Enhanced Primary Productivity in Fenced Desert Grasslands of China through Mowing and Vegetation Cover Interaction
Abstract
1. Introduction
2. Materials and Methods
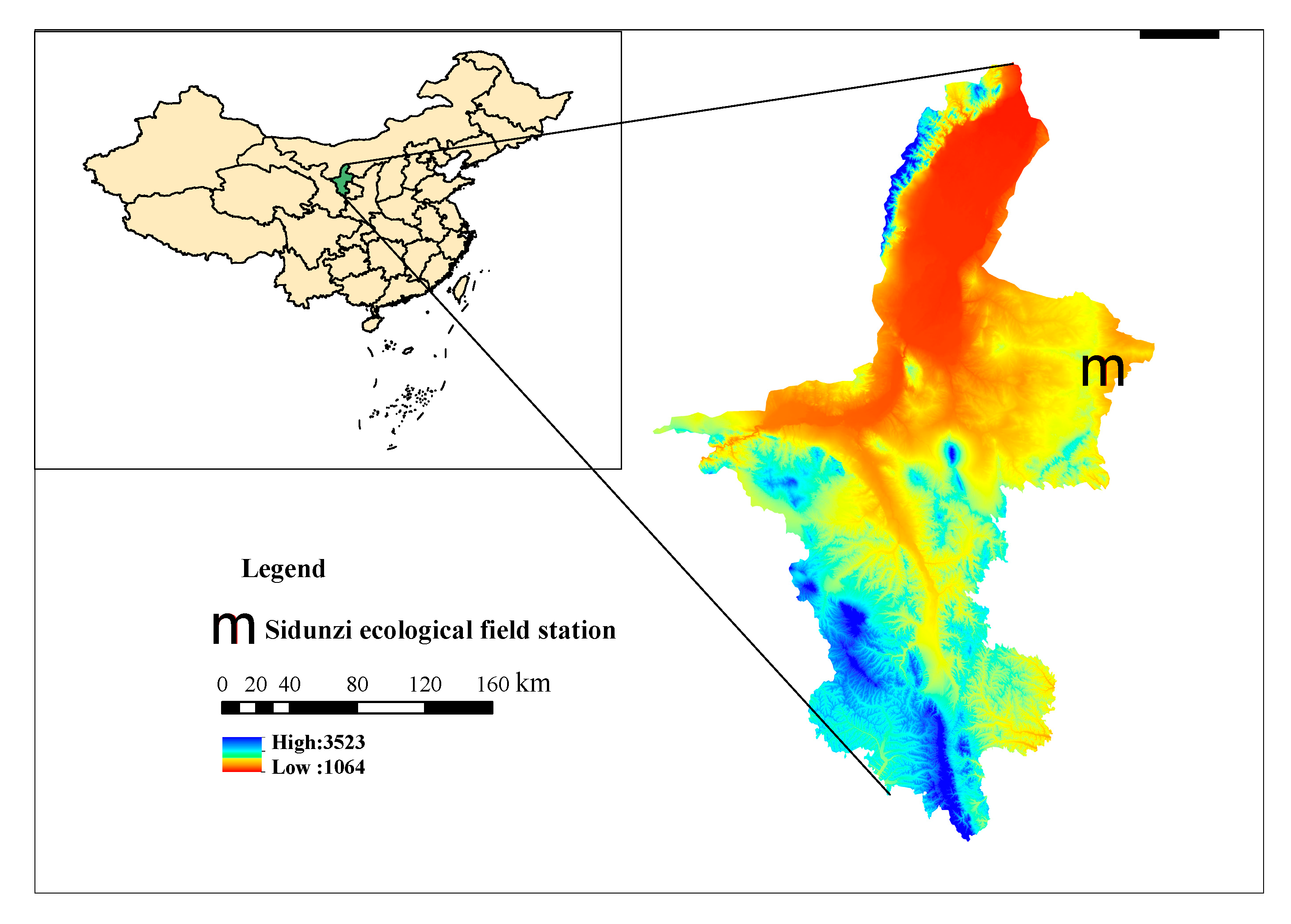
2.1. Study Site
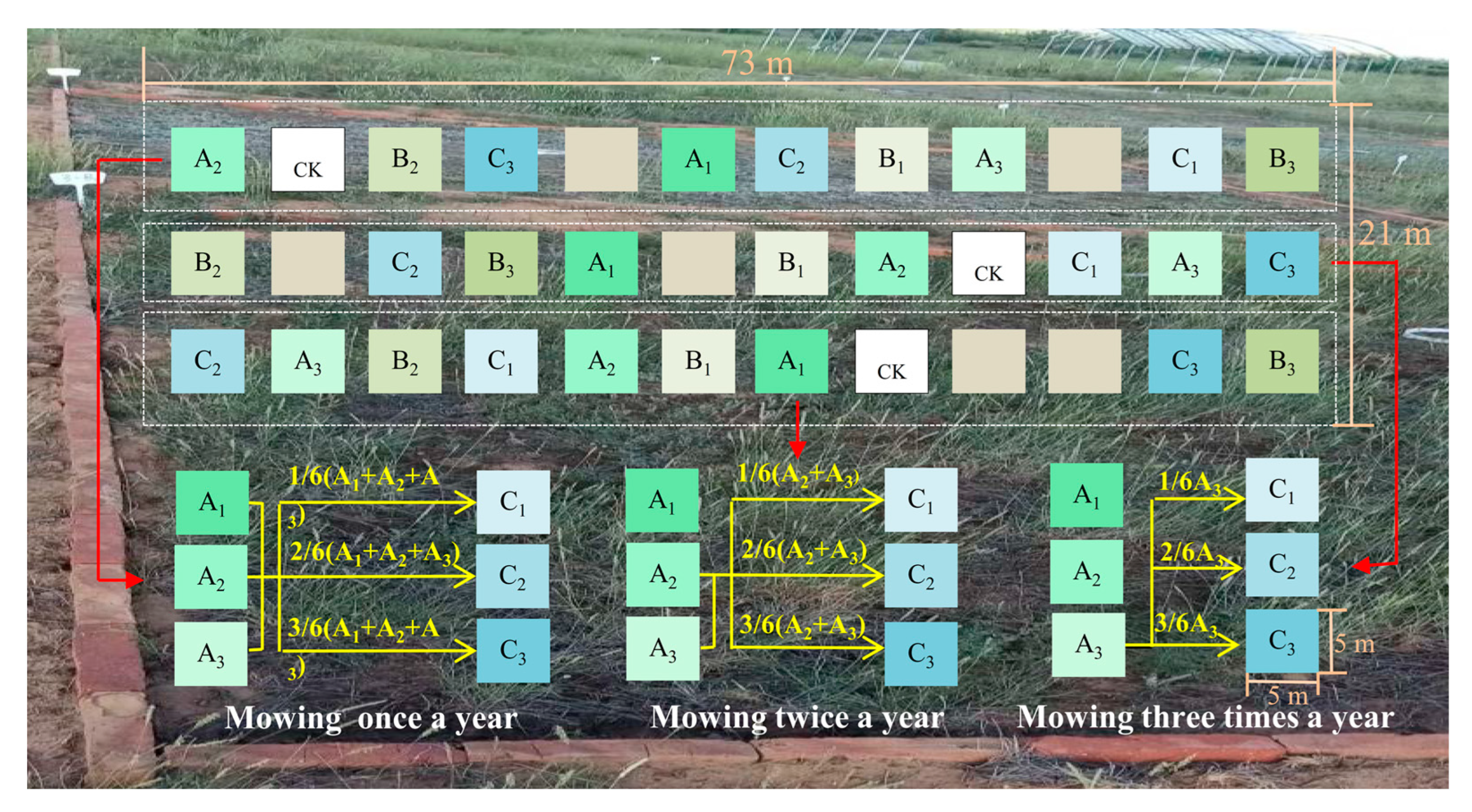
2.2. Experimental Design
2.3. Data Collection and Analysis
2.4. Statistical Analysis
3. Results
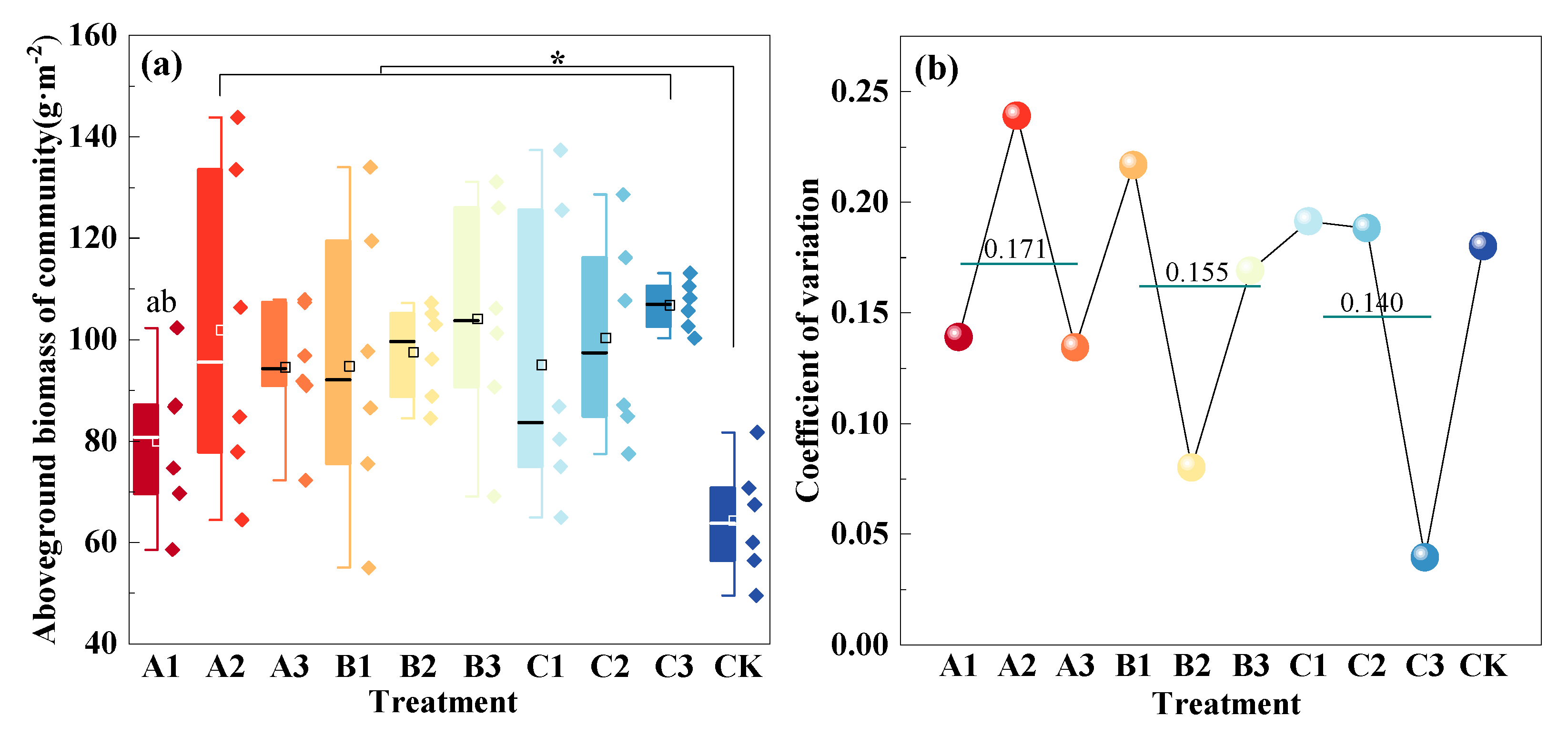
3.1. Community Aboveground Productivity with Mowing and Covering
3.2. Relationship between Species Diversity and Biomass under Mowing and Covering
3.3. Compensation Effects of Mowing and Covering
3.4. Compensability of Community Productivity of Mowing and Covering
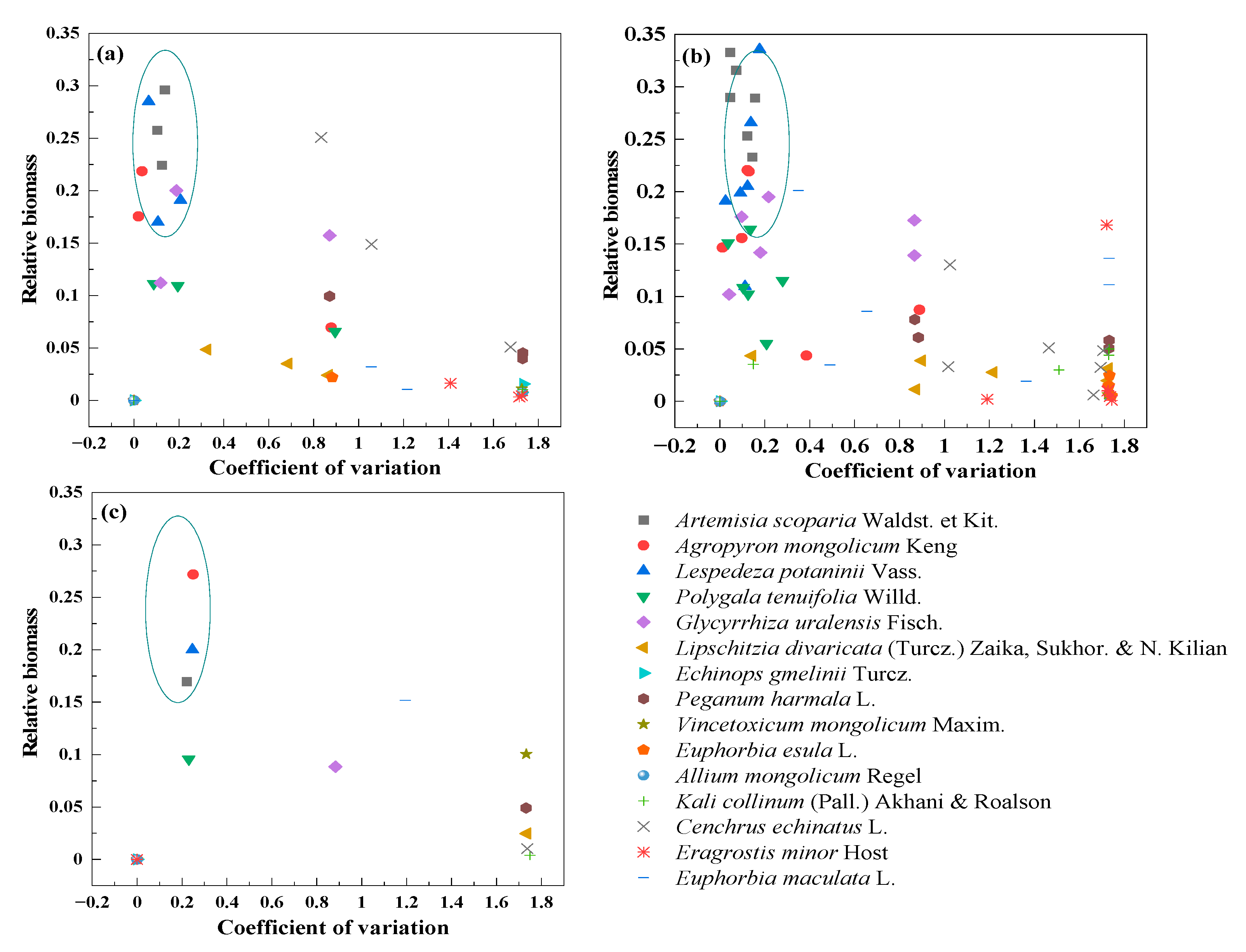
3.5. Compensation of Dominant and Sub-Dominant Species with Mowing and Covering
3.6. Compensation between Dominant and Other Plant Species with Mowing and Covering
3.7. Compensability between Functional Plant Groups with Mowing and Covering
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.B.; Zamanian, K.; Schleuss, P.M.; Zarebanadkouki, M.; Kuzyakov, Y. Degradation of Tibetan grasslands: Consequences for carbon and nutrient cycles. Agric. Ecosyst. Environ. 2018, 252, 93–104. [Google Scholar] [CrossRef]
- Mcsherry, M.E.; Ritchie, M.E. Effects of grazing on grassland soil carbon: A global review. Glob. Chang. Biol. 2013, 19, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Reinermann, S.; Asam, S.; Kuenzer, C. Remote Sensing of Grassland Production and Management-A Review. Remote Sens. 2020, 12, 1949. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.; Sun, W.; Zhu, J.; Liu, G.; Zhou, W.; Zhang, Q.; Shi, P.; Liu, X.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Du, G.Z.; Liu, Z.H.; Thirgood, S. Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau. Plant Soil. 2009, 319, 115–126. [Google Scholar] [CrossRef]
- Yao, X.X.; Wu, J.P.; Gong, X.Y.; Lang, X.; Wang, C.L.; Song, S.Z.; Ahmad, A.A. Effects of long term fencing on biomass, coverage, density, biodiversity and nutritional values of vegetation community in an alpine meadow of the Qinghai-Tibet Plateau. Ecol. Eng. 2019, 130, 80–93. [Google Scholar] [CrossRef]
