The Use of Plant Growth Promoting Rhizobacteria to Reduce Greenhouse Gases in Strawberry Cultivation under Different Soil Moisture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Experiment and Plant Material and Growth Conditions Employed
2.2. Experimental Factors
2.3. GHG Emission Measurement Methods
2.4. Statistical Methods
3. Results
3.1. Emission of CO2
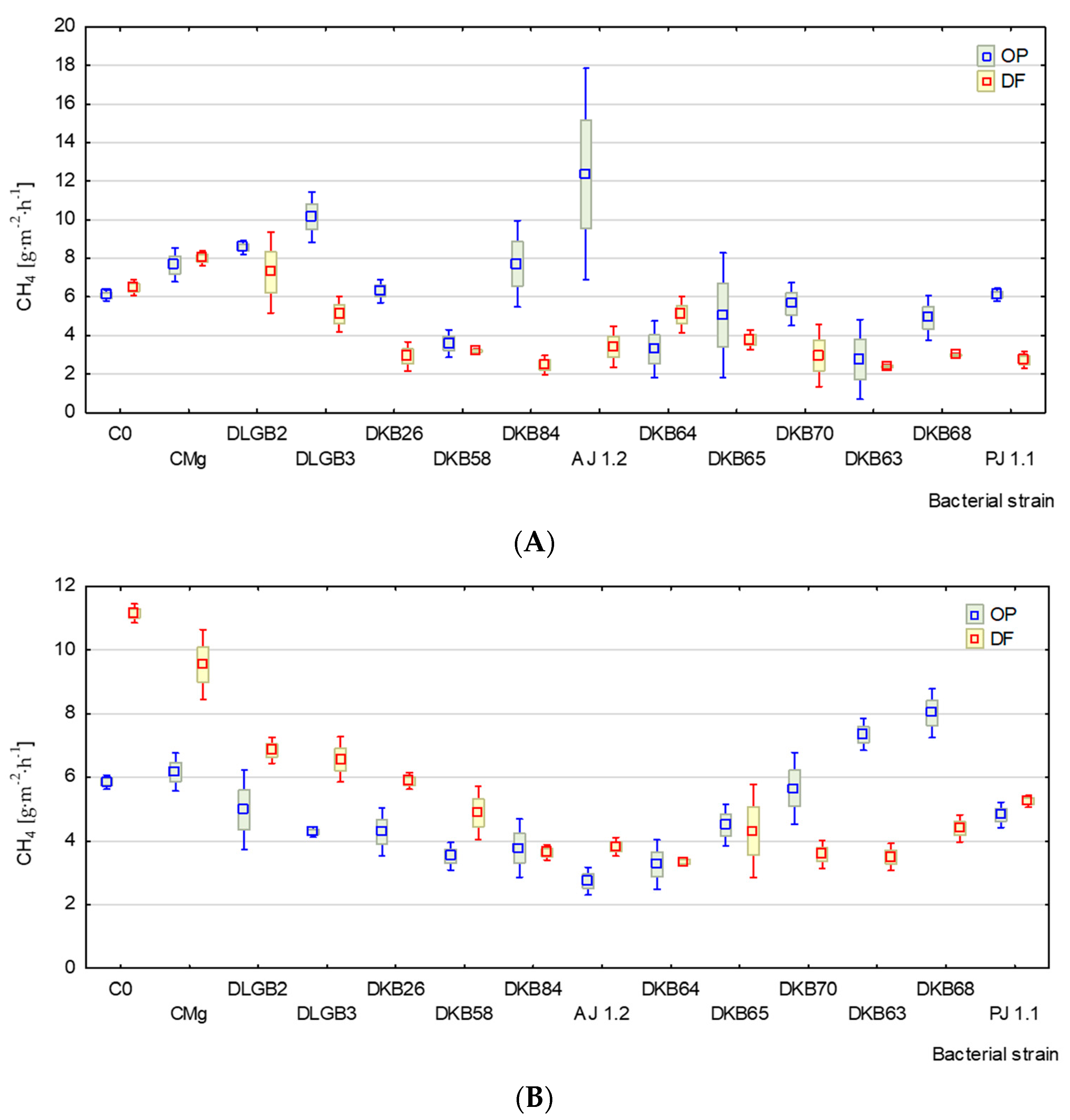
3.2. Emission of CH4
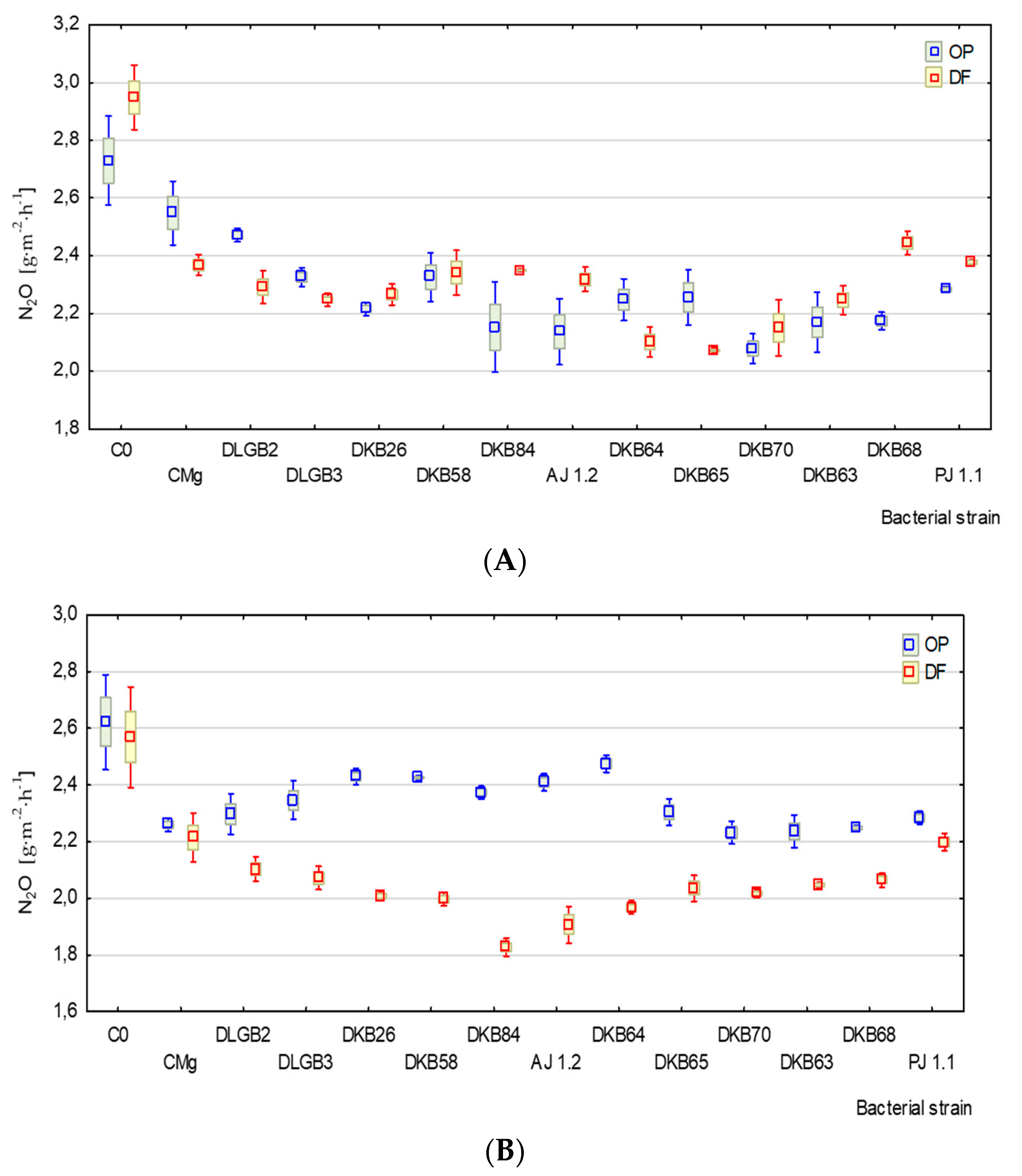
3.3. Emission of N2O
3.4. Emission of NH3
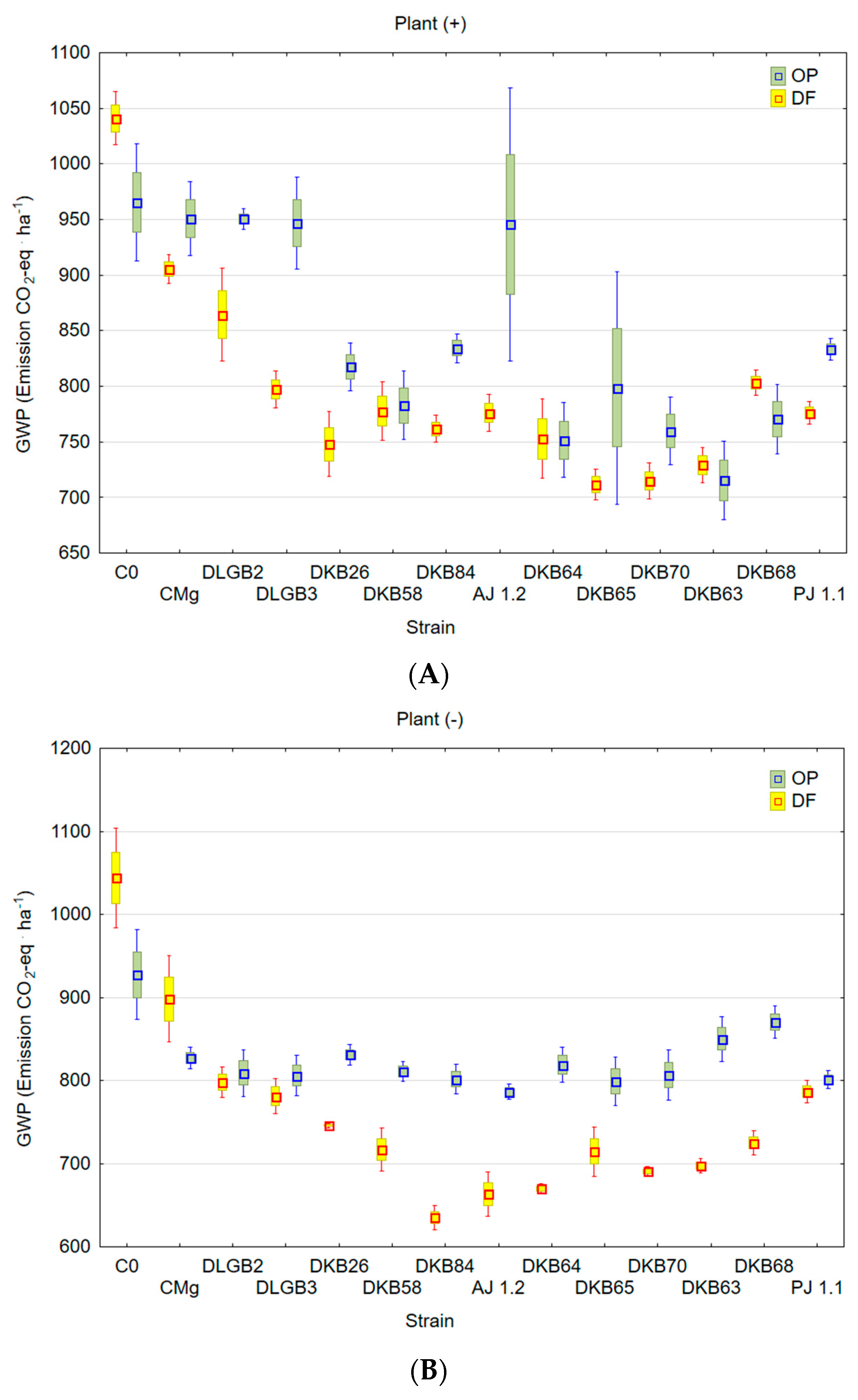
3.5. GWP
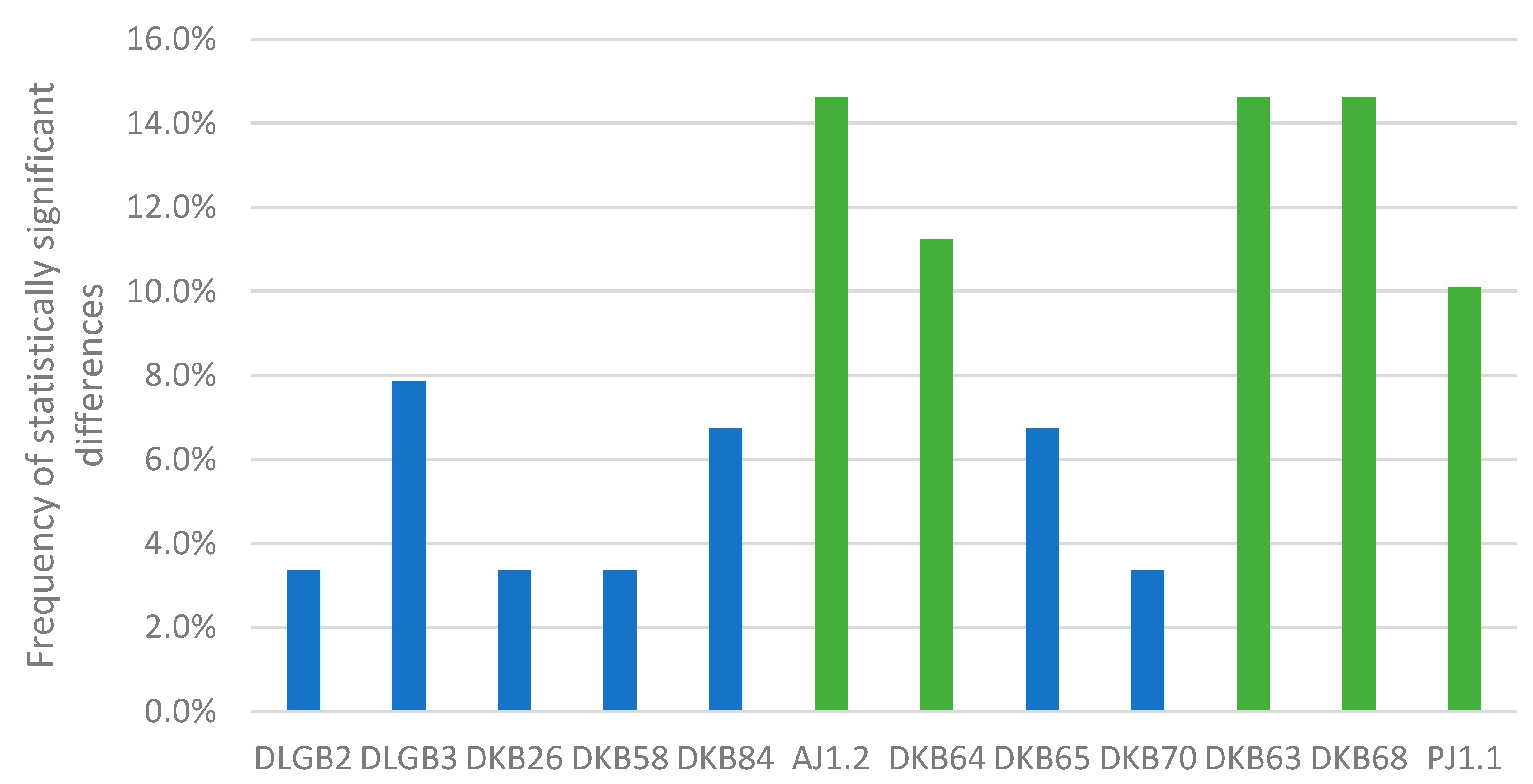
3.6. Recommendations
3.7. Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC, 2019). Climate Change and Land. An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef]
- Witkowska-Dąbrowska, M. Changes in the volume of air emissions from gases and selected pollutants originating from agricultural production in Poland and in the EU—Analyses using sustainable development indicators (in Polish). Probl. World Agric. 2018, 18, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.B.; Wollenberg, E.; van Vuuren, D. National contributions to climate change mitigation from agriculture: Allocating a global target. Clim. Policy 2018, 18, 1271–1285. [Google Scholar] [CrossRef] [Green Version]
- Loboguerrero, A.M.; Campbell, B.M.; Cooper, P.J.M.; Hansen, J.W.; Rosenstock, T.; Wollenberg, E. Food and earth systems: Priorities for climate change adaptation and mitigation for agriculture and food systems. Sustainability 2019, 11, 1372. [Google Scholar] [CrossRef] [Green Version]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Erde 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change (IPCC, 2007). Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; ISBN 978-0-521-88009-1. [Google Scholar]
- Ali, S.; Xie, L. Plant growth promoting and stress mitigating abilities of soil born microorganisms. Recent Pat. Food Nutr. Agric. 2020, 11, 96–104. [Google Scholar] [CrossRef]
