Antioxidant Potentials of Different Genotypes of Cowpea (Vigna unguiculata L. Walp.) Cultivated in Bulgaria, Southern Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sampling and Extract Preparation
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Determination of Radical Scavenging Capacity via the DPPH Method
2.6. Determination of Crude Protein Content via the Kjeldahl Method
2.7. Statistical Analysis
3. Results and Discussion
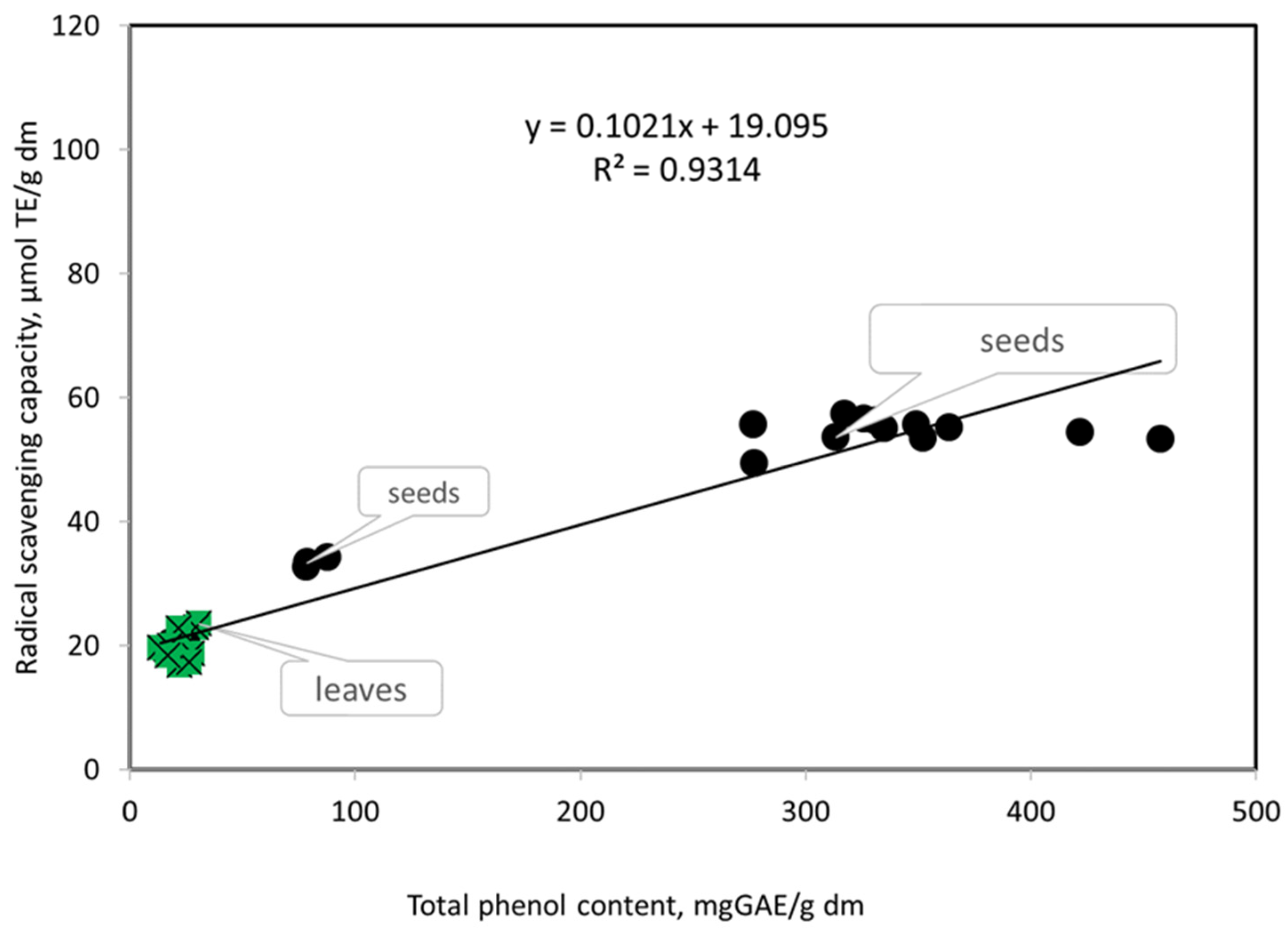
3.1. Antioxidant Potentials of Extracts Prepared from the Leaves of the Studied Genotypes of Vigna unguiculata L.
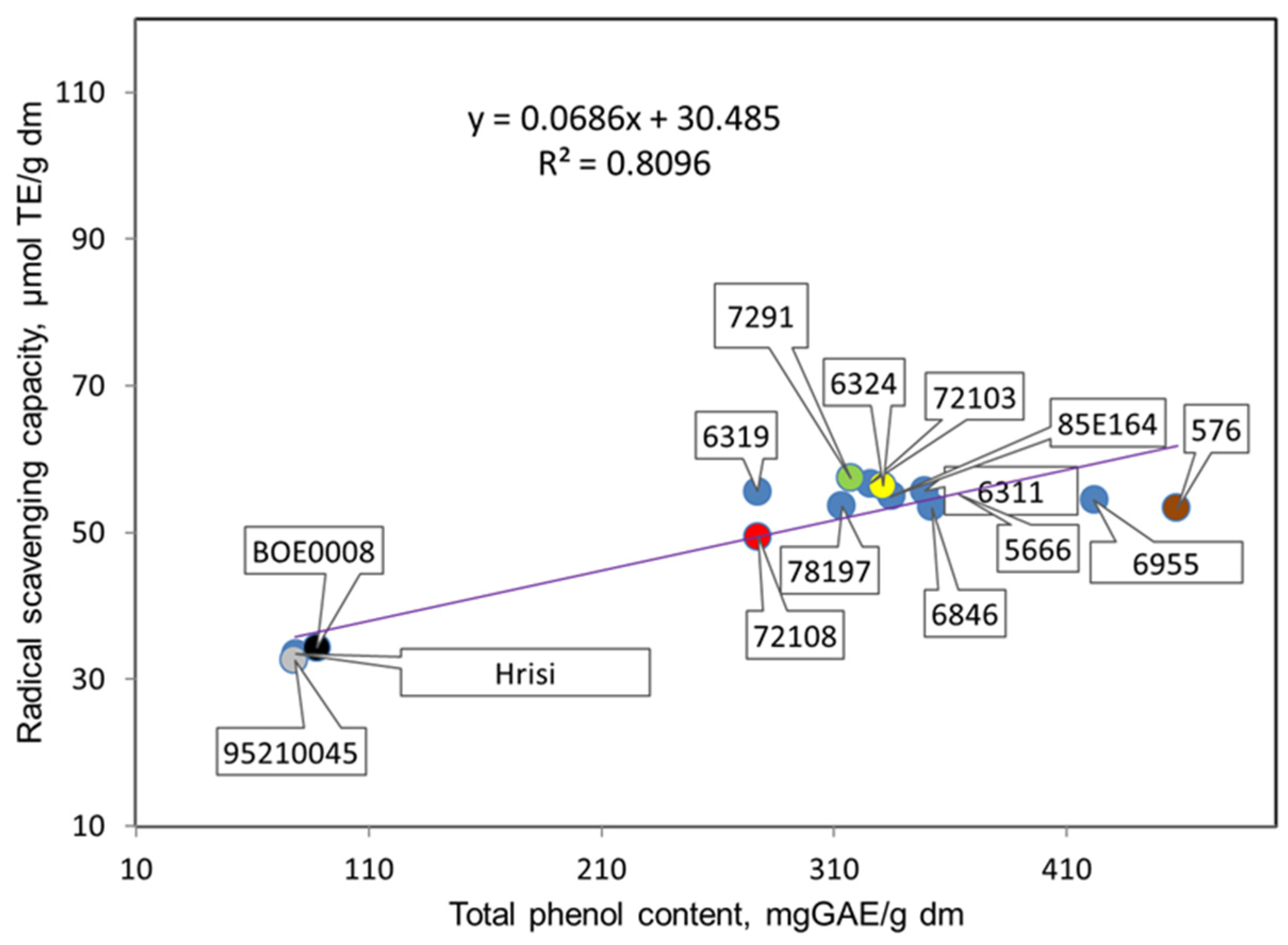
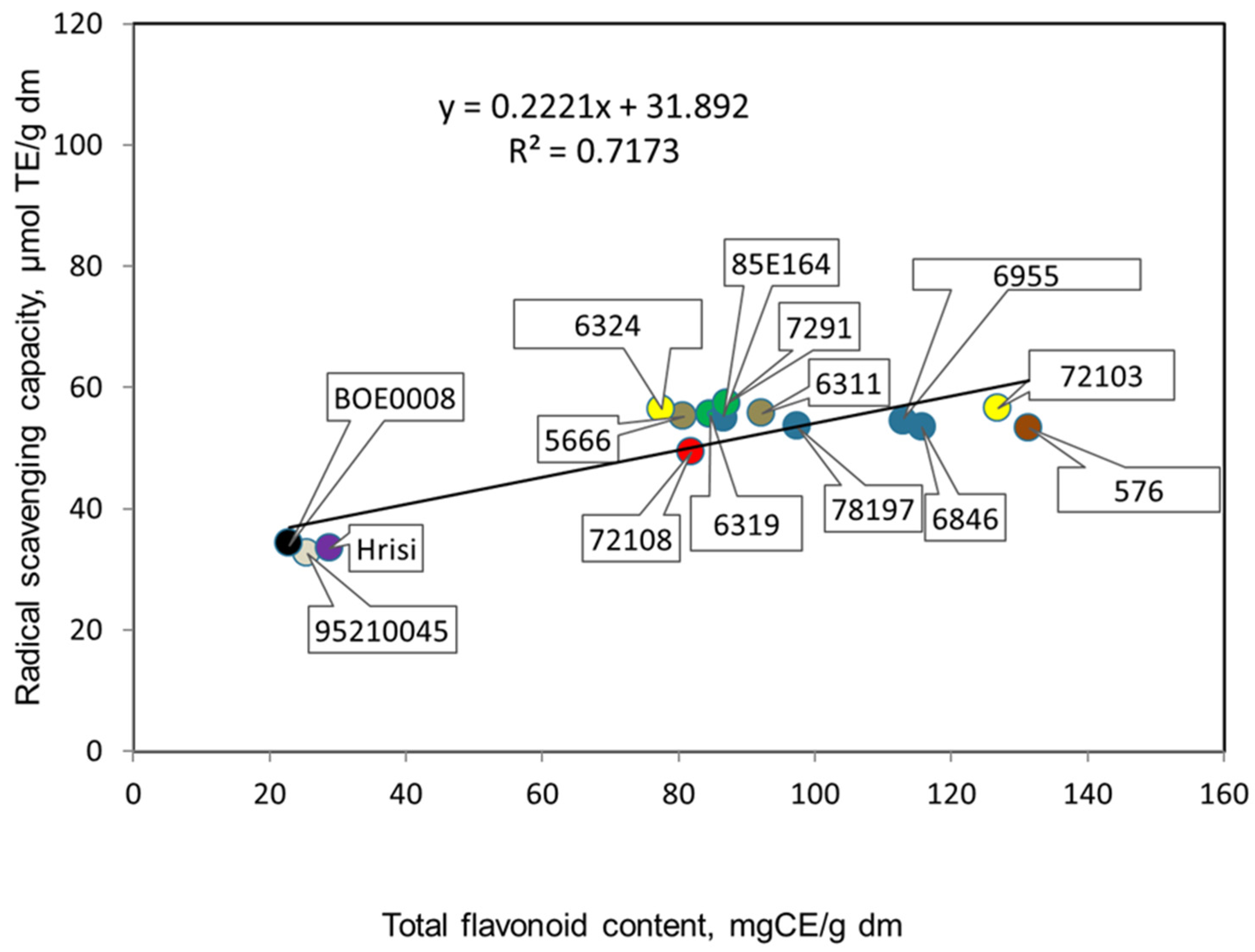
3.2. Antioxidant Potentials of Extracts Prepared from Seeds of the Studied Genotypes of V. Unguiculata L.
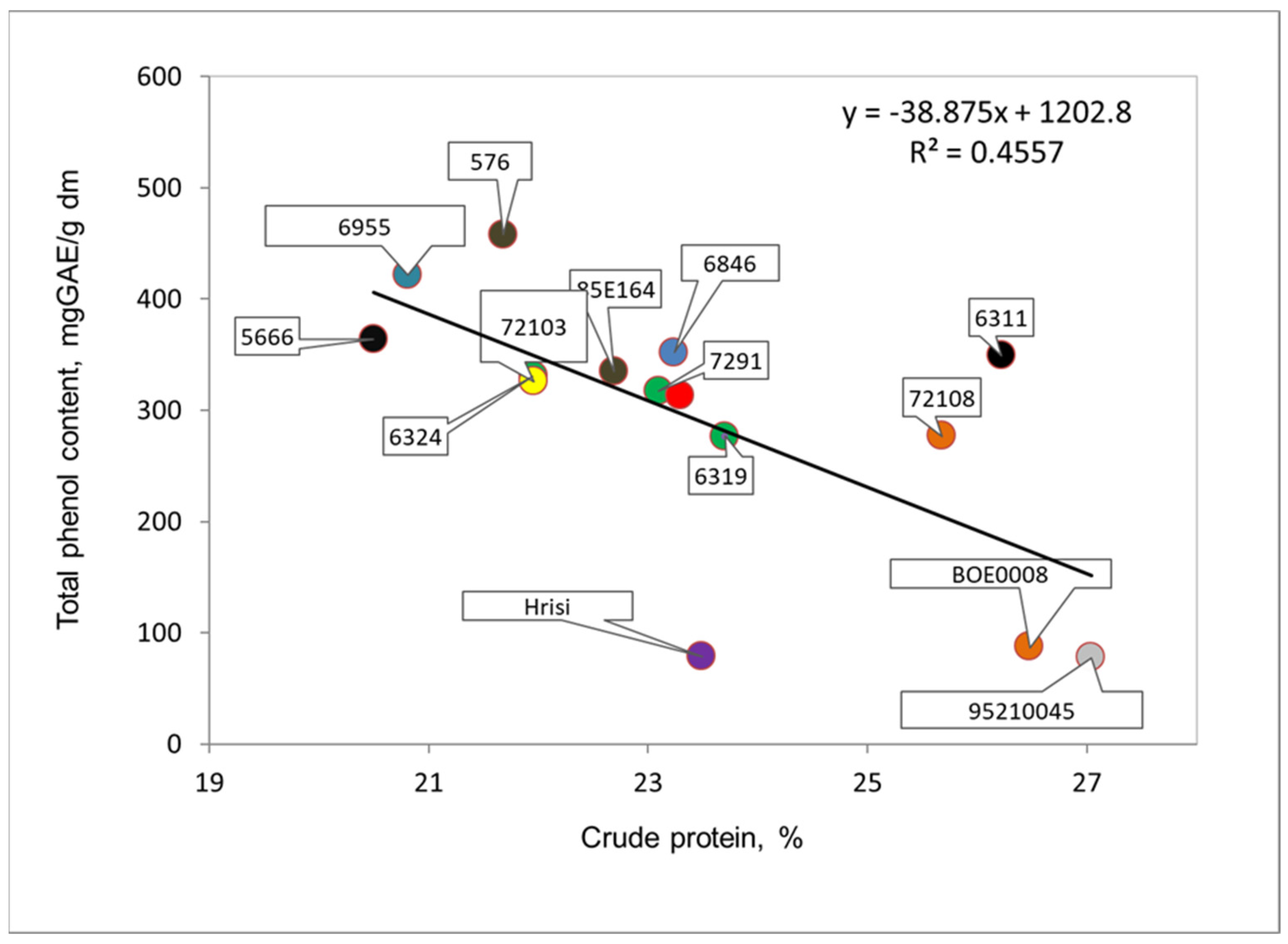
