Promotion of Growth of Alfalfa by Erwinia persicina Cp2 Exopolysaccharides under NaCl Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Measurement of Relevant Indicators
2.3.1. Germination Index Determination
2.3.2. Measurement of Growth Indicators
2.3.3. Determination of Chlorophyll Content
2.3.4. Determination of Oxidative Damage
2.3.5. Determination of Osmoregulatory Substances
2.3.6. Determination of Antioxidant Enzyme Activity
2.4. Statistical Analysis
3. Results
3.1. NaCl Concentration Screening Test
3.2. Effects of Different Concentrations of Cp2 EPS on Seed Germination of Alfalfa under Salt Stress
3.3. Effects of Different Concentrations of Cp2 EPS on Growth of Alfalfa Seedlings under Salt Stress
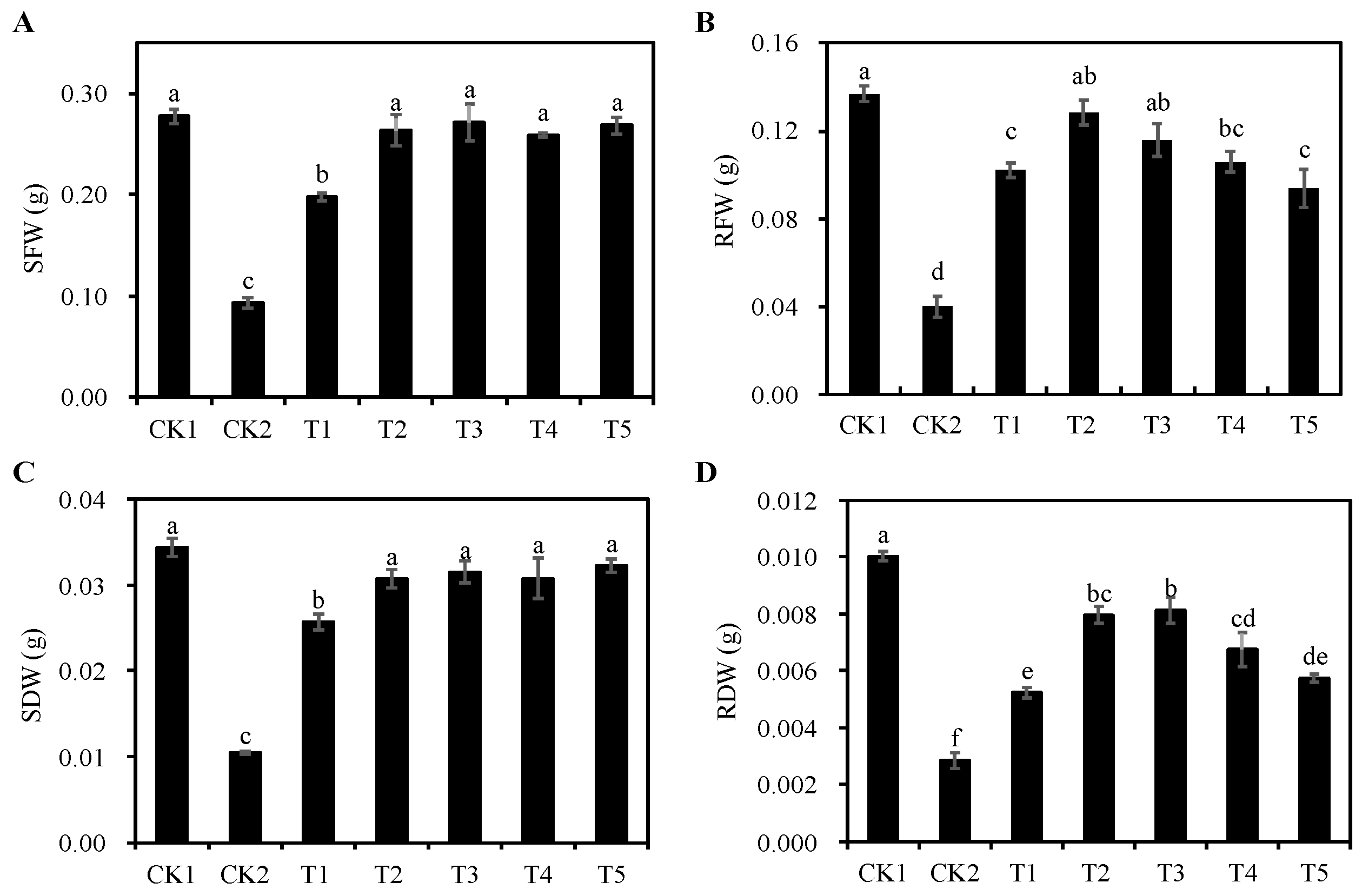
3.4. Effects of Different Concentrations of Cp2 EPS on Fresh and Dry Weight of Alfalfa under Salt Stress
3.5. Effects of Different Concentrations of Cp2 EPS on Root Morphology of Alfalfa under Salt Stress
3.6. Effects of Different Concentrations of Cp2 EPS on Chlorophyll Content of Alfalfa under Salt Stress
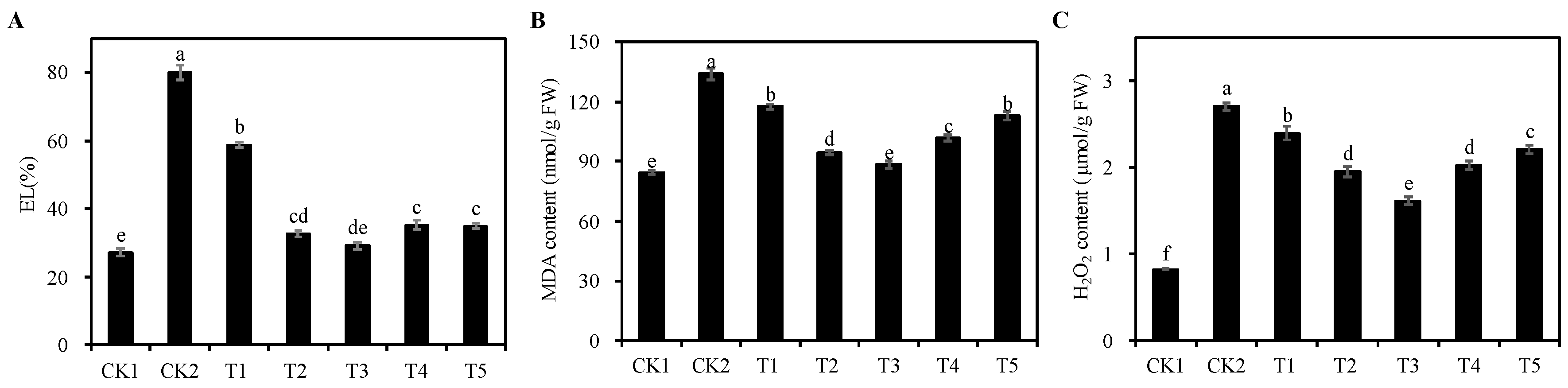
3.7. Oxidative Damage of Different Concentrations of Cp2 EPS on Alfalfa Seedlings under Salt Stress
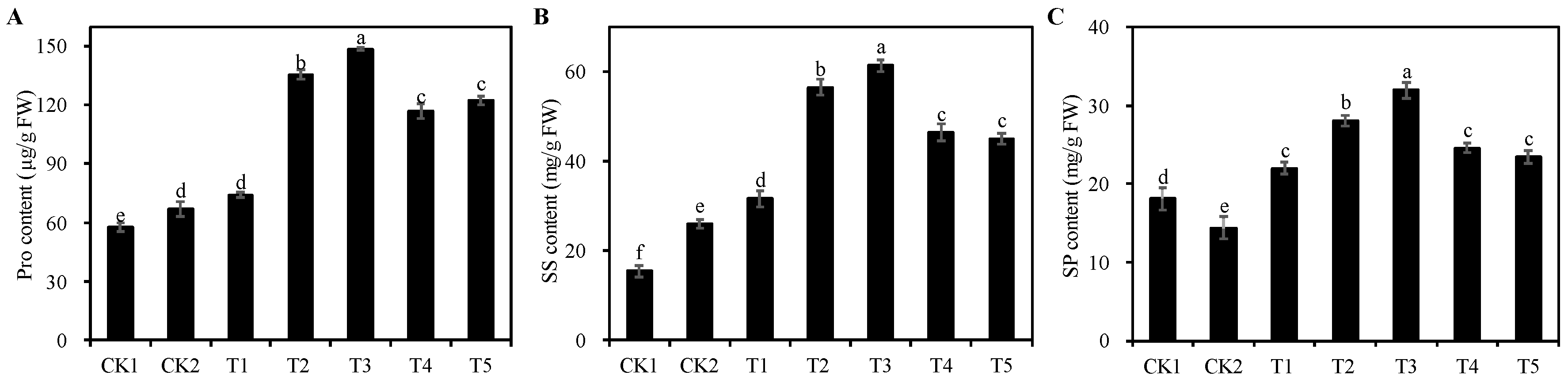
3.8. Effects of Different Concentrations of Cp2 EPS on Osmoregulatory Substances in Alfalfa Seedlings under Salt Stress
3.9. Effects of Different Concentrations of Cp2 EPS on Antioxidant Enzyme Activities of Alfalfa Seedlings under Salt Stress
3.10. Correlation Analysis and Principal Component Analysis
3.11. Comprehensive Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, K.; Qi, Y.; Chen, F.; Meng, Y.J.; Luo, X.F.; Shuai, H.W.; Zhou, W.G.; Ding, J.; Du, J.B.; Liu, J.; et al. Salt stress represses soybean seed germination by negatively regulating GA biosynthesis while positively mediating ABA biosynthesis. Front. Plant Sci. 2017, 8, 1372. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Lombardi, T.; Lupi, B. Effect of salinity on the germination and growth of Hordeum secalinum Schreber (Poaceae) in relation to the seeds after-ripening time. Biology 2006, 113, 37–42. [Google Scholar]