- Hou, D.J.; He, W.M.; Liu, C.C.; Qiao, X.G.; Guo, K. Litter accumulation alters the abiotic environment and drives community successional changes in two fenced grasslands in Inner Mongolia. Ecol. Evol. 2019, 9, 9214–9224. [Google Scholar] [CrossRef]
- Sun, J.; Liu, M.; Fu, B.J.; Kemp, D.; Zhao, W.W.; Liu, G.H.; Han, G.D.; Wilkes, A.; Lu, X.Y.; Chen, Y.C.; et al. Reconsidering the efficiency of grazing exclusion using fences on the Tibetan Plateau. Sci. Bull. 2020, 65, 1405–1414. [Google Scholar] [CrossRef]
- Baoyin, T.; Li, F.Y.; Minggagud, H.; Bao, Q.; Zhong, Y.J.L.E. Mowing succession of species composition is determined by plant growth forms, not photosynthetic pathways in Leymus chinensis grassland of Inner Mongolia. Landsc. Ecol. 2015, 30, 1795–1803. [Google Scholar] [CrossRef]
- Chang, L.; Han, F.X.; Chai, S.X.; Cheng, H.B.; Yang, D.L.; Chen, Y.Z. Straw strip mulching affects soil moisture and temperature for potato yield in semiarid regions. Agron. J. 2020, 112, 1126–1139. [Google Scholar] [CrossRef]
- Pykala, J. Mitigating human effects on European biodiversity through traditional animal husbandry. Conserv. Biol. 2000, 14, 705–712. [Google Scholar] [CrossRef]
- Yang, Y.J.; Du, W.; Cui, Z.Y.; Lei, S.; Lei, T.; Lv, J.L. Effects of plastic film mulching on soil water use efficiency and wheat yield in the Loess Plateau of China. Arid. Land. Res. Manag. 2020, 34, 405–418. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Loreau, M.; He, N.P.; Zhang, G.M.; Han, X.G. Mowing exacerbates the loss of ecosystem stability under nitrogen enrichment in a temperate grassland. Funct. Ecol. 2017, 31, 1637–1646. [Google Scholar] [CrossRef]
- Yang, G.J.; Lu, X.T.; Stevens, C.J.; Zhang, G.M.; Wang, H.Y.; Wang, Z.W.; Zhang, Z.J.; Liu, Z.Y.; Han, X.G. Mowing mitigates the negative impacts of N addition on plant species diversity. Oecologia 2019, 189, 769–779. [Google Scholar] [CrossRef]