3.3. Crude Protein Content of Seeds of the Studied Cowpea Genotypes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abebe, K.B.; Alemayehu, T.M. A review of the nutritional use of cowpea (Vigna unguiculata L. Walp) for human and animal diets. J. Agric. Food Res. 2022, 10, 100383. [Google Scholar] [CrossRef]
- Hall, E.A.; Cissé, N.; Thiaw, S.; Elawad, H.O.A.; Ehlers, J.D.; Ismail, A.M.; Fery, R.L.; Roberts, P.A.; Kitch, L.W.; Murdock, L.L.; et al. Development of cowpea cultivars and germplasm by the bean/cowpea CRSP. Field Crops Res. 2003, 82, 103–134. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Maia, F.M.M.; Farias, D.F.; Campello, C.C.; Carvalho, A.F.O.; Moreira, R.A.; de Oliveira, J.T.A. Protein fractions, amino acid composition and antinutritional constituents of high-yielding cowpea cultivars. J. Food Compos. Anal. 2010, 23, 54–60. [Google Scholar] [CrossRef]
- Stoilova, T. Cowpea (V. unguiculata L.)—Origin, distribution, characteristic and use. Plant Sci. 2013, 50, 87–90. [Google Scholar]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agrifood system: Nutritional advantages and constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef]