- Qureshi, M.I.; Abgin, M.Z.; Ahmad, J.; Muhammad, I. Effect of long-term salinity on cellular antioxidants, compatible solute and fatty acid profile of Sweet Annie (Artemisia annua L.). Phytochemistry 2013, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, properties and industrial food application of lactic acid bacteria-derived exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Alami, Y.; Achouak, W.; Marol, C.; Heulin, T. Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. strain isolated from sunflower roots. Appl. Environ. Microbiol. 2000, 66, 3393–3398. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz. J. Microbiol. 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils. 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Nunkaew, T.; Kantachote, D.; Nitoda, T.; Kanzaki, H.; Ritchie, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341. [Google Scholar] [CrossRef]
- Yang, F.R.; Chen, J.L.; Ye, S.H.; Liu, Z.F. Characterization of antioxidant activity of exopolysaccharides from endophytic Lysinibacillus sphaericus Ya6 under osmotic stress conditions. Process Biochem. 2022, 113, 87–96. [Google Scholar] [CrossRef]
- Guo, S.; Ma, X.; Cai, W.; Wang, Y.; Gao, X.; Fu, B.; Li, S. Exogenous proline improves salt tolerance of alfalfa through modulation of antioxidant capacity, ion homeostasis, and proline metabolism. Plants 2022, 11, 2994. [Google Scholar] [CrossRef] [PubMed]
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Sun, T.; Li, Z.Z. Alfalfa-corn rotation and row placement affects yield, water use, and economic returns in Northeast China. Field Crops Res. 2019, 241, 07558. [Google Scholar] [CrossRef]
- Lúa, J.; Roda, C.; Zanetti, M.E.; Blanco, F.A. Compatibility between legumes and Rhizobia for the establishment of a successful nitrogen-fixing symbiosis. Genes 2018, 9, 125. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Tayade, R.; Asekova, S.; Song, T.G.; Shannon, G.J.; Lee, D.J. Harnessing the potential of forage legumes, alfalfa, soybean, and cowpea for sustainable agriculture and global food security. Front. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef]
- Song, Y.G.; Lv, J.; Ma, Z.Q.; Dong, W. The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regul. 2019, 89, 239–249. [Google Scholar] [CrossRef]
- Singer, S.D.; Hannoufa, A.; Acharya, S. Molecular improvement of alfalfa for enhanced productivity and adaptability in a changing environment. Plant Cell Env. 2018, 41, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Van, Z.E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Xiong, X. Salt Tolerance Mechanism of Alfalfa under Non-Uniform Salt Stress; Nanjing Agricultural University: Nanjing, China, 2019; p. 13. [Google Scholar] [CrossRef]