- Dawson, T.P.; Perryman, A.H.; Osborne, T.M. Modelling impacts of climate change on global food security. Clim. Chang. 2016, 134, 429–440. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [Green Version]
- Bieniasz, M.; Konieczny, A.; Błaszczyk, J.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Pniak, M. Titanium organic complex improves pollination and fruit development of remontant strawberry cultivars under high-temperature conditions. Agriculture 2022, 12, 1795. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Kadkhodaei, S.; Vaculík, M. Physiological and biochemical responses of commercial strawberry cultivars under optimal and drought stress conditions. Plants 2023, 12, 496. [Google Scholar] [CrossRef]
- Mosa, W.F.A.E.G.; Paszt, L.S.; Frąc, M.; Trzciński, P.; Treder, W.; Klamkowski, K. The role of biofertilizers in improving vegetative growth, yield and fruit quality of apple. Hort. Sci. 2018, 45, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Goel, R.; Suyal, D.C.; Kumar, V.; Jain, L.; Soni, R. Stress-tolerant beneficial microbes for sustainable agricultural production. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Panpatte, D., Jhala, Y., Shelat, H., Vyas, R., Eds.; Springer: Singapore, 2018; Volume 7, pp. 141–159. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.J.; Peijnenburg, W.J.G.M.; Zhu, Y.C.; Zhang, M.; Sun, L.W.; Fu, Z.W.; Qian, H.F. Investigation of rhizospheric microbial communities in wheat, barley, and two rice varieties at the seedling stage. J. Agric. Food Chem. 2018, 66, 2645–2653. [Google Scholar] [CrossRef]