- Xiong, S.; Yao, X.; Li, A. Antioxidant Properties of Peptide from Cowpea Seed. Int. J. Food Prop. 2013, 16, 1245–1256. [Google Scholar] [CrossRef]
- Antova, G.A.; Stoilova, T.D.; Ivanova, M.M. Proximate and lipid composition of cowpea (Vigna unguiculata L.) cultivated in Bulgaria. J. Food Compos. Anal. 2014, 33, 146–152. [Google Scholar] [CrossRef]
- Sosulski, F.W.; Dabrowski, K.J. Composition of free and hydrolyzable phenolic acids in the flours and hulls of ten legume species. J. Agric. Food Chem. 1984, 32, 131–133. [Google Scholar] [CrossRef]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of broad bean seed extract and its phenolic composition. J. Funct. Foods 2017, 38, 656–662. [Google Scholar] [CrossRef]
- Gan, R.Y.; Deng, Z.Q.; Yan, A.X.; Shah, N.P.; Lui, W.Y.; Chan, C.L.; Corke, H. Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. LWT-Food Sci. Technol. 2016, 73, 168–177. [Google Scholar] [CrossRef]
- Liyanage, R.; Perera, O.S.; Weththasinghe, P.; Jayawardana, B.C.; Vidanaarachchi, J.K.; Sivakanesan, R. Nutritional properties and antioxidant content of commonly consumed cowpea cultivars in Sri Lanka. J. Food Legum. 2014, 27, 215–217. [Google Scholar]
- El-Mergawi, R.; Taie, H.A.A. Phenolic composition and antioxidant activity of raw seeds, green seeds and sprouts of ten faba bean (Vicia faba) cultivars consumed in Egypt. Int. J. Pharm. Biol. Sci. 2014, 5, 609–617. [Google Scholar]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of Phenolic Compounds, Carotenoids, Amino Acid Composition, In Vitro Antioxidant and Anti-Diabetic Activities in the Leaves of Seven Cowpea (Vigna unguiculata). Cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, G.V.; Sedibe, M.M.; Mofokeng, M.A. Phenotyping cowpea accessions at the seedling stage for drought tolerance in controlled environments. Open Agric. 2022, 7, 433–444. [Google Scholar] [CrossRef]
- Ravelombola, W.; Shi, A.; Chen, S.; Xiong, H.; Yang, Y.; Cui, Q.; Olaoye, D.; Mou, B. Evaluation of cowpea for drought tolerance at seedling stage. Euphytica 2020, 216, 123. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Tzanova, M.T.; Gerdzhikova, M.A.; Grozeva, N.H.; Terzieva, S.R. Antioxidant Activity and Total Phenolic Content of Five Salvia Species from Bulgaria. Bulg. Chem. Commun. 2019, 51, 90–94. [Google Scholar]
- Dinev, T.; Tzanova, M.; Velichkova, K.; Dermendzhieva, D.; Beev, G. Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus calamus L.; Chlorella vulgaris Beijerinck, Lemna minuta Kunth and Scenedesmus dimorphus (Turpin) Kützing. Appl. Sci. 2021, 11, 4745. [Google Scholar] [CrossRef]
- ISO 5983-1:2005; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 1: Kjeldahl Method. Technical Committee: Geneva, Switzerland, 2005.