- Mao, P.S.; Zhang, Y.; Huang, Q.; Mao, C.L.; Tang, J.; Sun, M. Physiological responses of Aohan alfalfa seeds to Melatonin priming under alkaline salt stress. Chin. J. Grassl. 2020, 42, 30–36. [Google Scholar] [CrossRef]
- Yao, B.; Huang, R.; Zhang, Z.F.; Shi, S.L. Seed-borne Erwinia persicina affects the growth and physiology of alfalfa (Medicago sativa L.). Front. Microbiol. 2022, 13, 891188. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Song, J.; Shi, W.W.; Liu, R.R.; Xu, Y.G.; Sui, N.; Zhou, J.C.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Ehab, A.I. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- Shobhit, R.V.; Vikas, K.P.; Jay, S.S. Plant growth promoting Curtobacterium albidum strain SRV4: An agriculturally important microbe to alleviate salinity stress in paddy plants. Ecol. Indic. 2019, 105, 553–562. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, L.; Ma, Y.; Lei, P.; Wang, R.; Gu, Y.; Li, S.; Zhang, F.; Xu, H. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int. J. Biol. Macromol. 2022, 209, 396–404. [Google Scholar] [CrossRef]
- Cramer, G.R.; Läuchli, A.; Epstein, E. Effects of NaCl and CaCl2 on ion activities in complex nutrient solutions and root growth of cotton. Plant Physiol. 1986, 81, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Luo, Y.T.; Li, Z.F.; Wang, J.M.; Wei, G.H. Role of exopolysaccharide in salt stress resistance and cell motility of Mesorhizobium alhagi CCNWXJ12-2T. Appl. Microbiol. Biotechnol. 2017, 101, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Chai, J.L.; Zhang, Y.C.; Zhang, C.; Lei, Y.; Li, Q.P.; Yao, T. Halotolerant rhizobacteria mitigate the effects of salinity stress on maize growth by secreting exopolysaccharides. Environ. Exp. Bot. 2022, 204, 105098. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity. Food Chem. 2008, 111, 642–647. [Google Scholar] [CrossRef]
- Din, B.U.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Munis, M.F.H.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, physiological and molecular markers for salt-stressed plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef]