- Trzciński, P.; Frąc, M.; Lisek, A.; Przybył, M.; Frąc, M.; Sas-Paszt, L. Growth promotion of raspberry and strawberry plants by bacterial inoculants. Acta Sci. Pol. Hortorum Cultus 2021, 20, 71–82. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Gałązka, A.; Feledyn-Szewczyk, B.; Marzec-Grządziel, A.; Sas-Paszt, L.; Gryta, A.; Trzciński, P.; Zdunek, A.; Frąc, M. The use of interactions between microorganisms in strawberry cultivation (Fragaria × ananassa Duch.). Front. Plant Sci. 2021, 12, 780099. [Google Scholar] [CrossRef]
- Disi, J.O.; Mohammad, H.K.; Lawrence, K.; Kloepper, J.; Fadamiro, H. A soil bacterium can shape belowground interactions between maize, herbivores and entomopathogenic nematodes. Plant Soil 2019, 437, 83–92. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Jones, J.; Shantharaj, D.; Liles, M.R.; Kloepper, J.W. Pectin-rich amendment enhances soybean growth promotion and nodulation mediated by Bacillus velezensis strains. Plants 2019, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Duy, M.; Hoi, N.; Ve, N.; Thuc, L.; Trang, N. Influence of Cellulomonas flavigena, Azospirillum sp. and Psudomonas sp. on rice growth and yield grown in submerged soil amended in rice straw. In Recent Trends in PGPR Research for Sustainable Crop Productivity; Sayyed, R.Z., Reddy, M.S., Al-Turki, A.I., Eds.; Scientific Publishers: Jodhpur, India, 2016; pp. 238–242. [Google Scholar]
- Hossain, M.; Ran, C.; Liu, K.; Ryu, C.-M.; Rasmussen-Ivey, C.; Williams, M.; Hassan, M.; Choi, S.-K.; Jeong, H.; Newman, M.; et al. Deciphering the conserved genetic loci implicated in plant disease control through comparative genomics of Bacillus amyloliquefaciens subsp. plantarum. Front. Plant Sci. 2015, 6, 631. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, H.; Bagyaraj, D.; Selvakumar, G.; Sundaram, S. Novel plant growth promoting rhizobacteria—Prospects and potential. Appl. Soil Ecol. 2015, 95, 38–53. [Google Scholar] [CrossRef]
- Paliwoda, D.; Mikiciuk, G.; Mikiciuk, M.; Kisiel, A.; Sas-Paszt, L.; Miller, T. Effects of rhizosphere bacteria on strawberry plants (Fragaria × ananassa Duch.) under water deficit. Int. J. Mol. Sci. 2022, 23, 10449. [Google Scholar] [CrossRef]
