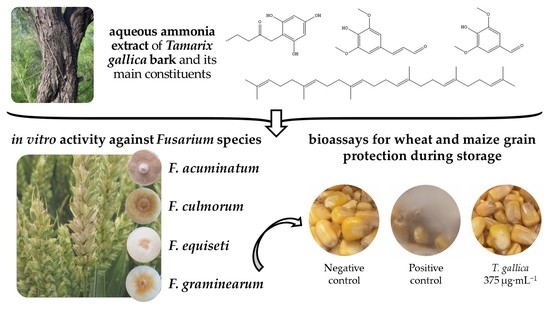
Phytochemical Profile and Activity against Fusarium Species of Tamarix gallica Bark Aqueous Ammonia Extract
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Fungal Isolates
2.3. Plant Material and Extraction Procedure
2.4. Extract Characterization
2.5. In Vitro Antifungal Activity Evaluation
2.6. Preparation of Conidial Suspension of F. graminearum
2.7. Stored Wheat and Maize Grain Protection Assays
2.8. In Vitro Germination Assays
2.9. Statistics
3. Results
3.1. Bark Vibrational Characterization
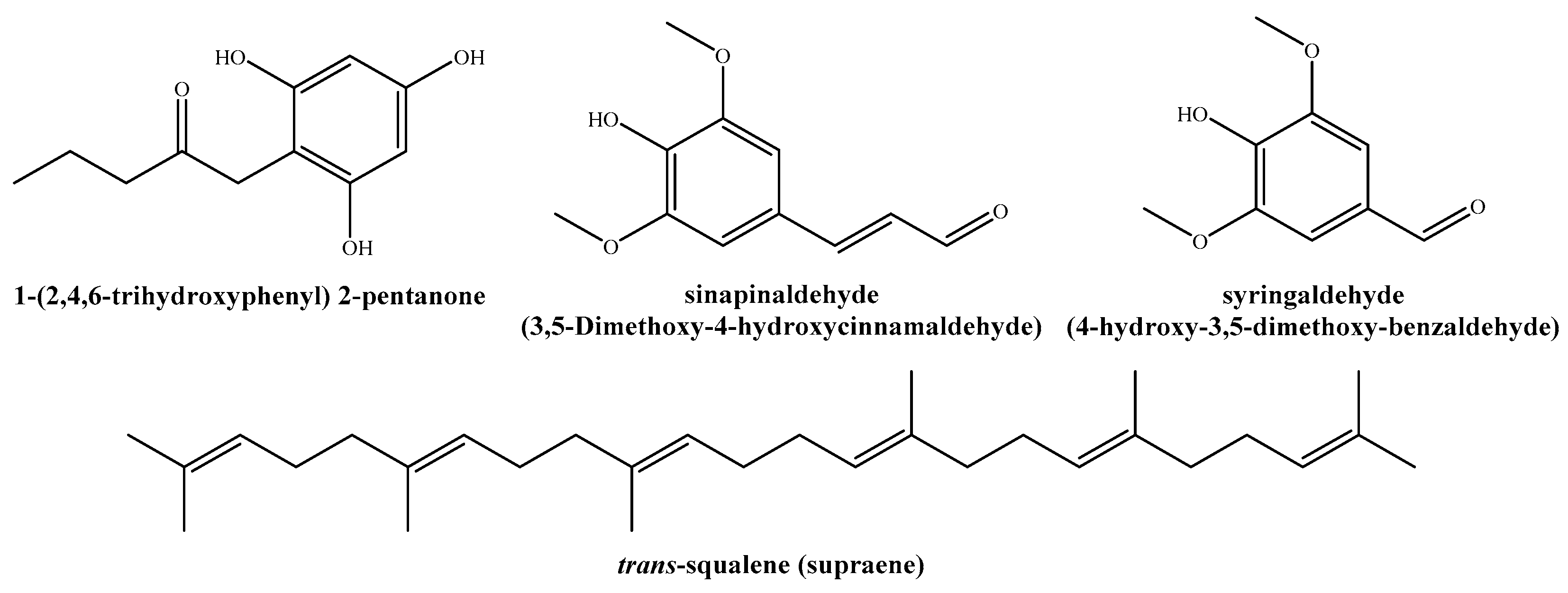
3.2. Bark Extract Constituents
3.3. Extract Antifungal Activity
3.3.1. In Vitro Activity
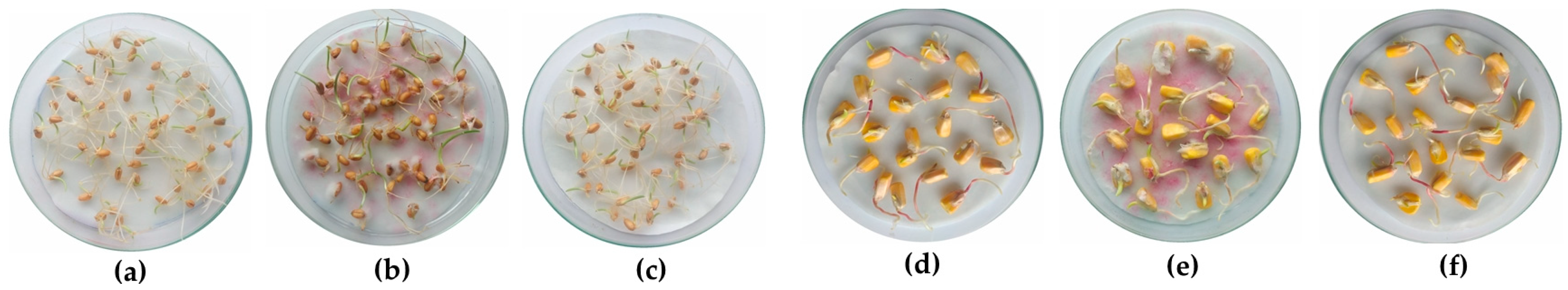
3.3.2. Protection of Wheat and Maize Grains
3.4. Germination Assays
4. Discussion
4.1. On the Phytochemical Composition and Mode of Action
4.2. Antimicrobial Activity Comparison
4.2.1. Comparison with Other Tamarix gallica Extracts
4.2.2. Comparison with other Tamaricaceae Family Bark Extracts
4.2.3. Comparison with Conventional Fungicides
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Q.; Zhu, Z.; Wang, B.; Chen, M. Recent progress on the salt tolerance mechanisms and application of tamarisk. Int. J. Mol. Sci. 2022, 23, 3325. [Google Scholar] [CrossRef]
- Han, Z.; Yin, W.; Zhang, J.; Niu, S.; Ren, L. Active anti-erosion protection strategy in tamarisk (Tamarix aphylla). Sci. Rep. 2013, 3, 3429. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, L.; Pan, B. Biological and ecological characteristics of Tamarix L. and its effect on the ecological environment. Sci. China Ser. D Earth Sci. 2002, 45, 18–22. [Google Scholar] [CrossRef]
- Dawalibi, V.; Monteverdi, M.; Moscatello, S.; Battistelli, A.; Valentini, R. Effect of salt and drought on growth, physiological and biochemical responses of two Tamarix species. Iforest Biogeosci. For. 2015, 8, 772–779. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 112245. [Google Scholar] [CrossRef]
- Elamin, M. A review of biological and pharmacological activities from the aerial part of tamarisk. Int. J. Pharm. Res. Allied Sci. 2016, 5, 22–36. [Google Scholar]
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Bakrouf, A.; Magné, C.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009, 47, 2083–2091. [Google Scholar] [CrossRef]
- Lee, J.M.; Yim, M.-J.; Lee, D.-S.; Lee, M.S.; Park, Y.G.; Jeon, J.H.; Choi, G. Comparison of biological activities of Korean halophytes. Nat. Prod. Sci. 2018, 24, 247–252. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as medicinal plants against human infectious diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Said, S.; Noureddine, G.; Eddine, L.S.; Abdelmadjid, G.; Djamel, B.; Tliba, A. Phenolic content, HPLC analysis and antioxidant activity extract from Tamarix gallica and Tamarix articulata growing in Southeast of Algeria. Res. J. Pharm. Technol. 2018, 11, 3826–3832. [Google Scholar] [CrossRef]