- Thorne, M.A.; Frank, D.A. The effects of clipping and soil moisture on leaf and root morphology and root respiration in two temperate and two tropical grasses. Plant Ecol. 2009, 200, 205–215. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J.J.N. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Collins, S.L.; Knapp, A.K.; Briggs, J.M.; Blair, J.M.; Steinauer, E.M. Modulation of diversity by grazing and mowing in native tallgrass prairie. Science 1998, 280, 745–747. [Google Scholar] [CrossRef]
- Socher, S.A.; Prati, D.; Boch, S.; Muller, J.; Baumbach, H.; Gockel, S.; Hemp, A.; Schoning, I.; Wells, K.; Buscot, F.; et al. Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic Appl. Ecol. 2013, 14, 126–136. [Google Scholar] [CrossRef]
- Storkey, J.; Macdonald, A.J.; Poulton, P.R.; Scott, T.; Kohler, I.H.; Schnyder, H.; Goulding, K.W.T.; Crawley, M.J. Grassland biodiversity bounces back from long-term nitrogen addition. Nature 2015, 528, 401–404. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, S.P.; Lin, G.H. Compensatory growth responses to clipping defoliation in Leymus chinensis (Poaceae) under nutrient addition and water deficiency conditions. Plant Ecol. 2008, 196, 85–99. [Google Scholar] [CrossRef]
- Li, M.R.; Wang, L.L.; Li, J.J.; Peng, Z.L.; Wang, L.; Zhang, X.F.; Xu, S.J. Grazing exclusion had greater effects than nitrogen addition on soil and plant community in a desert steppe, Northwest of China. BMC Plant Biol. 2022, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.X.; Yang, Y.; An, S.S.; Zhu, Z.L. Effects of different vegetation restoration measures on soil aggregate stability and erodibility on the Loess Plateau, China. Catena 2020, 185, 104294. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Liu, J.; Fu, Q.; Xu, Q.; Idimesheva, O. Soil water and temperature characteristics under different straw mulching and tillage measures in the black soil region of China. J. Soil. Water Conserv. 