- Gutiérrez-Uribe, J.A.; Romo-Lopez, I.; Serna-Saldívar, S.O. Phenolic composition and mammary cancer cell inhibition of extracts of whole cowpeas (Vigna unguiculata) and its anatomical parts. J. Funct. Foods 2011, 3, 290–297. [Google Scholar] [CrossRef]
- Sombié, P.A.E.D.; Compaoré, M.; Coulibaly, A.Y.; Ouédraogo, J.T.; Tignégré, J.-B.D.L.S.; Kiendrébéogo, M. Antioxidant and Phytochemical Studies of 31 Cowpeas (Vigna unguiculata (L. Walp.)) Genotypes from Burkina Faso. Foods 2018, 7, 143. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Phenolic compounds in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chem. 2012, 133, 156–162. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Banerjee, N.; Noratto, G.D.; Angel-Morales, G.; Hachibamba, T.; Awika, J.M.; Mertens-Talcott, S.U. Polyphenolic extracts from cowpea (Vigna unguiculata) protect colonic myofibroblasts (CCD18Co cells) from lipopolysaccharide (LPS)-induced inflammation–modulation of microRNA 126. Food Funct. 2015, 6, 145–153. [Google Scholar] [CrossRef]
- Okafor, J.N.C.; Meyer, M.; Le Roes-Hill, M.; Jideani, V.A. Flavonoid and Phenolic Acid Profiles of Dehulled and Whole Vigna subterranea (L.) Verdc Seeds Commonly Consumed in South Africa. Molecules 2022, 27, 5265. [Google Scholar] [CrossRef]
- Salawu, S.O.; Bester, M.J.; Duodu, K.G. Phenolic composition and bioactive properties of cell wall preparations and whole grains of selected cereals and legumes. J. Food Biochem. 2014, 38, 62–72. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Yang, L.Y.; Dykes, L.; Awika, J.M. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Dykes, L.; Awika, J.M. Ultra performance liquid chromatography-tandem quadrupole mass spectrometry profiling of anthocyanins and flavonols in Cowpea (Vigna unguiculata) of varying genotypes. J. Agric. Food Chem. 2012, 60, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Nderitu, A.M.; Dykes, L.; Awika, J.M.; Minnaar, A.; Duodu, K.G. Phenolic composition and inhibitory effect against oxidative DNA damage of cooked cowpeas as affected by simulated in vitro gastrointestinal digestion. Food Chem. 2013, 141, 1763–1771. [Google Scholar] [CrossRef]
- Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive Applications of Polyphenols in Dentistry—A Review. Int. J. Mol. Sci. 2021, 22, 4892. [Google Scholar] [CrossRef]
- Dicko, M.H.; Gruppen, H.; Barro, C.; van Berkel, W.J.H.; Voragen, A.G.J. Impact of phenolics and related enzymes in sorghum varieties for the resistance and susceptibility to biotic and abiotic stresses. J. Chem. Ecol. 2005, 31, 2671–2688. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.H.; Wan, X.S.; Newberne, P.; Kennedy, A.R. Bowman-birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig. Dis. Sci. 1999, 44, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Thumbrain, D.; Dwarka, D.; Gerrano, A.S.; Mellem, J.J. Antioxidant and apoptotic potential of protein isolates derived from Vigna unguiculata (L.) Walp. Int. J. Food Sci. Technol. 2020, 55, 2813–2823. [Google Scholar] [CrossRef]
- Pusztai, A.; Grant, G.; Buchan, W.C.; Bardocz, S.; de Carvalho, A.F.F.U.; Ewen, S.W.B. Lipid accumulation in obese Zucker rats is reduced by inclusion of raw kidney bean (Phaseolus vulgaris) in the diet. Br. J. Nutr. 1998, 79, 213–221. [Google Scholar] [CrossRef] [PubMed]