- Burczyk, P.; Marciniak, A.M. Static Chamber for Measuring the Emission and Immission of Gases from the Soil Surface. Institute of Technology and Life Sciences—National Research Institute, Falenty (PL). Patent 224053, G01N 1/22 (2006.01), 8 December 2011. [Google Scholar]
- Burczyk, P.; Gamrat, R.; Gałczyńska, M. The use of the photoacoustic field gas monitor for measurement of the concentration of gases in measurements of dinitrogen oxide emission from grassland’s soils. Pol. J. Environ. Stud. 2008, 17, 105–108. [Google Scholar]
- Hewitt, C.N. Methods of Environmental Data Analysis; Springer: Dordrecht, The Netherlands, 1992. [Google Scholar] [CrossRef]
- Dorman, C. Environmental Data Analysis; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC, 2013): Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar] [CrossRef] [Green Version]
- EPA (U.S. Environmental Protection Agency). Inventory of US Greenhouse Gas Emissions and Sinks: 1990–2016. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2016 (accessed on 19 October 2022).
- Lynch, J.; Cain, M.; Pierrehumbert, R.; Allen, M. Demonstrating GWP*: A means of reporting warming-equivalent emissions that captures the contrasting impacts of short- and long-lived climate pollutants. Environ. Res. Lett. 2020, 15, 044023. [Google Scholar] [CrossRef]
- Szeląg-Sikora, A.; Niemiec, M.; Sikora, J.; Chowaniak, M. Possibilities of designating swards of grasses and small-seed legumes from selected organic farms in Poland for feed. In Proceedings of the IX International Scientific Symposium Farm Machinery and Processes Management in Sustainable Agriculture, Lublin, Poland, 22–24 November 2017. [Google Scholar] [CrossRef]
- Mohanty, S.; Swain, C.K. Role of microbes in climate smart agriculture. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Panpatte, D., Jhala, Y., Shelat, H., Vyas, R., Eds.; Springer: Singapore, 2018; pp. 129–140. [Google Scholar] [CrossRef]