2021, 76, 256–262. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Chai, S.X.; Tian, H.H.; Chai, Y.W.; Li, Y.W.; Chang, L.; Cheng, H.B. Straw strips mulch on furrows improves water use efficiency and yield of potato in a rainfed semiarid area. Agric. Water Manag. 2019, 211, 142–151. [Google Scholar] [CrossRef]
- Suo, G.D.; Xie, Y.S.; Zhang, Y.; Luo, H. Long-term effects of different surface mulching techniques on soil water and fruit yield in an apple orchard on the Loess Plateau of China. Sci. Hortic. 2019, 246, 643–651. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, Y.; Lin, H.X.; Feng, H.; Dyck, M. Effects of different mulching technologies on evapotranspiration and summer maize growth. Agric. Water Manag. 2018, 201, 309–318. [Google Scholar] [CrossRef]
- Grman, E.; Lau, J.A.; Schoolmaster, D.R.; Gross, K.L. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol. Lett. 2010, 13, 1400–1410. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Ecology—Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Sasaki, T.; Lauenroth, W.K. Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 2011, 166, 761–768. [Google Scholar] [CrossRef]
- Yu, J.F.; Zhang, Y.; Wang, Y.T.; Luo, X.; Liang, X.Q.; Huang, X.M.; Zhao, Y.X.; Zhou, X.Y.; Li, J.P. Ecosystem photosynthesis depends on increased water availability to enhance carbon assimilation in semiarid desert steppe in northern China. Glob. Ecol. Conserv. 2022, 38, e02202. [Google Scholar] [CrossRef]
- Xue, R.; Yang, Q.; Miao, F.H.; Wang, X.Z.; Shen, Y.Y. Slope aspect influences plant biomass, soil properties and microbial composition in alpine meadow on the Qinghai-Tibetan Plateau. J. Soil. Sci. Plant Nut. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Maschinski, J.; Whitham, T.G. The continuum of plant responses to herbivory: The influence of plant association, nutrient availability, and timing. Am. Soc. Nat. 1989, 134, 1–19. [Google Scholar] [CrossRef]