- Boulaaba, M.; Snoussi, M.; Saada, M.; Mkadmini, K.; Smaoui, A.; Abdelly, C.; Ksouri, R. Antimicrobial activities and phytochemical analysis of Tamarix gallica extracts. Ind. Crops Prod. 2015, 76, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Gurung, S.; Hansen, J.M.; Bonman, J.M.; Gironella, A.I.N.; Adhikari, T.B. Multiple disease resistance to four leaf spot diseases in winter wheat accessions from the USDA National Small Grains Collection. Crop Sci. 2012, 52, 1640–1650. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key global actions for mycotoxin management in wheat and other small grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Feksa, H.R.; Do Couto, H.T.Z.; Garozi, R.; De Almeida, J.L.; Gardiano, C.G.; Tessmann, D.J. Pre- and postinfection application of strobilurin-triazole premixes and single fungicides for control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Crop Prot. 2019, 117, 128–134. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Q.; Zhang, G.; Wu, J.; Zhu, F.; Yang, H.; Zhuang, Y. Carbendazim-resistance of Gibberella zeae associated with fusarium head blight and its management in Jiangsu Province, China. Crop Prot. 2019, 124, 104866. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J. Statistical optimization of aqueous ammonia pretreatment and enzymatic hydrolysis of corn cob powder for enhancing sugars production. Biochem. Eng. J. 2021, 174, 108106. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Casanova-Gascón, J.; Barriuso-Vargas, J.J.; Balduque-Gil, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Valorization of Quercus suber L. bark as a source of phytochemicals with antimicrobial activity against apple tree diseases. Plants 2022, 11, 3415. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid conjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef]
- Olesen, M.; Duijn, B.v.; Boelt, B. Introduction of New Methods: Spectral Imaging. Seed Test. Int. 2014, 147, 10–13. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Tamarix gallica leaf extract mediated novel route for green synthesis of CuO nanoparticles and their application for N-arylation of nitrogen-containing heterocycles under ligand-free conditions. RSC Adv. 2015, 5, 40628–40635. [Google Scholar] [CrossRef]
- Boulaaba, M.; Tsolmon, S.; Ksouri, R.; Han, J.; Kawada, K.; Smaoui, A.; Abdelly, C.; Isoda, H. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology 2013, 65, 927–936. [Google Scholar] [CrossRef]
- Nisar, J.; Ali Shah, S.M.; Ayaz, S.; Akram, M.; Rashid, A.; Mustafa, I.; Nisar, Z. In vitro comparative evaluation of Tamarix gallica extracts for antioxidant and antidiabetic activity. Exp. Biol. Med. 2022, 15353702221139208. [Google Scholar] [CrossRef]
- Lefahal, M.; Makhloufi, E.-H.; Trifa, W.; Ayad, R.; El Hattab, M.; Benahmed, M.; Keskin, M.; Akkal, S. The cosmetic potential of the medicinal halophyte Tamarix gallica L. (Tamaricaceae) growing in the eastern part of Algeria: Photoprotective and antioxidant activities. Comb. Chem. High Throughput Screen. 2021, 24, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Al-Otibi, F.; Moria, G.A.; Alharbi, R.I.; Yassin, M.T.; Al-Askar, A.A. The antifungal properties of Tamarix aphylla extract against some plant pathogenic fungi. Microorganisms 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Socolsky, C.; Salvatore, A.; Asakawa, Y.; Bardón, A. Bioactive new bitter-tasting p-hydroxystyrene glycoside and other constituents from the fern Elaphoglossum spathulatum. Arkivoc 2003, 10, 347–355. [Google Scholar] [CrossRef]