- Sapkota, A.; Haghverdi, A.; Avila, C.C.E.; Ying, S.C. Irrigation and greenhouse gas emissions: A review of field-based studies. Soil Syst. 2020, 4, 20. [Google Scholar] [CrossRef]
- Norberg, L.; Hellman, M.; Berglund, K.; Hallin, S.; Berglund, Ö. Methane and nitrous oxide production from agricultural peat soils in relation to drainage level and abiotic and biotic factors. Front. Environ. Sci. 2021, 9, 631112. [Google Scholar] [CrossRef]
- Nie, T.; Huang, J.; Zhang, Z.; Chen, P.; Li, T.; Dai, C. The inhibitory effect of a water-saving irrigation regime on CH4 emission in Mollisols under straw incorporation for 5 consecutive years. Agric. Water Manag. 2023, 278, 108163. [Google Scholar] [CrossRef]
- Jhala, Y.K.; Vyas, R.V.; Shelat, H.N.; Patel, H.K.; Patel, H.K.; Patel, K.T. Isolation and characterization of methane utilizing bacteria from wetland paddy ecosystem. World J. Microbiol. Biotechnol. 2014, 30, 1845–1860. [Google Scholar] [CrossRef]
- Shakoor, A.; Xu, Y.; Wang, Q.; Chen, N.; He, F.; Zuo, H.; Yin, H.; Yan, X.; Ma, Y.; Yang, S. Effects of fertilizer application schemes and soil environmental factors on nitrous oxide emission fluxes in a rice-wheat cropping system, east China. PLoS ONE 2018, 13, e0202016. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Li, F.; Deng, A.; Feng, X.; Fang, F.; Zhang, W. Integrated assessment of the impact of enhanced-efficiency nitrogen fertilizer on N2O emission and crop yield. Agric. Ecosyst. Environ. 2016, 231, 218–228. [Google Scholar] [CrossRef]
- Kroeze, C.; Mosier, A.; Bouwman, L. Closing the global N2O budget: A retrospective analysis 1500-1994. Glob. Biogeochem. Cycles 1999, 13, 1–8. [Google Scholar] [CrossRef]
- Hu, H.W.; Trivedi, P.; He, J.Z.; Singh, B.K. Microbial nitrous oxide emissions in dryland ecosystems: Mechanisms, microbiome and mitigation. Environ. Microbiol. 2017, 19, 4808–4828. [Google Scholar] [CrossRef] [Green Version]
