Effects of Drought Hardening and Saline Water Irrigation on the Growth, Yield, and Quality of Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Agronomic Practices
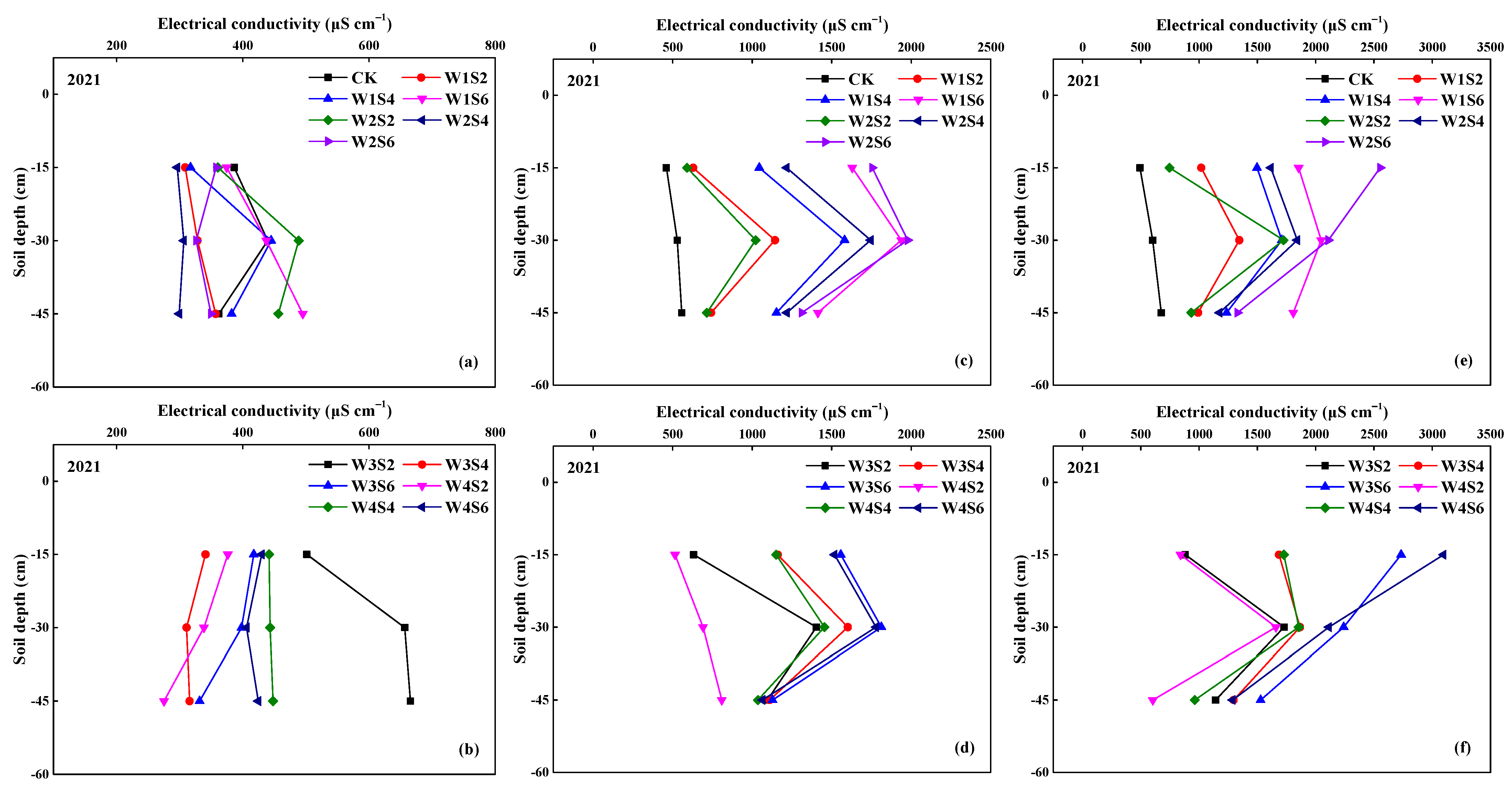