- Hjalten, J.; Danell, K.; Ericson, L.J.E. Effects of simulated herbivory and intraspecific competition on the compensatory ability of birches. Ecology 1993, 74, 1136–1142. [Google Scholar] [CrossRef]
- Wang, Y.T.; Shen, Y.J.; Xie, Y.Z.; Ma, H.B.; Li, W.C.; Luo, X.; Zhang, H.; Zhang, Y.; Li, J.P. Changes in precipitation have both direct and indirect effects on typical steppe aboveground net primary productivity in Loess Plateau, China. Plant Soil. 2022, 484, 503–515. [Google Scholar] [CrossRef]
- Schmid, J.S.; Huth, A.; Taubert, F. Impact of mowing frequency and temperature on the production of temperate grasslands: Explanations received by an individual-based model. Oikos 2022, 9, e09108. [Google Scholar] [CrossRef]
- Wan, Z.Q.; Yang, J.Y.; Gu, R.; Liang, Y.; Yan, Y.L.; Gao, Q.Z.; Yang, J. Influence of Different Mowing Systems on Community Characteristics and the Compensatory Growth of Important Species of the Stipa grandis Steppe in Inner Mongolia. Sustainability 2016, 8, 1121. [Google Scholar] [CrossRef]
- Polley, H.W.; Wilsey, B.J.; Derner, J.D. Dominant species constrain effects of species diversity on temporal variability in biomass production of tallgrass prairie. Oikos 2007, 116, 2044–2052. [Google Scholar] [CrossRef]
- Zhao, T.Q.; Zhang, F.; Suo, R.Z.; Zhen, J.H.; Qiao, J.R.; Zhao, M.L.; Bai, K.Y.; Zhang, B. The importance of functional diversity in regulating forage biomass and nutrition: Evidence from mowing in semiarid grasslands. Restor. Ecol. 2022, 31, e13742. [Google Scholar] [CrossRef]
- Wang, Y.H.; Niu, X.X.; Zhao, L.Q.; Liang, C.Z.; Miao, B.L.; Zhang, Q.; Zhang, J.H.; Schmid, B.; Ma, W.H. Biotic stability mechanisms in Inner Mongolian grassland. P. Roy. Soc. B Biol. Sci. 2020, 287, 20200675. [Google Scholar] [CrossRef]
- Hazi, J.; Penksza, K.; Barczi, A.; Szentes, S.; Papay, G. Effects of Long-Term Mowing on Biomass Composition in Pannonian Dry Grasslands. Agronomy 2022, 12, 1107. [Google Scholar] [CrossRef]
- Ruprecht, E.; Szabo, A. Grass litter is a natural seed trap in long-term undisturbed grassland. J. Veg. Sci. 2012, 23, 495–504. [Google Scholar] [CrossRef]
