PsFT, PsTFL1, and PsFD Are Involved in Regulating the Continuous Flowering of Tree Peony (Paeonia × lemoinei ‘High Noon’)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sequence Isolation
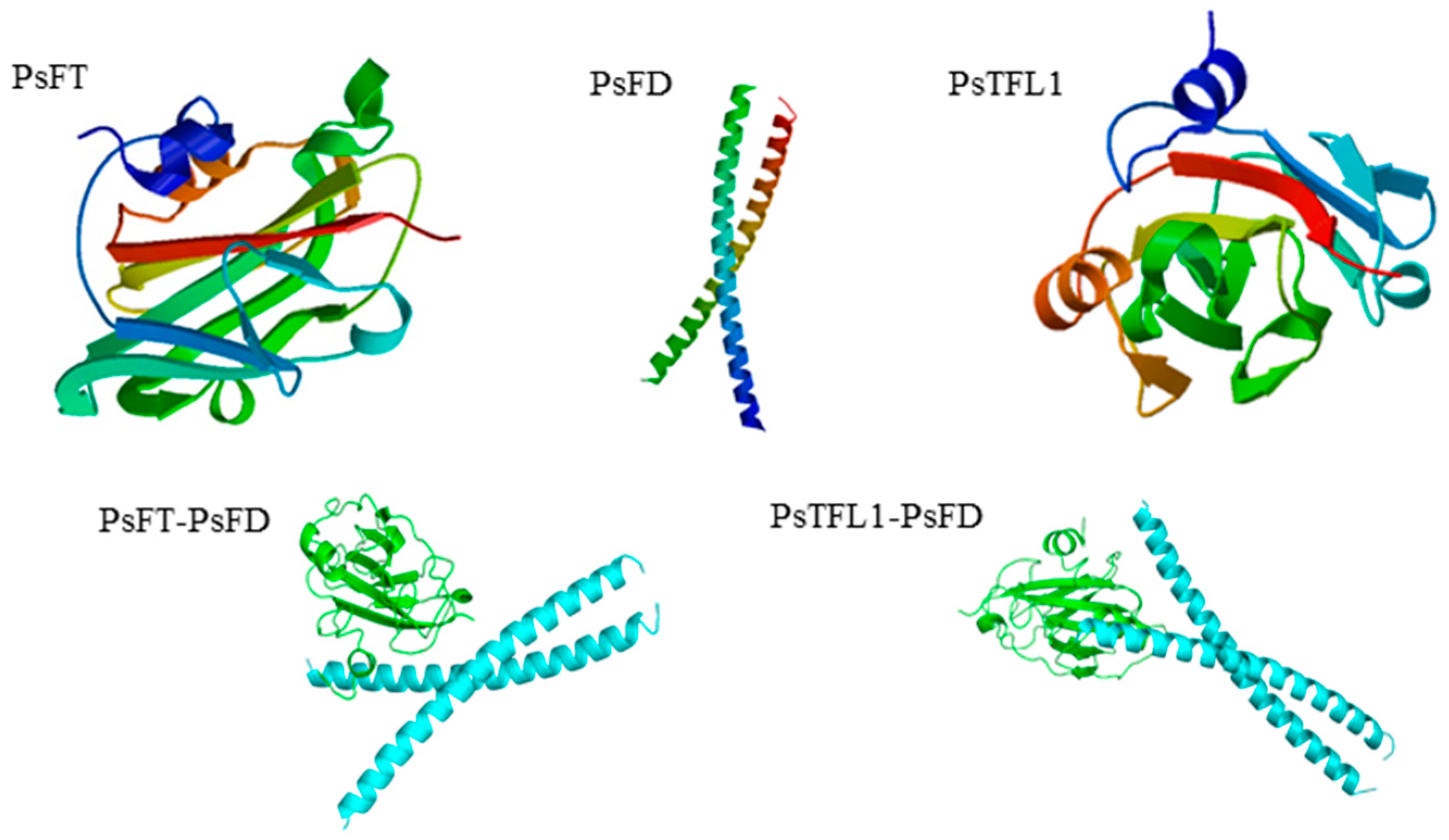
2.3. Protein Structure and Phylogenetic Analysis
2.4. Expression Analysis
2.5. Subcellular Localization
2.6. Plant Transformation
2.7. Yeast 2-Hybrid (Y2H) Analysis
2.8. Bimolecular Fluorescence Complementation (BiFC)
2.9. Co-IP Assay
2.10. Sequencing Analysis and Primer Synthesis
3. Results
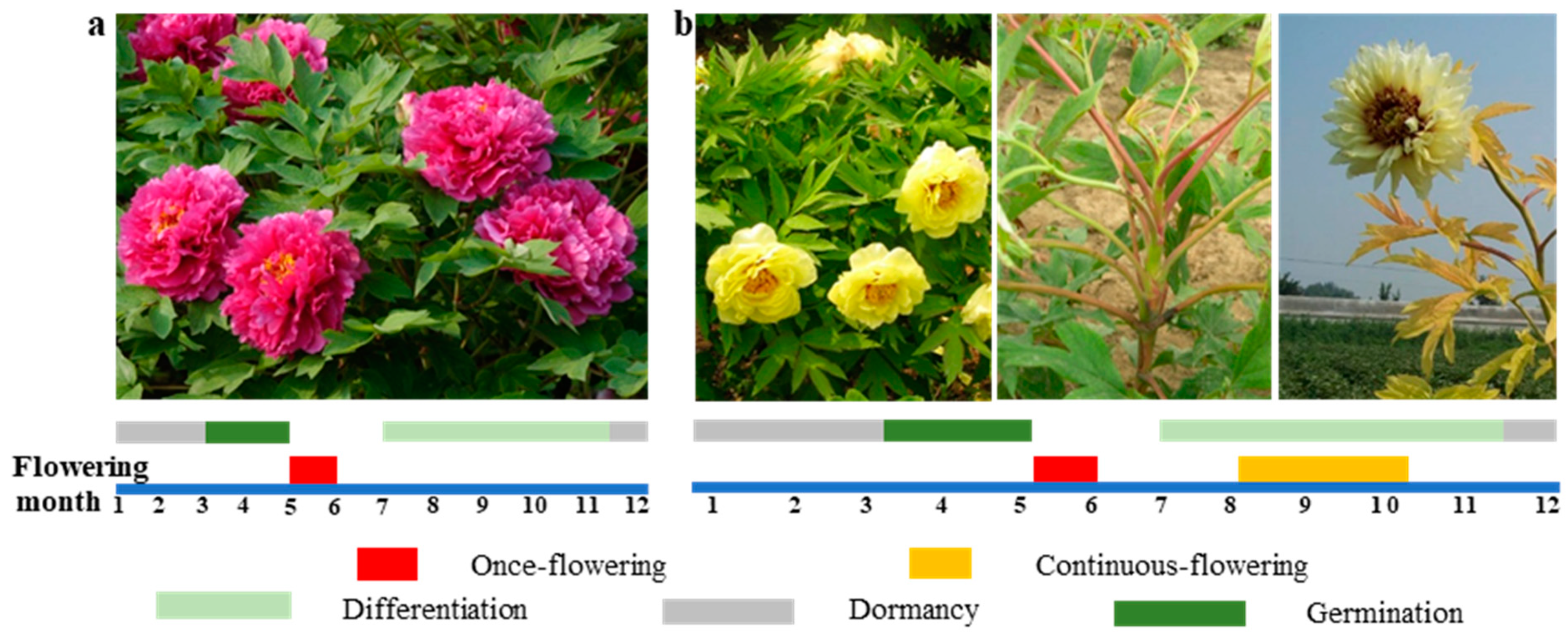
3.1. Comparison of Tree Peony Flowering Traits
3.2. Sequencing Analyses of FD and TFL1 in Tree Peonies
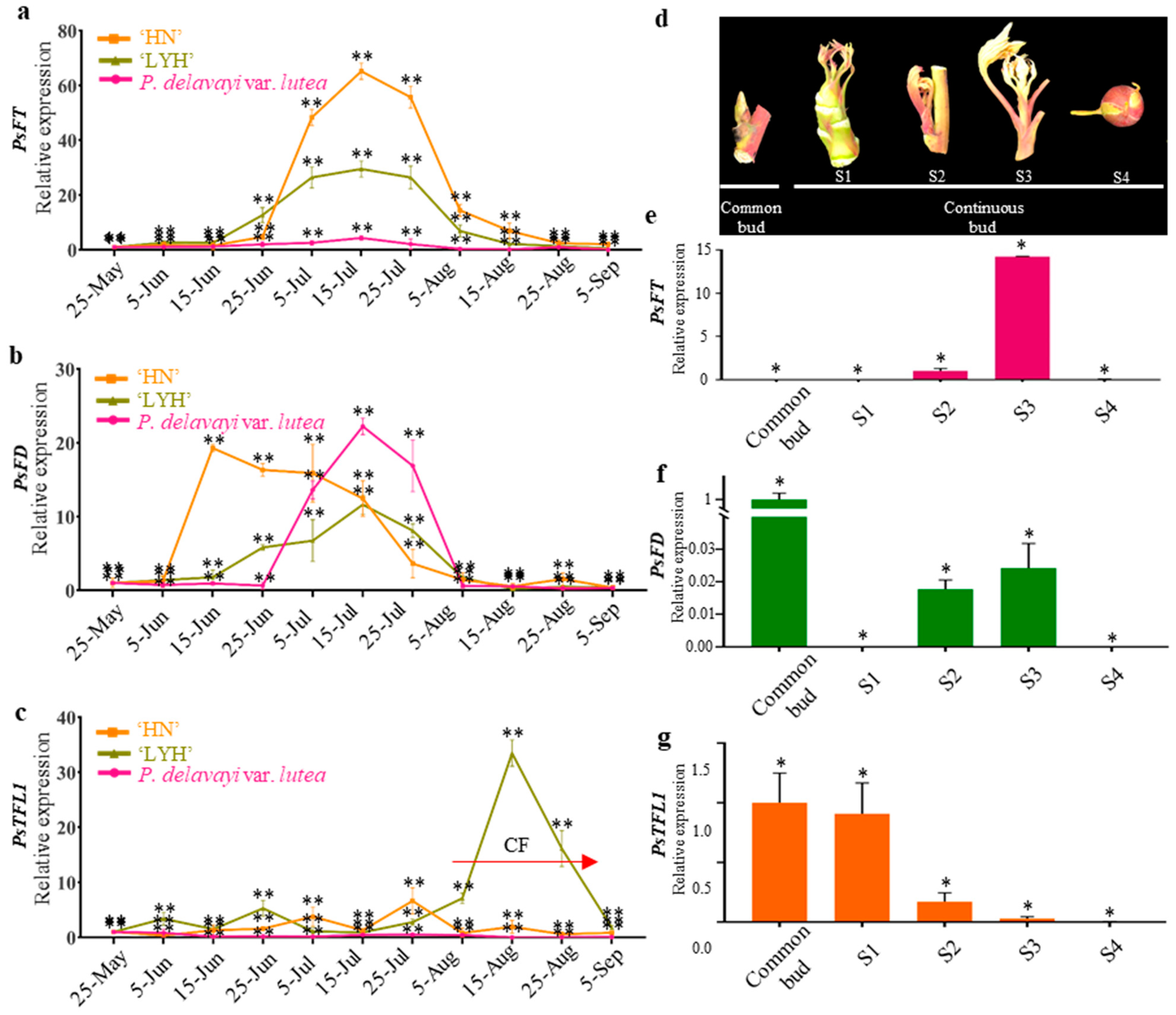
3.3. Expression of PsTFL1, PsFD, and PsFT in Different Flowering Cultivars
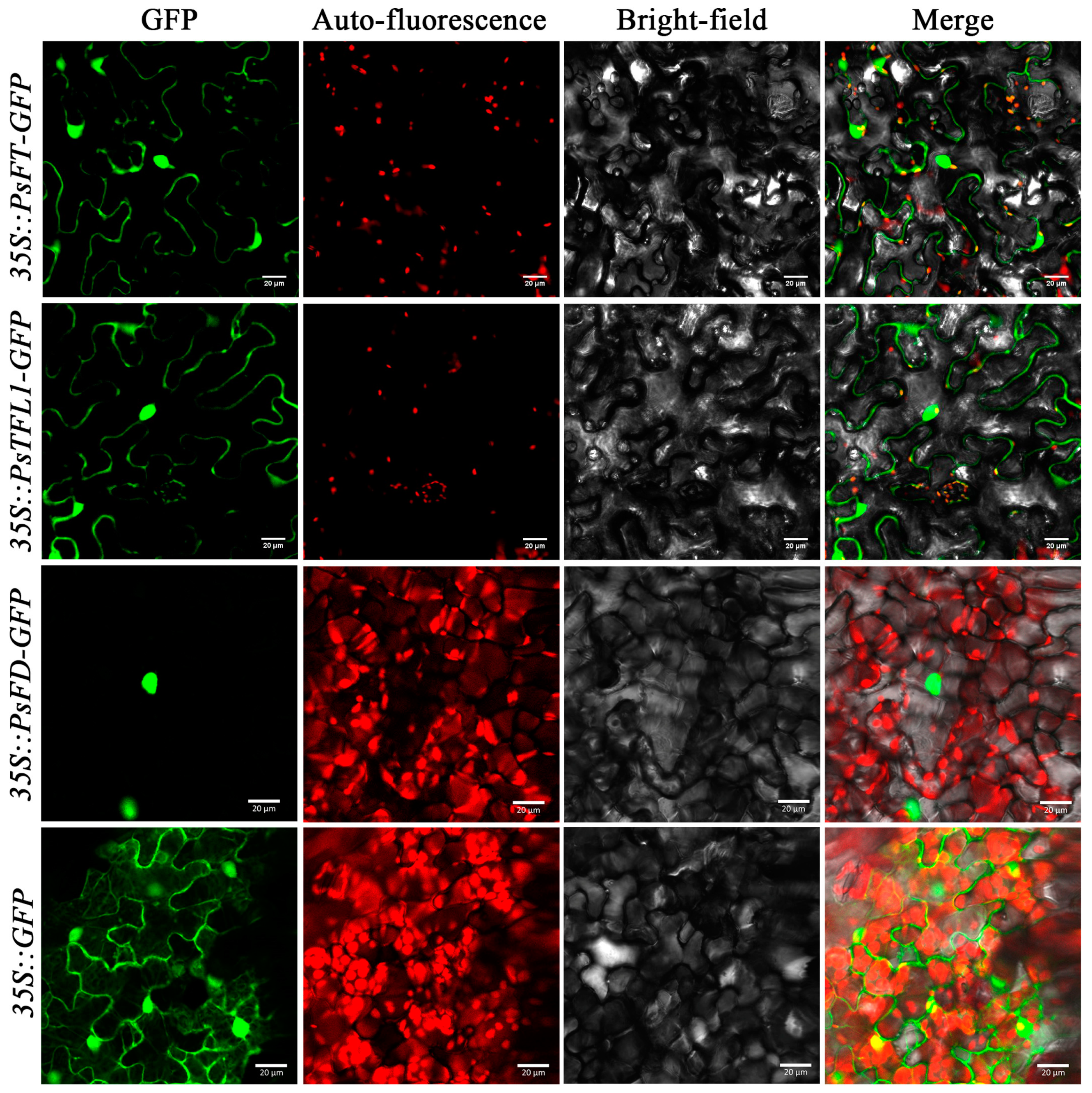
3.4. Subcellular Localization of PsTFL1, PsFD, and PsFT
3.5. Ectopic Expression of PsTFL1 in A. thaliana
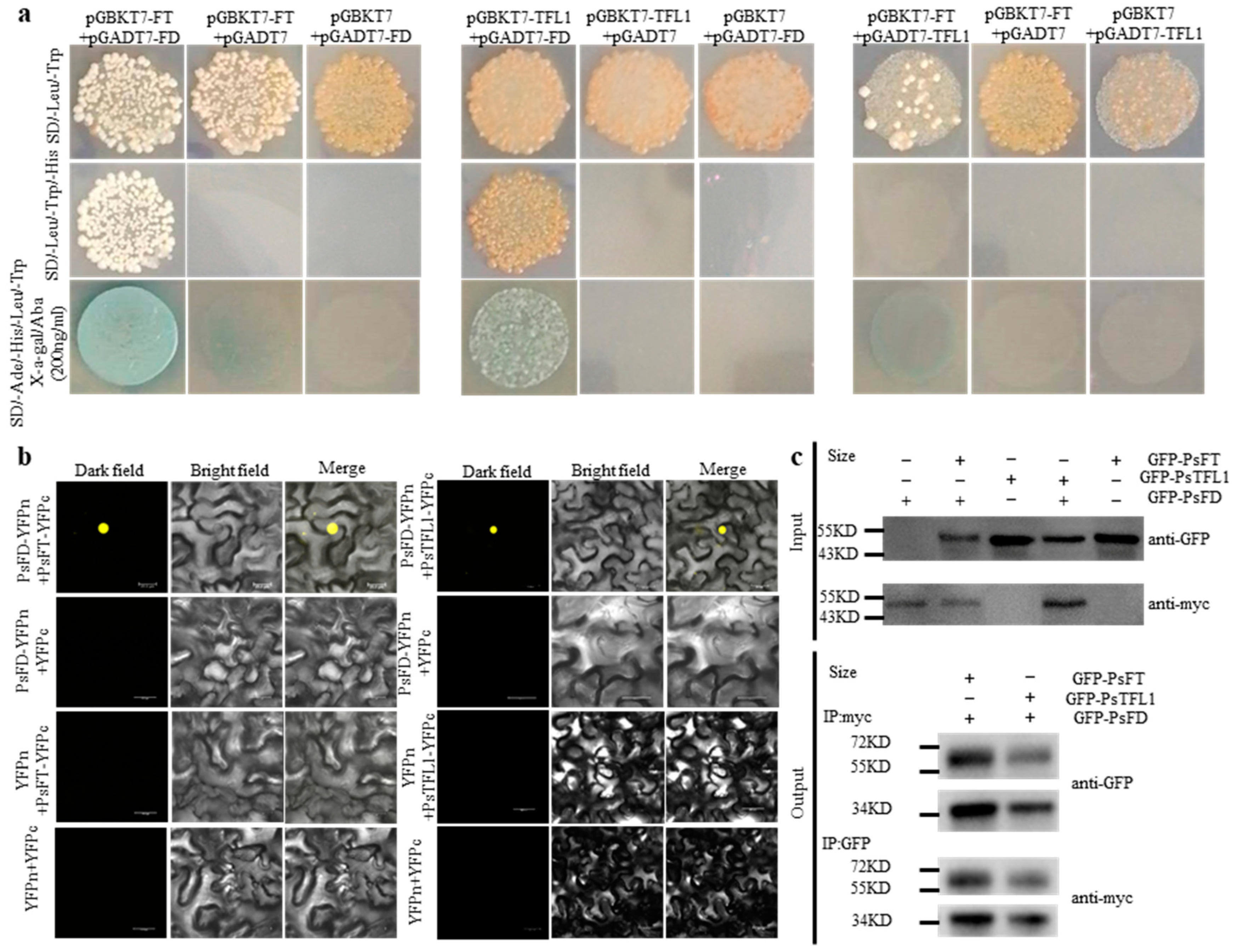
3.6. PsFT and PsTFL1 Physically Interact with PsFD
4. Discussion
4.1. The Different Roles of PsFT-PsFD and PsTFL1-PsFD in Flowering Control
4.2. The Role of PsFT, PsTFL1, and PsFD in Regulating CF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bai, M.; Liu, J.Y.; Fan, C.G.; Chen, Y.Q.; Chen, H.; Lu, J.; Sun, J.J.; Ning, G.G. KSN heterozygosity is associated with continuous flowering of Rosa rugosa Purple branch. Hortic. Res. 2021, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.G.; Gendall, A.R.; Dean, C. When to switch to flowering. Annu. Rev. Cell Dev. Biol. 1999, 15, 19–25. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Gao, H.; Yang, Y.; Yang, S.; Luo, L.; Yu, C.; Wang, J.; Cheng, T.; Zhang, Q.; Pan, H. Differentially expressed genes related to flowering transition between once- and continuous-flowering Roses. Biomolecules 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Beruto, M.; Xue, J.Q.; Zhu, F.Y.; Liu, C.J.; Yan, Y.M.; Zhang, X.X. Molecular cloning and potential function prediction of homologous SOC1 genes in tree peony. Plant Cell Rep. 2015, 34, 1459–1471. [Google Scholar] [CrossRef]
- Blumel, M.; Dally, N.; Jung, C. Flowering time regulation in crops-what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015, 32, 121–129. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Coelho, C.P.; Minow, M.A.; Chalfun-Júnior, A.; Colasanti, J. Putative sugarcane FT/TFL1 genes delay flowering time and alter reproductive architecture in Arabidopsis. Front. Plant Sci. 2014, 5, 221. [Google Scholar] [CrossRef]
- Banfield, M.J.; Brady, R.L. The structure of Antirrhinum centroradialis protein (CEN) suggests a role as a kinase regulator. J. Mol. Biol. 2000, 297, 1159–1170. [Google Scholar] [CrossRef]
- Randoux, M.; Davière, J.M.; Jeauffre, J.; Thouroude, T.; Pierre, S.; Toualbia, Y.; Perrotte, J.; Reynoird, J.P.; Jammes, M.J.; Oyant, L.H.; et al. RoKSN, a floral repressor, forms protein complexes with RoFD and RoFT to regulate vegetative and reproductive development in rose. New Phytol. 2014, 202, 161–173. [Google Scholar] [CrossRef]