- Alagammal, M.; Tresina, P.; Mohan, V. GC-MS determination of bioactive components of Polygala javana DC. Int. J. Curr. Pharm. Res. 2012, 4, 42–44. [Google Scholar]
- Rabiu, Z.; Hamzah, M.A.A.M.; Hasham, R.; Zakaria, Z.A. Characterization and antiinflammatory properties of fractionated pyroligneous acid from palm kernel shell. Environ. Sci. Pollut. Res. 2021, 28, 40535–40543. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Zhang, M.; Ge, S.; Mo, B.; Li, S.; Ohkoshi, M. Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci. 2017, 24, 399–404. [Google Scholar] [CrossRef]
- Kamal, K.M.; Hashim, Y.Z.H.Y.; Sani, M.S.A.; Rahim, N.A.; Maifiah, M.H.M.; Jihadi, N.I.M. Combination of polymyxin B and Aquilaria malaccensis extract enhanced the killing and inhibited the growth of Acinetobacter baumannii and Klebsiella pneumoniae. Malays. J. Microbiol. 2021, 18, 27–36. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Custódio, L.; Mecha, D.; Zengin, G.; Cziáky, Z.; Sotkó, G.; Pereira, C.G. Nutritional and phyto-therapeutic value of the halophyte Cladium mariscus L. (Pohl.): A special focus on seeds. Plants 2022, 11, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.; Saad, A.; Ahmed, W.; Refahy, L.; Nasr, S. HPLC-DAD-ESI-MS/MS characterization of bioactive secondary metabolites from Strelitzia nicolai leaf extracts and their antioxidant and anticancer activities in vitro. Pharmacogn. Res. 2018, 10, 368–378. [Google Scholar] [CrossRef]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrella, I.; Pinto, E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J. Mass Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Ralph, J.; Marques, G.; Gutiérrez, A.; Martínez, Á.T.; del Río, J.C. Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J. Agric. Food. Chem. 2013, 61, 2434–2445. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Influences of cinnamic aldehydes on H+ extrusion activity and ultrastructure of Candida. J. Med. Microbiol. 2013, 62, 232–240. [Google Scholar] [CrossRef]
- Zare, B.; Sepehrizadeh, Z.; Faramarzi, M.A.; Soltany-Rezaee-Rad, M.; Rezaie, S.; Shahverdi, A.R. Antifungal activity of biogenic tellurium nanoparticles against Candida albicans and its effects on squalene monooxygenase gene expression. Biotechnol. Appl. Biochem. 2014, 61, 395–400. [Google Scholar] [CrossRef]
- Suman, T.Y.; Elumalai, D.; Kaleena, P.K.; Rajasree, S.R.R. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind. Crops Prod. 2013, 47, 239–245. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.-L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food. Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef]
- Wei, F.H.; Chen, F.L.; Tan, X.M. Gas chromatographic-mass spectrometric analysis of essential oil of Jasminum officinale L var grandiflorum flower. Trop. J. Pharm. Res. 2015, 14, 149–152. [Google Scholar] [CrossRef]