- Bakken, L.R.; Frostegård, Å. Sources and sinks for N2O, can microbiologist help to mitigate N2O emissions? Environ. Microbiol. 2017, 19, 4801–4805. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Wang, M.; Wang, Y.; Shen, R.; Gou, J.; Li, J.; Jin, J.; Li, L. Impacts of soil moisture on nitrous oxide emission from croplands: A case study on the rice-based agro-ecosystem in Southeast China. Chemosphere-Glob. Chang. Sci. 2000, 2, 207–224. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. Atmos. 2004, 109, D17302. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.A.; Putz, M.; Spor, A.; Bru, D.; Breuil, M.C.; Hallin, S.; Philippot, L. Non-denitrifying nitrous oxide-reducing bacteria—An effective N2O sink in soil. Soil Biol. Biochem. 2016, 103, 376–379. [Google Scholar] [CrossRef]
- Jones, C.M.; Graf, D.R.H.; Bru, D.; Philippot, L.; Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: A potential nitrous oxide sink. ISME J. 2013, 7, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Medhi, K.; Fagodiya, R.K.; Subrahmanyam, G.; Mondal, R.; Raja, P.; Malyan, S.K.; Gupta, D.K.; Gupta, C.K.; Pathak, H. Molecular and ecological perspectives of nitrous oxide producing microbial communities in agro-ecosystems. Rev. Environ. Sci. Biotechnol. 2020, 19, 717–750. [Google Scholar] [CrossRef]
- Bueno, E.; Mania, D.; Mesa, S.; Bedmar, E.J.; Frostegård, Å.; Bakken, L.R.; Delgado, M.J. Regulation of the emissions of the greenhouse gas nitrous oxide by the soybean endosymbiont Bradyrhizobium diazoefficiens. Int. J. Mol. Sci. 2022, 23, 1486. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Escolar, C.; Gallardo, A.; Ochoa, V.; Gozalo, B.; Prado-Comesaña, A. Direct and indirect impacts of climate change on microbial and biocrust communities alter the resistance of the N cycle in a semiarid grassland. J. Ecol. 2014, 102, 1592–1605. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Russo, G.; Melucci, F.M. Intensification in olive growing reduces global warming potential under both integrated and organic farming. Sustainability 2022, 14, 6389. [Google Scholar] [CrossRef]
- Core Writing Team; Pachauri, R.K.; Meyer, L.A. (Eds.) Intergovernmental Panel on Climate Change (IPCC, 2014): Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2015. [Google Scholar]
- Naser, H.M.; Nagata, O.; Sultana, S.; Hatano, R. Carbon sequestration and contribution of CO2, CH4 and N2O fluxes to global warming potential from paddy-fallow fields on mineral soil beneath peat in central Hokkaido, Japan. Agriculture 2020, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Clark, S. Organic farming and climate change: The need for innovation. Sustainability 2020, 12, 7012. [Google Scholar] [CrossRef]




| Gas | Statistic | DF Plant (+) * | OP Plant (+) | DF Plant (−) | OP Plant (−) |
|---|---|---|---|---|---|
| CO2 | Mean ± SD | 1921 ± 185 | 2096 ± 189 | 1886 ± 107 | 1953 ± 126 |
| Range | 1777–2899 | 1748–2619 | 1724–2211 | 1765–2426 | |
| CV | 10% | 9% | 6% | 6% | |
| N2O | Mean ± SD | 2.32 ± 0.21 | 2.29 ± 0.2 | 2.07 ± 0.18 | 2.35 ± 0.13 |
| Range | 2.01–3.11 | 1.94–2.97 | 1.78–2.83 | 2.14–2.85 | |
| CV | 9% | 9% | 9% | 5% | |
| CH4 | Mean ± SD | 4.2 ± 2.09 | 6.44 ± 3.49 | 5.48 ± 2.41 | 4.94 ± 1.69 |
| Range | 1.45–10.64 | 0–19.68 | 2.8–11.53 | 2.3–9.22 | |
| CV | 50% | 54% | 44% | 34% | |
| NH3 | Mean ± SD | 0.56 ± 0.07 | 0.54 ± 0.11 | 0.5 ± 0.08 | 0.56 ± 0.08 |
| Range | 0.43–0.85 | 0.24–0.85 | 0.37–0.77 | 0.37–0.76 | |
| CV | 13% | 20% | 15% | 15% | |
| GWP | Mean ± SD | 797.1 ± 89.4 | 844.5 ± 104.7 | 754.8 ± 108.3 | 824.7 ± 45.7 |
| Range | 691–1073 | 645–1131 | 615–1132 | 758–997 | |
| CV | 24% | 28% | 19% | 15% |
| Variable | Sum of Squares | df | Mean Square Error | F | p |