| No | Catalogue No. | Origin | Color of Flower | Plant Habit/Type | Color of Seeds | Shape of Seed |
|---|---|---|---|---|---|---|
| 1 | 6311 | Bulgaria | purple | erect | black | rhomboid |
| 2 | 6319 | Bulgaria | purple | erect | beige | kidney-shaped |
| 3 | 6955 | Bulgaria | purple | semi erect | beige with brown hilum | rhomboid |
| 4 | 5666 | Bulgaria | purple | erect | brown | egg-shaped |
| 5 | 7291 | Bulgaria | purple | erect | gray with brown trim | egg-shaped |
| 6 | 72108 | Bulgaria | purple | prostrate | gray with brown trim | egg-shaped |
| 7 | 85E164 | Bulgaria | purple | erect | brown | kidney-shaped |
| 8 | 576 | Bulgaria | white | semi erect | brown | rhomboid |
| 9 | 72103 | Bulgaria | purple | semi erect | beige | egg-shaped |
| 10 | 6846 | Bulgaria | purple | prostrate | beige | kidney-shaped |
| 11 | 78197 | Bulgaria | purple | prostrate | gray | egg-shaped |
| 12 | 6324 | Bulgaria | purple | erect | beige with black hilum | kidney-shaped |
| 13 | 95210045 | Turkey | white | semi erect | white with brown hilum | kidney-shaped |
| 14 | Hrisi | Sadovo, Bulgaria | white | erect | white with black hilum | kidney-shaped |
| 15 | BOE0008 | Petrich, Bulgaria | white | erect | white with black hilum | egg-shaped |
| Genotype, Catalogue No. | Color of Seeds | Total Phenolic Content, Mg GAE/g dm | Total Flavonoid Content, Mg CE/g dm | Radical Scavenging Capacity, µmol TE/g dm |
|---|---|---|---|---|
| 6311 | black | 31.0 ± 0.5 * a ** | 17.4 ± 0.9 a | 23.6 ±1.3 a |
| 6319 | beige | 29.3 ± 0.4 a | 18.4 ± 0.5 a | 22.9 ± 0.5 a |
| 6955 | beige with brown hilum | 24.3 ± 0.4 a | 14.7 ± 0.7 a | 17.3 ± 0.5 a |
| 5666 | brown | 27.6 ± 0.9 a,c | 17.9 ± 0.3 b,c | 18.7 ± 1.7 a |
| 7291 | gray with brown trim | 21.8 ± 0.4 b,c | 14.8 ± 0.3 a,c | 22.7 ± 0.5 a |
| 72108 | gray with brown trim | 25.5 ± 0.6 a | 16.6 ± 0.4 a | 20.2 ± 0.6 a |
| 85E164 | brown | 22.3 ± 0.5 a | 12.2 ± 0.7 a | 16.9 ± 0.25 a |
| 576 | brown | 26.7 ± 0.6 a,c | 16.7 ± 0.6 b,c | 17.3 ± 0.4 a |
| 72103 | beige | 21.9 ± 0.3 a | 12.5 ± 0.6 a | 20.3 ± 0.5 a |
| 6846 | beige | 13.4 ± 0.5 a,b | 13.4 ± 0.6 a,b | 19.6 ± 0.4 a |
| 78197 | gray | 18.0 ± 0.3 a | 11.3 ± 0.4 a | 19.9 ± 0.3 a |
| 6324 | beige with black hilum | 15.2 ± 0.7 a | 9.4 ± 0.5 a | 19.0 ± 0.4 a |
| 95210045 | white with brown hilum | 17.2 ± 0.5 b,c | 9.3 ± 0.4 a,c | 18.6 ± 0.9 a |
| Hrisi | white with black hilum | 17.1 ± 0.4 b,c | 9.8 ± 0.7 a,c | 18.4 ± 0.7 a |
| BOE0008 | white with black hilum | 18.0 ± 0.4 a | 11.3 ± 0.6 a | 20.5 ± 0.4 a |
| min | 13.4 ± 0.5 | 9.3 ± 0.4 | 16.9 ± 0.3 | |
| max | 31.0 ± 0.5 | 18.4 ± 0.5 | 23.6 ± 1.2 | |
| mean * | color of seed | 22.0 ± 0.5 a | 13.7 ± 0.5 a | 19.7 ± 0.6 a |
| Variables | Radical Scavenging Capacity, µmol TE/g dm * | TPC, mgGAE/g dm * | TFC, mgCE/g dm * | Radical Scavenging Capacity, µmol TE/g dm ** | TPC, mgGAE/g dm ** | TFC, mgCE/g dm ** | Protein, % ** |
|---|---|---|---|---|---|---|---|
| Radical scavenging capacity, µmol TE/g dm * | 1 | 0.322 | 0.377 | 0.209 | −0.084 | −0.021 | 0.429 |
| TPC, mgGAE/g dm * | 0.322 | 1 | 0.873 | 0.418 | 0.432 | 0.328 | −0.085 |
| TFC, mgCE/g dm * | 0.377 | 0.873 | 1 | 0.535 | 0.562 | 0.480 | −0.176 |
| Radical scavenging capacity, µmol TE/g dm ** | 0.209 | 0.418 | 0.535 | 1 | 0.900 | 0.847 | −0.601 |