- Li, Z.L.; Peng, Q.; Dong, Y.S.; Guo, Y. The influence of increased precipitation and nitrogen deposition on the litter decomposition and soil microbial community structure in a semiarid grassland. Sci. Total Environ. 2022, 844, 157115. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, L.C.M.; Connolly, S.R.; He, F.J.E.L. Understanding diversity–stability relationships: Towards a unified model of portfolio effects. Ecol. Lett. 2013, 16, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Muraina, T.O.; Xu, C.; Yu, Q.; Yang, Y.D.; Jing, M.H.; Jia, X.T.; Jaman, M.S.; Dam, Q.; Knapp, A.K.; Collins, S.L.; et al. Species asynchrony stabilises productivity under extreme drought across Northern China grasslands. J. Ecol. 2021, 109, 1665–1675. [Google Scholar] [CrossRef]
- Michaud, A.; Plantureux, S.; Amiaud, B.; Carrere, P.; Cruz, P.; Duru, M.; Dury, B.; Farruggia, A.; Fiorelli, J.L.; Kerneis, E.; et al. Identification of the environmental factors which drive the botanical and functional composition of permanent grasslands. J. Agric. Sci. 2012, 150, 219–236. [Google Scholar] [CrossRef]
- Gilhaus, K.; Boch, S.; Fischer, M.; Holzel, N.; Kleinebecker, T.; Prati, D.; Rupprecht, D.; Schmitt, B.; Klaus, V.H. Grassland management in Germany: Effects on plant diversity and vegetation composition. Tuexenia 2017, 377, 379–397. [Google Scholar] [CrossRef]
- Bell, J.L.; Sloan, L.C.; Snyder, M.A. Regional changes in extreme climatic events: A future climate scenario. J. Clim. 2004, 17, 81–87. [Google Scholar] [CrossRef]
- Fang, J.Y.; Piao, S.L.; Zhou, L.M.; He, J.S.; Wei, F.Y.; Myneni, R.B.; Tucker, C.J.; Tan, K. Precipitation patterns alter growth of temperate vegetation. Geophys. Res. Lett. 2005, 32, L21411. [Google Scholar] [CrossRef]
- Finegan, B.; Delgado, D. Structural and Floristic Heterogeneity in a 30-Year-Old Costa Rican Rain Forest Restored on Pasture Through Natural Secondary Succession. Restor. Ecol. 2000, 8, 380–393. [Google Scholar] [CrossRef]