|---|---|---|---|---|---|
| CO2 | 2,146,922 | 3 | 715,640.6 | 29.43622 | >0.001 |
| N2O | 4 | 3 | 1.4 | 40.02875 | >0.001 |
| CH4 | 224 | 3 | 74.7 | 11.83576 | >0.001 |
| NH3 | 0 | 3 | 0.1 | 8.89481 | >0.001 |
| C0 | 38.7 | 3.0 | 12.9 | 4.792 | 0.003 |
| CMg | 35.5 | 3.0 | 11.8 | 17.352 | >0.001 |
| DLGB2 | 18.2 | 3.0 | 6.1 | 18.168 | >0.001 |
| DLGB3 | 21.9 | 3.0 | 7.3 | 30.772 | >0.001 |
| DKB26 | 4.8 | 3.0 | 1.6 | 5.410 | 0.002 |
| DKB58 | 15.5 | 3.0 | 5.2 | 9.873 | >0.001 |
| DKB84 | 3.5 | 3.0 | 1.2 | 2.381 | 0.073 |
| AJ 1.2 | 8.9 | 3.0 | 3.0 | 7.891 | >0.001 |
| DKB64 | 3.8 | 3.0 | 1.3 | 4.146 | 0.008 |
| DKB65 | 8.4 | 3.0 | 2.8 | 3.710 | 0.013 |
| DKB70 | 14.0 | 3.0 | 4.7 | 7.891 | >0.001 |
| DKB63 | 11.3 | 3.0 | 3.8 | 13.676 | >0.001 |
| DKB68 | 0.6 | 3.0 | 0.2 | 0.529 | 0.663 |
| PJ 1.1 | 9.6 | 3.0 | 3.2 | 2.638 | 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paliwoda, D.; Mikiciuk, G.; Mikiciuk, M.; Miller, T.; Kisiel, A.; Sas-Paszt, L.; Kozioł, A.; Brysiewicz, A. The Use of Plant Growth Promoting Rhizobacteria to Reduce Greenhouse Gases in Strawberry Cultivation under Different Soil Moisture Conditions. Agronomy 2023, 13, 754. https://doi.org/10.3390/agronomy13030754
Paliwoda D, Mikiciuk G, Mikiciuk M, Miller T, Kisiel A, Sas-Paszt L, Kozioł A, Brysiewicz A. The Use of Plant Growth Promoting Rhizobacteria to Reduce Greenhouse Gases in Strawberry Cultivation under Different Soil Moisture Conditions. Agronomy. 2023; 13(3):754. https://doi.org/10.3390/agronomy13030754
Chicago/Turabian StylePaliwoda, Dominika, Grzegorz Mikiciuk, Małgorzata Mikiciuk, Tymoteusz Miller, Anna Kisiel, Lidia Sas-Paszt, Agnieszka Kozioł, and Adam Brysiewicz. 2023. "The Use of Plant Growth Promoting Rhizobacteria to Reduce Greenhouse Gases in Strawberry Cultivation under Different Soil Moisture Conditions" Agronomy 13, no. 3: 754. https://doi.org/10.3390/agronomy13030754