- Anandan, A.; Eswaran, R.; Doss, A.; Sangeetha, G.; Anand, S. Chemical compounds investigation of Lucas aspera leaves-a potential folklore medicinal plant. Asian J. Pharm. Clin. Res. 2012, 5, 86–88. [Google Scholar]
- Masuda, A.; Akiyama, S.-I.; Kuwano, M.; Ikekawa, N. Potentiation of antifungal effect of amphotericin B by squalene, an intermediate for sterol biosynthesis. J. Antibiot. 1982, 35, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E. Mechanisms of action of systemic antifungal agents. J. Am. Acad. Dermatol. 1993, 28, S28–S34. [Google Scholar] [CrossRef]
- Belter, A.; Skupinska, M.; Giel-Pietraszuk, M.; Grabarkiewicz, T.; Rychlewski, L.; Barciszewski, J. Squalene monooxygenase–a target for hypercholesterolemic therapy. Biol. Chem. 2011, 392, 1053–1075. [Google Scholar] [CrossRef]
- Nowosielski, M.; Hoffmann, M.; Wyrwicz, L.S.; Stepniak, P.; Plewczynski, D.M.; Lazniewski, M.; Ginalski, K.; Rychlewski, L. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J. Chem. Inf. Model. 2011, 51, 455–462. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Y.-S.; Lin, K.; Huang, X.; Chen, Y.-j.; Lai, D.; Kang, N.; Huang, L.; Weng, C.-F. A narrative review: The pharmaceutical evolution of phenolic syringaldehyde. Biomed. Pharmacother. 2022, 153, 113339. [Google Scholar] [CrossRef] [PubMed]
- Fillat, A.; Gallardo, O.; Vidal, T.; Pastor, F.; Díaz, P.; Roncero, M. Enzymatic grafting of natural phenols to flax fibres: Development of antimicrobial properties. Carbohydr. Polym. 2012, 87, 146–152. [Google Scholar] [CrossRef]
- Musthafa, S.A.; Dabdoub, W.; Sadiq, M.; Munuswamy-Ramanujam, G. Syringaldehyde isolated from Abutilon indicum Linn. leaves exhibits broad spectrum anti-microbial activity. Mater. Today Proc. 2022, 50, 335–339. [Google Scholar] [CrossRef]
- Bibi, S.; Afzal, M.; Aziz, N.; Din, B.; Khan, M.; Khan, A.; Komal, H. Antifungal activity of Tamarix aphylla (L.) Karst. stem-bark extract against some pathogenic fungi. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 541–545. [Google Scholar]
- Iqbal, A.; Begum, N.; Rabbi, F.; Akhtar, N.; Khan, W.M.; Shah, Z. In-vitro antimicrobial, antioxidant and enzyme inhibitory activities of fixed oil extracted from stem bark of Tamarix aphylla. Pharm. Chem. J. 2022, 56, 1116–1122. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.l.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, hispidulin, and cirsimaritin identified in Tamarix ramosissima barks from southern Xinjiang and their antioxidant and antimicrobial activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef]
- Mikaeili, A.; Karimi, I.; Modarresi, M.; Shahbazi, A.; Jalilian, N. Antimycotic activity of aqueous extract of Tamarix ramosissima Ledeb. bark on in vitro and in vivo guinea pig model of dermatophytosis. Int. J. Life Sci. Pharma Res. 2018, 8, 8–15. [Google Scholar]
