.27 | 1.55 ± 0.05 | 5.81 ± 0.2 | 3.69 ± 0.2 |
| Soil Texture | - | Sandy Loam | Clay Sandy Loam | Sandy | Medium Sandy-Loam |
| Powerhouse | Gaskfarm | |
|---|---|---|
| pH | 8.5 | 8.6 |
| Total N (% by weight) | 8.33 ± 0.09 | 5.33 ± 0.07 |
| Total C (% by weight) | 27.91 ± 1.11 | 38.34 ± 2.58 |
| Total P (% by weight) | 0.99 ± 0.33 | 0.76 ± 0.62 |
| NO3− and NO2−-N (mg g−1) | 24.53 ± 9.07 | 11.23 ± 3.68 |
| Plant available N (% by weight) | 74 ± 27 | 36 ± 11 |
| Nutrients (mg kg−1 Dry Matter) | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| Tissue | Control | D120 | S120 | D480 | S480 | p-Value | |
| Ca | Grain | 350 ± 10 | 290 ± 40 | 280 ± 9 | 290 ± 10 | 310 ± 10 | 0.492 |
| K | Grain | 6000 ± 300 | 4840 ± 40 | 4900 ± 30 | 4350 ± 60 | 5100 ± 300 | 0.002 |
| Mg | Grain | 780 ± 70 | 930 ± 80 | 980 ± 60 | 840 ± 50 | 1040 ± 40 | 0.034 |
| Ca | Shoot | 5000 ± 1000 | 7000 ± 1000 | 7000 ± 1000 | 16,000 ± 2000 | 17,000 ± 2000 | 0.001 |
| K | Shoot | 25,000 ± 1000 | 27,700 ± 700 | 23,000 ± 1000 | 32,100 ± 800 | 27,000 ± 2000 | 0.331 |
| Mg | Shoot | 1100 ± 300 | 1300 ± 700 | 1200 ± 700 | 800 ± 800 | 1800 ± 400 | 0.249 |
| Nutrients (mg kg−1 Dry Matter) | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| Tissue | Control | D120 | S120 | D480 | S480 | p-Value | |
| Ca | Grain | 1500 ± 200 | 2100 ± 500 | 1300 ± 500 | 1700 ± 100 | 2600 ± 300 | 0.012 |
| K | Grain | 6000± 600 | 5000 ± 2000 | 6000 ± 1000 | 6000± 1000 | 5000 ± 1000 | 0.072 |
| Mg | Grain | 1900 ± 600 | 2000 ± 1000 | 1900 ± 200 | 2000 ± 200 | 2700 ± 700 | 0.319 |
| Ca | Shoot | 15,000 ± 400 | 151,000 ± 900 | 16,000 ± 1000 | 25,000± 1600 | 33,200 ± 3000 | 0.075 |
| K | Shoot | 28,000± 4000 | 29,000 ± 3000 | 37,000± 3000 | 46,000± 3000 | 23,300 ± 4000 | 0.035 |
| Mg | Shoot | 4700± 300 | 4300 ± 600 | 5200 ± 400 | 4700 ± 300 | 6100 ± 700 | 0.220 |
| Traits | Powerhouse Experiment 1 | Gaskfarm Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|---|---|
| S480 | C | S480 | C | S480 | C | |
| Plant height | - | ✓ | - | ✓ | - | ✓ |
| Tiller number | - | ✓ | ✓ | ✓ | ✓ | ✓ |
| HI | x | ✓ | ✓ | x | ||
| Straw biomass | ✓ | ✓ | - | ✓ | ✓ | - |
| Grain biomass | - | ✓ | - | ✓ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallat-Sanchez, J.; Smith, J.; Norton, G.J. Impact of Adding Anaerobic Digestate to Soil and Consequences on Crop Performance. Agronomy 2023, 13, 2889. https://doi.org/10.3390/agronomy13122889
Hallat-Sanchez J, Smith J, Norton GJ. Impact of Adding Anaerobic Digestate to Soil and Consequences on Crop Performance. Agronomy. 2023; 13(12):2889. https://doi.org/10.3390/agronomy13122889
Chicago/Turabian StyleHallat-Sanchez, Juana, Jo Smith, and Gareth J. Norton. 2023. "Impact of Adding Anaerobic Digestate to Soil and Consequences on Crop Performance" Agronomy 13, no. 12: 2889. https://doi.org/10.3390/agronomy13122889
APA StyleHallat-Sanchez, J., Smith, J., & Norton, G. J. (2023). Impact of Adding Anaerobic Digestate to Soil and Consequences on Crop Performance. Agronomy, 13(12), 2889. https://doi.org/10.3390/agronomy13122889