- Mira, M.M.; Katarzyna, C.; Robert, D.H.; Claudio, S. In vitro differentiation of tracheary elements is induced by suppression of Arabidopsis phytoglobins. Plant Physiol. Biochem. 2019, 135, 141–148. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from Bacillus amyloliquefaciens C-1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Arroussi, H.E.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; Mernissi, N.E.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J. Appl. Phycol. 2018, 30, 2929–2941. [Google Scholar] [CrossRef]






| Treatments | Germination Potential (%) | Germination Rate (%) | Germination Index |
|---|---|---|---|
| CK1 | 98.00 ± 0.76 a | 99.50 ± 0.50 a | 68.22 ± 0.40 a |
| CK2 | 80.50 ± 1.41 c | 85.00 ± 1.07 d | 49.17 ± 1.10 c |
| T1 | 89.00 ± 1.81 b | 93.50 ± 1.30 c | 59.37 ± 0.94 b |
| T2 | 95.50 ± 1.18 a | 97.50 ± 0.73 ab | 61.12 ± 0.91 b |
| T3 | 96.50 ± 1.59 a | 98.50 ± 0.73 ab | 61.86 ± 1.09 b |
| T4 | 96.00 ± 0.66 a | 98.00 ± 0.76 ab | 59.38 ± 0.83 b |
| T5 | 94.50 ± 1.68 a | 96.50 ± 1.18 b | 60.20 ± 1.24 b |
| Treatments | Average Root Diameter (mm) | Root Surface Area (cm2) | Root Volume (cm3) |
|---|---|---|---|
| CK1 | 0.323 ± 0.009 e | 3.329 ± 0.162 a | 0.027 ± 0.001 a |
| CK2 | 1.064 ± 0.026 a | 0.588 ± 0.032 e | 0.013 ± 0.002 c |
| T1 | 0.678 ± 0.019 b | 1.271 ± 0.062 d | 0.019 ± 0.001 b |
| T2 | 0.550 ± 0.015 d | 2.058 ± 0.050 c | 0.027 ± 0.002 a |
| T3 | 0.603 ± 0.014 c | 2.536 ± 0.146 b | 0.028 ± 0.001 a |
| T4 | 0.619 ± 0.019 bc | 1.627 ± 0.165 d | 0.020 ± 0.002 b |
| T5 | 0.647 ± 0.017 b | 1.360 ± 0.177 d | 0.018 ± 0.002 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Jin, Z.; Huang, R.; He, L.; Tian, W.; Zhao, L.; Zhang, Z. Promotion of Growth of Alfalfa by Erwinia persicina Cp2 Exopolysaccharides under NaCl Stress. Agronomy 2023, 13, 2129. https://doi.org/10.3390/agronomy13082129
Chen H, Jin Z, Huang R, He L, Tian W, Zhao L, Zhang Z. Promotion of Growth of Alfalfa by Erwinia persicina Cp2 Exopolysaccharides under NaCl Stress. Agronomy. 2023; 13(8):2129. https://doi.org/10.3390/agronomy13082129
Chicago/Turabian StyleChen, Haiyan, Zhenhai Jin, Rong Huang, Linxin He, Wangjun Tian, Liang Zhao, and Zhenfen Zhang. 2023. "Promotion of Growth of Alfalfa by Erwinia persicina Cp2 Exopolysaccharides under NaCl Stress" Agronomy 13, no. 8: 2129. https://doi.org/10.3390/agronomy13082129
APA StyleChen, H., Jin, Z., Huang, R., He, L., Tian, W., Zhao, L., & Zhang, Z. (2023). Promotion of Growth of Alfalfa by Erwinia persicina Cp2 Exopolysaccharides under NaCl Stress. Agronomy, 13(8), 2129. https://doi.org/10.3390/agronomy13082129