- Iwata, H.; Gaston, A.; Remay, A.; Thouroude, T.; Jeauffre, J.; Kawamura, K.; Oyant, L.H.; Araki, T.; Denoyes, B.; Foucher, F. The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J. 2012, 69, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Luo, L.; Fu, Q.T.; Niu, L.; Xu, Z.F. Isolation and functional characterization of JcFT, a FLOWERING LOCUS T (FT) homologous gene from the biofuel plant Jatropha curcas. BMC Plant Biol. 2014, 14, 125. [Google Scholar] [CrossRef]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Ahn, J.H.; Miller, D.; Winter, V.J.; Banfield, M.J.; Lee, J.H.; Yoo, S.Y.; Henz, S.R.; Brady, R.L.; Weigel, D. Divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006, 25, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hans, H.; Christian, J.; Molina, C. Mutations in single FT- and TFL1-paralogs of rapeseed (Brassica napus L.) and their impact on flowering time and yield components. Front. Plant Sci. 2014, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Kardailsky, I.; Shukla, V.K.; Ahn, J.H.; Dagenais, N.; Christensen, S.K.; Nguyen, J.T.; Chory, J.; Harrison, M.; Weigel, D. North European invasion by common ragweed is associated with early flowering and dominant changes in FT/TFL1 expression. J. Exp. Bot. 2018, 69, 2647–2658. [Google Scholar] [CrossRef]