- Van Staalduinen, M.A.; Dobarro, I.; Peco, B. Interactive effects of clipping and nutrient availability on the compensatory growth of a grass species. Plant Ecol. 2010, 208, 55–64. [Google Scholar] [CrossRef]
- Bai, Y.F.; Han, X.G.; Wu, J.G.; Chen, Z.Z.; Li, L.H. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Feng, J.C.; Loreau, M.; He, N.P.; Han, X.G.; Jiang, L. Nitrogen addition does not reduce the role of spatial asynchrony in stabilising grassland communities. Ecol. Lett. 2019, 22, 563–571. [Google Scholar] [CrossRef]

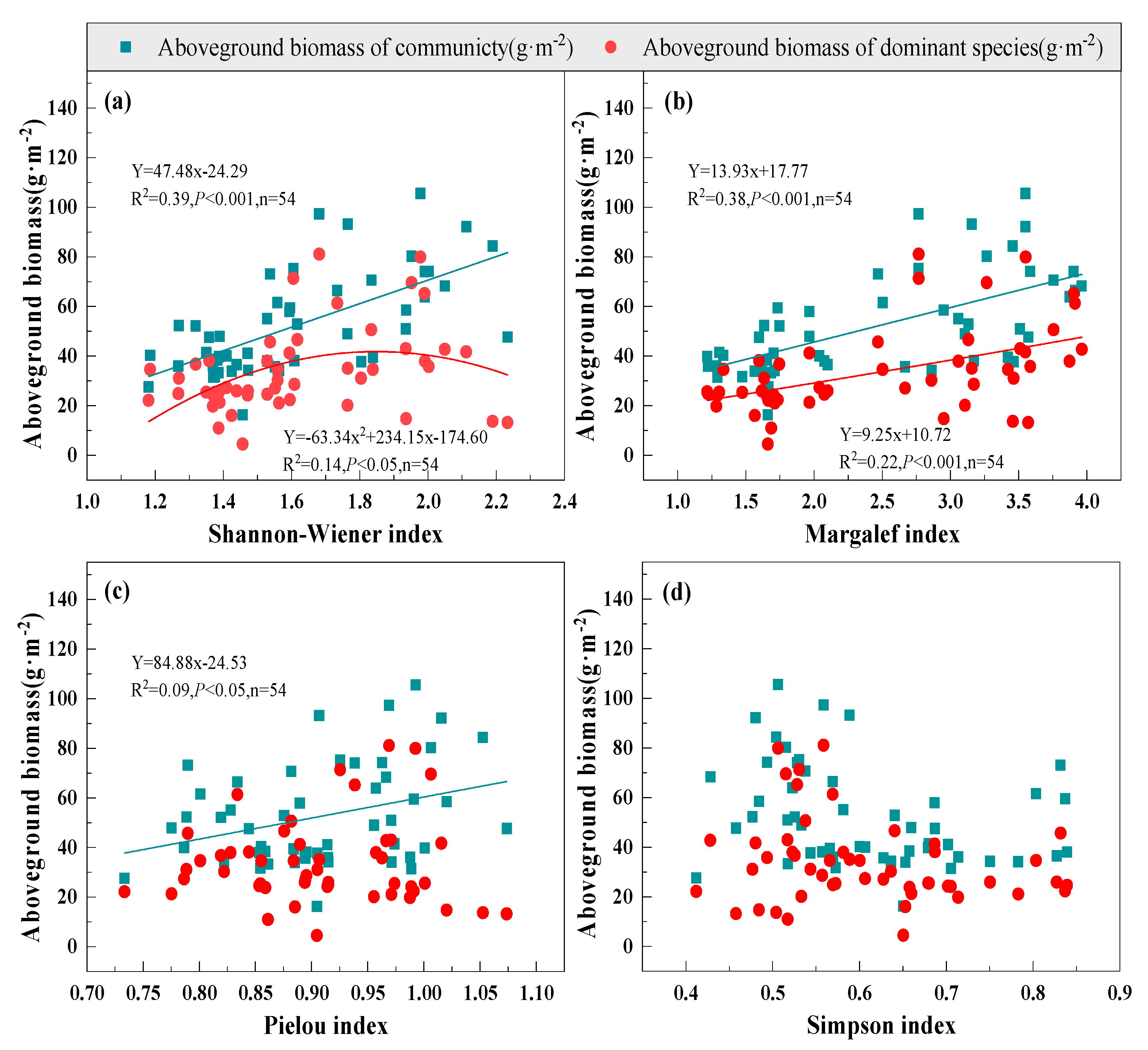
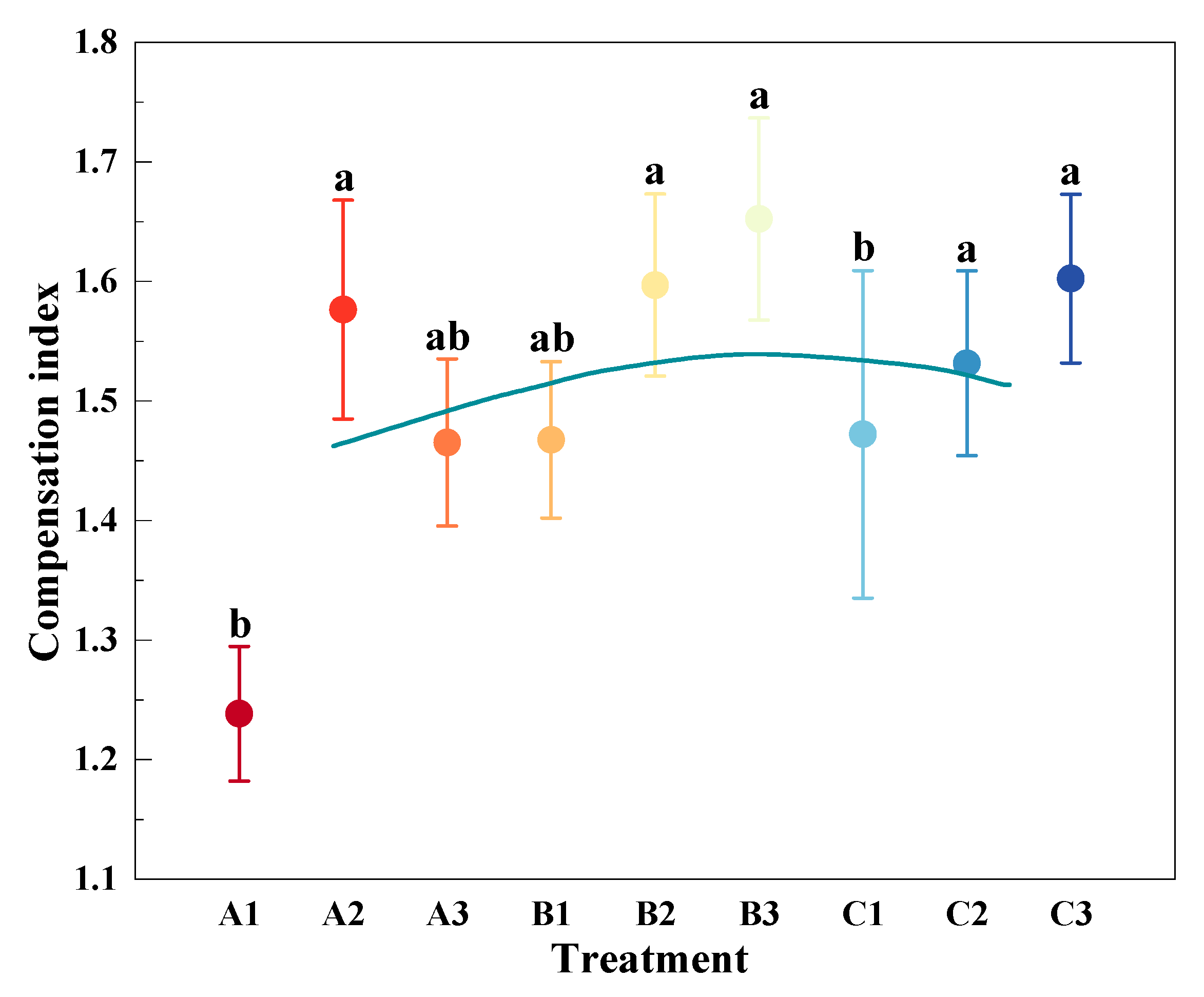

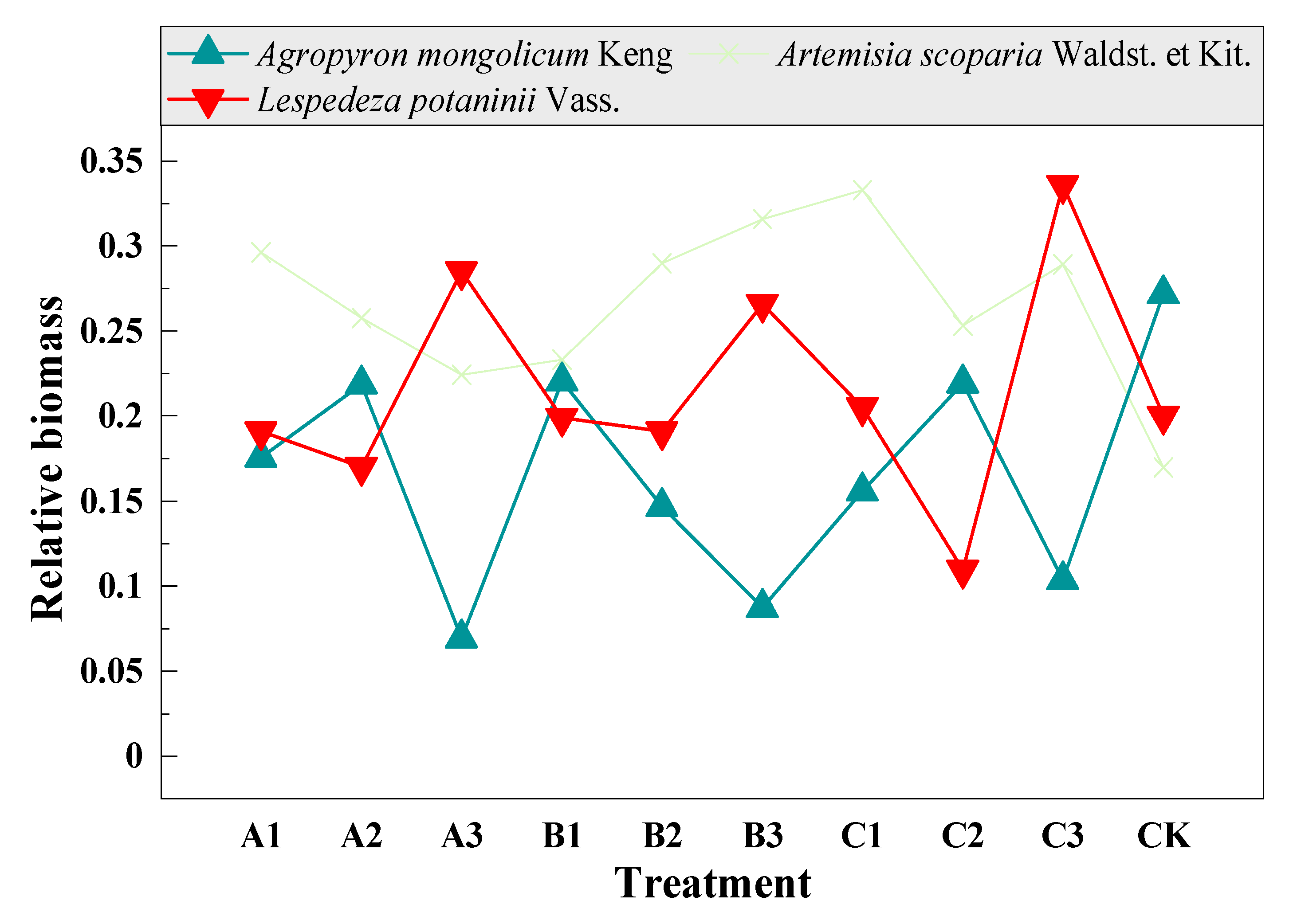
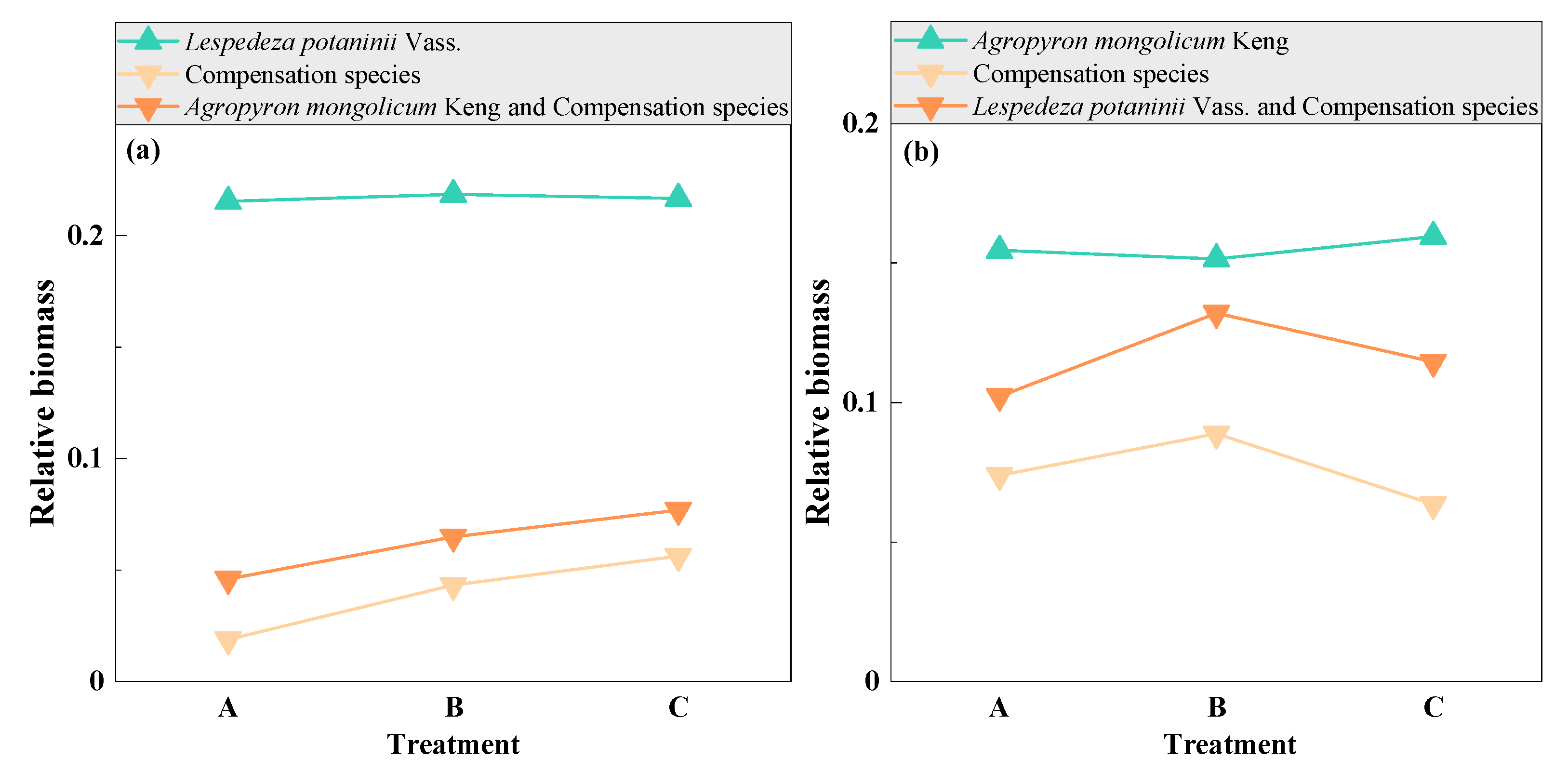
| Functional Group | Species | A1 | A2 | A3 | B1 | B2 | B3 | C1 | C2 | C3 | CK |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subshrub | Lespedeza potaninii Vass. | 0.11 | 0.11 | 0.10 | 0.11 | 0.13 | 0.14 | 0.08 | 0.09 | 0.14 | 0.08 |
| Perennial | Agropyron mongolicum Keng | 0.10 | 0.13 | 0.12 | 0.16 | 0.13 | 0.09 | 0.18 | 0.22 | 0.08 | 0.18 |
| Polygala tenuifolia Willd. | 0.06 | 0.03 | 0.04 | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 | 0.05 | 0.02 | |
| Glycyrrhiza uralensis Fisch. | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | |
| Lipschitzia divaricata (Turcz.) Zaika, Sukhor. and N. Kilian | 0.01 | 0.01 | 0.06 | - | 0.02 | 0.05 | 0.02 | 0.02 | - | 0.01 | |
| Echinops gmelinii Turcz. | - | - | - | - | - | - | - | - | - | 0.01 | |
| Peganum harmala L. | - | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.05 | 0.02 | 0.02 | |
| Vincetoxicum mongolicum Maxim. | 0.01 | - | - | 0.01 | - | - | 0.02 | 0.01 | - | - | |
| Annual or biennial | Artemisia scoparia Waldst. et Kit. | 0.49 | 0.46 | 0.41 | 0.43 | 0.35 | 0.50 | 0.40 | 0.39 | 0.45 | 0.36 |
| Euphorbia esula L. | 0.13 | 0.15 | 0.16 | 0.10 | 0.11 | 0.11 | 0.16 | 0.12 | 0.16 | 0.21 | |
| Allium mongolicum Regel | - | - | 0.01 | - | 0.01 | 0.01 | - | 0.01 | - | 0.02 | |