- Paul, P.A.; McMullen, M.P.; Hershman, D.E.; Madden, L.V. Meta-analysis of the effects of triazole-based fungicides on wheat yield and test weight as influenced by Fusarium head blight intensity. Phytopathology 2010, 100, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Nichea, M.J.; Monge, M.d.P.; Zachetti, V.G.L.; Chiacchiera, S.M.; Ramirez, M.L. Effect of fungicides commonly used for Fusarium head blight management on growth and fumonisin production by Fusarium proliferatum. Rev. Argent. Microbiol. 2021, 53, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, Y.; Orina, A.; Gavrilova, O.; Gagkaeva, T.; Glupov, V. The effect of fungicides on growth of Fusarium fungi in vitro. BIO Web Conf. 2020, 18, 00022. [Google Scholar] [CrossRef]
- Marín, P.; de Ory, A.; Cruz, A.; Magan, N.; González-Jaén, M.T. Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Valle-Algarra, F.M.; Mateo, R.; Jiménez, M.; Magan, N. Effect of fenpropimorph, prochloraz and tebuconazole on growth and production of T-2 and HT-2 toxins by Fusarium langsethiae in oat-based medium. Int. J. Food Microbiol. 2011, 151, 289–298. [Google Scholar] [CrossRef]
- Kulik, T.; Lojko, M.; Jestoi, M.; Perkowski, J. Sublethal concentrations of azoles induce tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012, 335, 58–67. [Google Scholar] [CrossRef] [Green Version]



| Bark | Leaves [28] | Assignment |
|---|---|---|
| 3393 | OH stretching; hydrogen bonds | |
| 3358 | OH group in phenolic compounds | |
| 2917 | 2925 | –CH2 asymmetric stretching of alkyls (cutine, wax, pectin) |
| 2850 | 2861 | –CH2 symmetric stretching (cutine and wax); CH2–(C6)– bending (cellulose) |
| 1732 | C=O stretching of alkyl ester | |
| 1628 | 1652 | C=O stretching (hemicellulose, bonded ketones, …); C=C stretching |
| 1594 | C=C stretching | |
| 1504 | 1519 | Aromatic skeletal. Typical of carotenoids |
| 1460 | 1442 | Symmetric aromatic ring stretching vibration (C=C ring); C–H deformation; O–CH3 stretching |
| 1421 | C–H deformation | |
| 1328 | CH in-plane bending in cellulose I and cellulose II | |
| 1223 | 1261 | Amide III; C–C–O asymmetric stretching acetylated glucomannan; C–O and OH of COOH; in-plane rocking vibration signal of the –CH2– group |
| 1153 | 1153 | C–O–C asymmetric stretching in cellulose I and cellulose II; C–C in-plane (β-carotene) |
| 1123 | H–C–O bond bending | |
| 1030 | 1052 | C–O stretching; O–H out plane bending |
| RT (min) | Area (%) | Assignment | Qual |
|---|---|---|---|
| 5.0425 | 7.5026 | 2,5-furandione, dihydro-3-methylene- | 91 |
| 9.1734 | 2.9246 | Benzofuran, 2,3-dihydro- | 68 |
| 11.0490 | 3.9897 | Phenol, 2,6-dimethoxy- | 96 |
| 11.7078 | 1.1151 | Vanillin | 96 |
| 12.2064 | 2.1478 | Ethanone, 1-(3-hydroxyphenyl)- | 90 |
| 13.7495 | 2.4736 | 4-methyl-2,5-dimethoxybenzaldehyde | 72 |
| 14.7764 | 1.8309 | 2,3,4,5-tetramethylbenzoic acid | 30 |
| 14.8595 | 8.1339 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | 96 |
| 15.0909 | 2.7329 | 4-methoxymethyl-6-methyl-1H-pyrazolo [3,4-b]pyridin-3-ylamine | 52 |
| 15.3105 | 1.6158 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- | 89 |
| 15.5005 | 0.9374 | Methyl tetradecanoate | 96 |
| 15.6845 | 7.2470 | Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | 92 |