- Flachowsky, H.; Szankowski, I.; Waidmann, S.; Peil, A.; Tränkner, C.; Hanke, M.V. The MdTFL1 gene of apple (Malus × domestica Borkh.) reduces vegetative growth and generation time. Tree Physiol. 2012, 32, 1288–1301. [Google Scholar] [CrossRef]
- Freiman, A.; Shlizerman, L.; Golobovitch, S.; Yablovitz, Z.; Korchinsky, R.; Cohen, Y.; Samach, A.; Chevreau, E.; Roux, P.L.; Patocchi, A.; et al. Development of a transgenic early flowering pear (Pyrus communis L.) genotype by RNAi silencing of PcTFL1-1 and PcTFL1-2. Planta 2012, 235, 1239–1251. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kobayashi, Y.; Goto, K.; Abe, M.; Araki, T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005, 46, 1175–1189. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- Khan, M.R.; Ai, X.Y.; Zhang, J.Z. Genetic regulation of flowering time in annual and perennial plants. Wiley Interdiscip. Rev. RNA 2014, 5, 347–359. [Google Scholar] [CrossRef]
- Harig, L.; Beinecke, F.A.; Oltmanns, J.; Muth, J.; Müller, O.; Rüping, B.; Twyman, R.M.; Fischer, R.; Prüfer, D.; Noll, G.A. Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J. 2012, 72, 908–921. [Google Scholar] [CrossRef]
- Alvarez, J.; Guli, C.L.; Yu, X.H.; Smyth, D.R. Terminal flower: A gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992, 2, 103–116. [Google Scholar] [CrossRef]