| Kali collinum (Pall.) Akhani and Roalson | 0.01 | - | 0.01 | - | 0.02 | 0.02 | 0.02 | 0.02 | - | 0.01 | |
| Cenchrus echinatus L. | 0.09 | 0.04 | 0.04 | 0.02 | 0.06 | 0.02 | 0.02 | 0.04 | 0.03 | 0.01 | |
| Eragrostis minor Host | 0.01 | 0.01 | 0.01 | 0.03 | 0.01 | - | 0.02 | 0.01 | 0.01 | - | |
| Euphorbia maculata L. | - | 0.04 | 0.03 | 0.09 | 0.10 | 0.10 | 0.03 | 0.10 | 0.05 | 0.08 |
| Ratio of Treated Biomass to Control Biomass | Compensatory Effect |
|---|---|
| G/C > 1 | Overcompensation |
| G/C = 1 | Equal compensation |
| G/C < 1 | Undercompensation |
| Subshrubs | Tufted Grasses | Rhizomatous Grasses | Herbs | Annual or Biennial Herbs | |
|---|---|---|---|---|---|
| Subshrubs | 1 | −0.57 (−0.53) | 0.67 * (0.15) | 0.53 (−0.90 **) | −0.74 * (0.40) |
| Tufted grasses | 1 | −0.92 ** (−0.13) | 0.52 (0.21) | 0.64 * (0.56) | |
| Rhizomatous grasses | 1 | −0.62 (−0.30) | −0.64 * (−0.54) | ||
| Herbs | 1 | 0.43 (−0.60) | |||
| Annual or biennial herbs | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Li, J.; Xie, Y.; Wang, Y.; Yu, J.; Liang, X. Enhanced Primary Productivity in Fenced Desert Grasslands of China through Mowing and Vegetation Cover Interaction. Agronomy 2023, 13, 2029. https://doi.org/10.3390/agronomy13082029
Luo X, Li J, Xie Y, Wang Y, Yu J, Liang X. Enhanced Primary Productivity in Fenced Desert Grasslands of China through Mowing and Vegetation Cover Interaction. Agronomy. 2023; 13(8):2029. https://doi.org/10.3390/agronomy13082029
Chicago/Turabian StyleLuo, Xu, Jianping Li, Yingzhong Xie, Yutao Wang, Jianfei Yu, and Xiaoqian Liang. 2023. "Enhanced Primary Productivity in Fenced Desert Grasslands of China through Mowing and Vegetation Cover Interaction" Agronomy 13, no. 8: 2029. https://doi.org/10.3390/agronomy13082029
APA StyleLuo, X., Li, J., Xie, Y., Wang, Y., Yu, J., & Liang, X. (2023). Enhanced Primary Productivity in Fenced Desert Grasslands of China through Mowing and Vegetation Cover Interaction. Agronomy, 13(8), 2029. https://doi.org/10.3390/agronomy13082029