| TPC, mgGAE/g dm ** | −0.084 | 0.432 | 0.562 | 0.900 | 1 | 0.921 | −0.675 |
| TFC, mgCE/g dm ** | −0.021 | 0.328 | 0.480 | 0.847 | 0.921 | 1 | −0.606 |
| Protein, % ** | 0.429 | −0.085 | −0.176 | −0.601 | −0.675 | −0.606 | 1 |
| Genotype, Catalogue No. | Color of Seeds | Total Phenolic Content, Mg GAE/g dm * | Total Flavonoid Content, Mg CE/g dm * | Radical Scavenging Capacity, µmol TE/g dm * | Protein, % * |
|---|---|---|---|---|---|
| 6311 | black | 349.0 ± 5.2 * a ** | 92.3 ± 0.4 a | 55.7 ± 0.4 a | 26.2 ± 2.1 a |
| 6319 | beige | 277.0 ± 3.0 a | 84.7 ± 0.5 a | 55.6 ± 0.6 a | 23.7 ± 1.8 a |
| 6955 | beige with brown hilum | 421.7 ± 3.5 a | 113.0 ± 1.9 a | 54.4 ± 0.3 a | 20.8 ± 1.6 a |
| 5666 | brown | 363.8 ± 7.2 a | 80.7 ± 0.9 a | 55.2 ± 0.4 a | 20.5 ± 1.4 a |
| 7291 | gray with brown trim | 317.3 ± 4.8 a | 87.0 ± 0.8 a | 57.4 ± 0.8 a | 23.1 ± 1.8 a |
| 72108 | gray with brown trim | 277.2 ± 7.8 a | 81.8 ± 0.4 a | 49.4 ± 0.9 a | 25.7 ± 2.0 a |
| 85E164 | brown | 334.8 ± 6.5 a | 86.6 ± 0.4 a | 55.0 ± 0.6 a | 22.7 ± 1.4 a |
| 576 | brown | 457.5 ± 5.2 a | 131.4 ± 1.9 a | 53.3 ± 0.7 a | 21.7 ± 1.5 a |
| 72103 | beige | 325.8 ± 4.3 a | 126.9 ± 2.7 a | 56.6 ± 0.4 a | 22.0 ± 1.6 a |
| 6846 | beige | 352.0 ± 6.5 a | 115.7 ± 2.3 a | 53.5 ± 0.7 a | 23.2 ± 1.7 a |
| 78197 | gray | 313.4 ± 5.4 a | 97.3 ± 0.9 a | 53.7 ± 0.4 a | 23.3 ± 1.7 a |
| 6324 | beige with black hilum | 331.0 ± 6.0 a,b | 77.5 ± 0.7 b | 56.3 ± 0.4 a | 22.0 ± 1.5 b,c |
| 95210045 | white with brown hilum | 78.2 ± 0.4 a,b | 25.5 ± 0.9 b | 32.6 ± 1.3 a | 27.0 ± 2.2 c |
| Hrisi | white with black hilum | 78.8 ± 0.6 a,d | 28.8 ± 1.0 b,a | 33.5 ± 1.2 a | 23.5 ± 1.7 c |
| BOE0008 | white with black hilum | 87.8 ± 1.9 c,a,b | 22.8 ± 0.7 b,d | 34.2 ± 1.0 a | 26.5 ± 2.1 c |
| min | 78.2 ± 0.4 | 22.8 ± 0.7 | 32.6 ± 1.3 | 20.5 ± 1.4 | |
| max | 457.5 ± 5.2 | 131.4 ± 1.9 | 57.4 ± 0.8 | 27.0 ± 2.2 | |
| mean * | Color of seed | 291.0 ± 4.6 a | 83.5 ± 1.1 a | 50.4 ± 0.7 a | 23.5 ± 1.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzanova, M.T.; Stoilova, T.D.; Todorova, M.H.; Memdueva, N.Y.; Gerdzhikova, M.A.; Grozeva, N.H. Antioxidant Potentials of Different Genotypes of Cowpea (Vigna unguiculata L. Walp.) Cultivated in Bulgaria, Southern Europe. Agronomy 2023, 13, 1684. https://doi.org/10.3390/agronomy13071684
Tzanova MT, Stoilova TD, Todorova MH, Memdueva NY, Gerdzhikova MA, Grozeva NH. Antioxidant Potentials of Different Genotypes of Cowpea (Vigna unguiculata L. Walp.) Cultivated in Bulgaria, Southern Europe. Agronomy. 2023; 13(7):1684. https://doi.org/10.3390/agronomy13071684
Chicago/Turabian StyleTzanova, Milena Tankova, Tsvetelina Dimitrova Stoilova, Mima Hristova Todorova, Neli Yovcheva Memdueva, Maria Asenova Gerdzhikova, and Neli Hristova Grozeva. 2023. "Antioxidant Potentials of Different Genotypes of Cowpea (Vigna unguiculata L. Walp.) Cultivated in Bulgaria, Southern Europe" Agronomy 13, no. 7: 1684. https://doi.org/10.3390/agronomy13071684
APA StyleTzanova, M. T., Stoilova, T. D., Todorova, M. H., Memdueva, N. Y., Gerdzhikova, M. A., & Grozeva, N. H. (2023). Antioxidant Potentials of Different Genotypes of Cowpea (Vigna unguiculata L. Walp.) Cultivated in Bulgaria, Southern Europe. Agronomy, 13(7), 1684. https://doi.org/10.3390/agronomy13071684