- Shannon, S.; Meeks-Wagner, D.R.A. mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 1991, 3, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Kardailsky, I.; Shukla, V.K.; Ahn, J.H.; Dagenais, N.; Christensen, S.K.; Nguyen, J.T.; Chory, J.; Harrison, M.; Weigel, D. Activation tagging of the floral inducer FT. Science 1999, 286, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, Y.; Money, T.; Bradley, D. A single amino acid converts a repressor to an activator of flowering. Proc. Natl. Acad. Sci. USA 2005, 21, 7748–7753. [Google Scholar] [CrossRef]
- Hanano, S.; Goto, K. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 2011, 23, 3172–3184. [Google Scholar] [CrossRef]
- Cheng, F.Y. Advances in the breeding of tree peonies and a cultivar system for the cultivar group. Int. J. Plant Breed. 2007, 1, 89–104. [Google Scholar]
- Zhou, H.; Cheng, F.Y.; Wang, R.; Zhong, Y.; He, C.Y. Transcriptome comparison reveals key candidate genes responsible for the unusual reblooming trait in tree peonies. PLoS ONE 2013, 8, e79996. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, F.Y.; Wu, J.; He, C.Y. Isolation and functional analysis of Flowering Locus T in tree peonies (PsFT). J. Am. Soc. Hortic. Sci. 2015, 140, 265–271. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Eshed, Y.; Lippman, Z.B. Revolutions in agriculture chart a course for targeted breeding of old and new crops. Science 2019, 366, eaax0025. [Google Scholar] [CrossRef]