| 16.0762 | 2.9054 | Benzoic acid, 4-hydroxy-3,5-dimethoxy-, hydrazide | 95 |
| 16.4917 | 3.7293 | Benzoic acid, 4-hydroxy-3,5-dimethoxy- | 98 |
| 16.6460 | 2.9655 | Aspidinol | 59 |
| 16.6994 | 1.6692 | 2-fluorenamine | 46 |
| 17.3938 | 1.3234 | 9-hexadecenoic acid, methyl ester, (Z)- | 95 |
| 17.5897 | 3.6982 | Hexadecanoic acid, methyl ester (or methyl palmitate) | 98 |
| 17.9102 | 2.2142 | n-hexadecanoic acid | 99 |
| 18.0705 | 1.4350 | Benzeneacetic acid, .alpha.-phenyl-, methyl ester | 72 |
| 18.2188 | 10.0460 | 3,5-dimethoxy-4-hydroxycinnamaldehyde | 98 |
| 18.2960 | 11.8101 | 2-pentanone, 1-(2,4,6-trihydroxyphenyl) | 53 |
| 19.2694 | 1.9702 | 11-octadecenoic acid, methyl ester | 99 |
| 19.4949 | 1.7364 | Methyl stearate | 99 |
| 25.0919 | 9.9556 | Supraene | 98 |
| Treatment | Effective Concentration | F. acuminatum | F. culmorum | F. equiseti | F. graminearum |
|---|---|---|---|---|---|
| T. gallica bark extract | EC50 | 568.8 | 272.8 | 440.2 | 238.3 |
| EC90 | 928.0 | 825.6 | 698.3 | 334.8 | |
| 1-(2,4,6-trihydroxyphenyl)-2-pentanone | EC50 | 147.9 | 81.5 | 114.3 | 95.8 |
| EC90 | 236.2 | 213.4 | 238.2 | 190.7 | |
| Sinapinaldehyde | EC50 | 257.1 | 117.0 | 209.4 | 169.9 |
| EC90 | 555.2 | 367.7 | 530.2 | 299.5 | |
| Trans-squalene | EC50 | 242.5 | 179.6 | 153.7 | 114.3 |
| EC90 | 507.3 | 380.2 | 393.5 | 258.0 | |
| Syringaldehyde | EC50 | 322.7 | 176.8 | 144.1 | 124.6 |
| EC90 | 601.4 | 374.8 | 316.1 | 246.6 |
| Commercial Fungicide | Pathogen | Radial Growth of Mycelium (mm) | Inhibition (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Control (PDA) | Rd/10 | Rd * | Rd × 10 | Rd/10 | Rd * | Rd × 10 | ||
| Mancozeb | F. acuminatum | 75 | 65 | 0 | 0 | 13.3 | 100 | 100 |
| F. culmorum | 75 | 75 | 5 | 0 | 0 | 93.3 | 100 | |
| F. equiseti | 75 | 70 | 25 | 0 | 6.7 | 66.7 | 100 | |
| F. graminearum | 75 | 75 | 5 | 0 | 0 | 93.3 | 100 | |
| Fosetyl-Al | F. acuminatum | 75 | 66.7 | 35 | 0 | 11.1 | 53.3 | 100 |
| F. culmorum | 75 | 75 | 0 | 0 | 0 | 100 | 100 | |
| F. equiseti | 75 | 75 | 60 | 26.7 | 0 | 20 | 64.4 | |
| F. graminearum | 75 | 33.3 | 0 | 0 | 55.6 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hernández, E.; González-García, V.; Correa-Guimarães, A.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Phytochemical Profile and Activity against Fusarium Species of Tamarix gallica Bark Aqueous Ammonia Extract. Agronomy 2023, 13, 496. https://doi.org/10.3390/agronomy13020496
Sánchez-Hernández E, González-García V, Correa-Guimarães A, Casanova-Gascón J, Martín-Gil J, Martín-Ramos P. Phytochemical Profile and Activity against Fusarium Species of Tamarix gallica Bark Aqueous Ammonia Extract. Agronomy. 2023; 13(2):496. https://doi.org/10.3390/agronomy13020496
Chicago/Turabian StyleSánchez-Hernández, Eva, Vicente González-García, Adriana Correa-Guimarães, José Casanova-Gascón, Jesús Martín-Gil, and Pablo Martín-Ramos. 2023. "Phytochemical Profile and Activity against Fusarium Species of Tamarix gallica Bark Aqueous Ammonia Extract" Agronomy 13, no. 2: 496. https://doi.org/10.3390/agronomy13020496