- Laurie, R.E.; Diwadkar, P.; Jaudal, M.; Zhang, L.; Hecht, V.; Wen, J.; Tadege, M.; Mysore, K.S.; Putterill, J.; Weller, J.L.; et al. The Medicago FLOWERING LOCUS homolog, MtFTa11, is a key regulator of flowering time. Plant Physiol. 2011, 156, 2207–2224. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.N.; Hou, X.H.; Zou, Y.P.; Han, T.S.; Niu, X.M.; Zhang, J.; Zhao, Z.; Todesco, M.; Balasubramanian, S.; et al. Parallel evolution of common allelic variants confers flowering diversity in Capsella rubella. Plant Cell 2018, 30, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP Protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.F.; Li, G.F.; Krom, N.; Tang, Y.H.; Wen, J.Q. Genetic regulation of flowering time and inflorescence architecture by MtFDa and MtFTa1 in Medicago truncatula. Plant Physiol. 2021, 185, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kotoda, N.; Hayashi, H.; Suzuki, M.; Igarashi, M.; Hatsuyama, Y.; Kidou, S.I.; Igasaki, T.; Nishiguchi, M.; Yano, K.; Shimizu, T.; et al. Molecular characterization of FLOWERING LOCUT T-like genes of apple (Malus × domestica Borkh.). Plant Cell Physiol. 2010, 5, 561–575. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Adams, J.P.; Kim, H.; No, K.; Ma, C.; Strauss, S.H.; Drnevichc, J.; Vanderveldea, L.; Ellisa, J.D.; Ricea, B.M.; et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc. Natl. Acad. Sci. USA 2011, 108, 10756–10761. [Google Scholar] [CrossRef]
- Endo, T.; Shimada, T.; Fujii, H.; Kobayashi, Y.; Araki, T.; Omura, M. Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res. 2005, 14, 703–712. [Google Scholar] [CrossRef]
- Reig, C.; Gil-Muñoz, F.; Vera-Sirera, F.; García-Lorca, A.; Martínez-Fuentes, A.; Mesejo, C.; Pérez-Amador, M.A.; Agustí, M. Bud sprouting and floral induction and expression of FT in loquat [Eriobotrya japonica (Thunb.) Lindl.]. Planta 2017, 246, 915–925. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Cheng, F.; Huang, H.; Geng, Z.; He, C. PsFT, PsTFL1, and PsFD Are Involved in Regulating the Continuous Flowering of Tree Peony (Paeonia × lemoinei ‘High Noon’). Agronomy 2023, 13, 2071. https://doi.org/10.3390/agronomy13082071
Zhang L, Cheng F, Huang H, Geng Z, He C. PsFT, PsTFL1, and PsFD Are Involved in Regulating the Continuous Flowering of Tree Peony (Paeonia × lemoinei ‘High Noon’). Agronomy. 2023; 13(8):2071. https://doi.org/10.3390/agronomy13082071
Chicago/Turabian StyleZhang, Limei, Fangyun Cheng, He Huang, Ziwen Geng, and Chaoying He. 2023. "PsFT, PsTFL1, and PsFD Are Involved in Regulating the Continuous Flowering of Tree Peony (Paeonia × lemoinei ‘High Noon’)" Agronomy 13, no. 8: 2071. https://doi.org/10.3390/agronomy13082071
APA StyleZhang, L., Cheng, F., Huang, H., Geng, Z., & He, C. (2023). PsFT, PsTFL1, and PsFD Are Involved in Regulating the Continuous Flowering of Tree Peony (Paeonia × lemoinei ‘High Noon’). Agronomy, 13(8), 2071. https://doi.org/10.3390/agronomy13082071