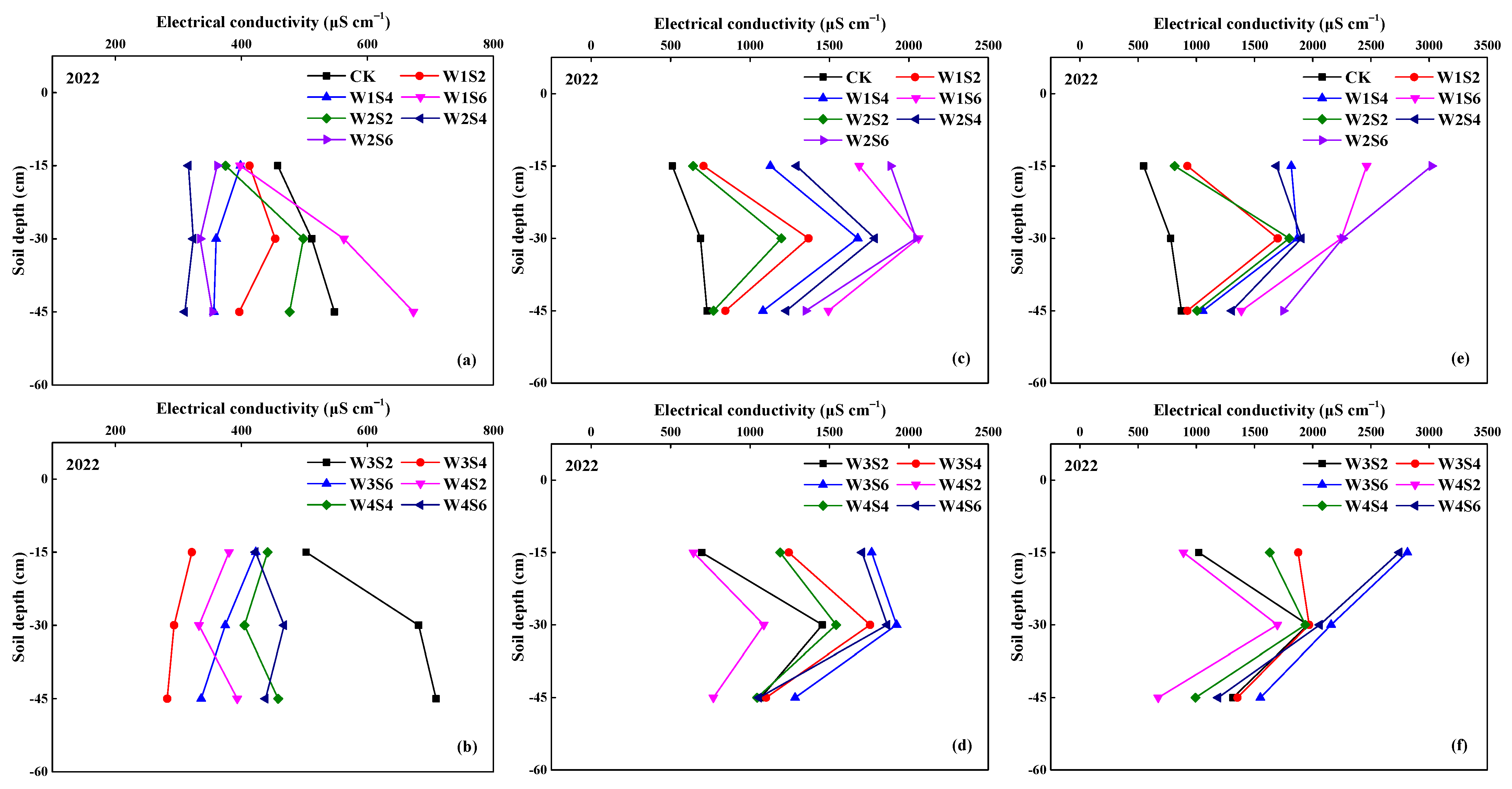
2.4. Measurements of Soil Salinity
2.5. Measurements of Growth and Physiological Parameters
2.6. Measurements of Yield, WUE and Quality
2.7. Statistical Analysis
3. Results
3.1. Soil Salinity (EC)
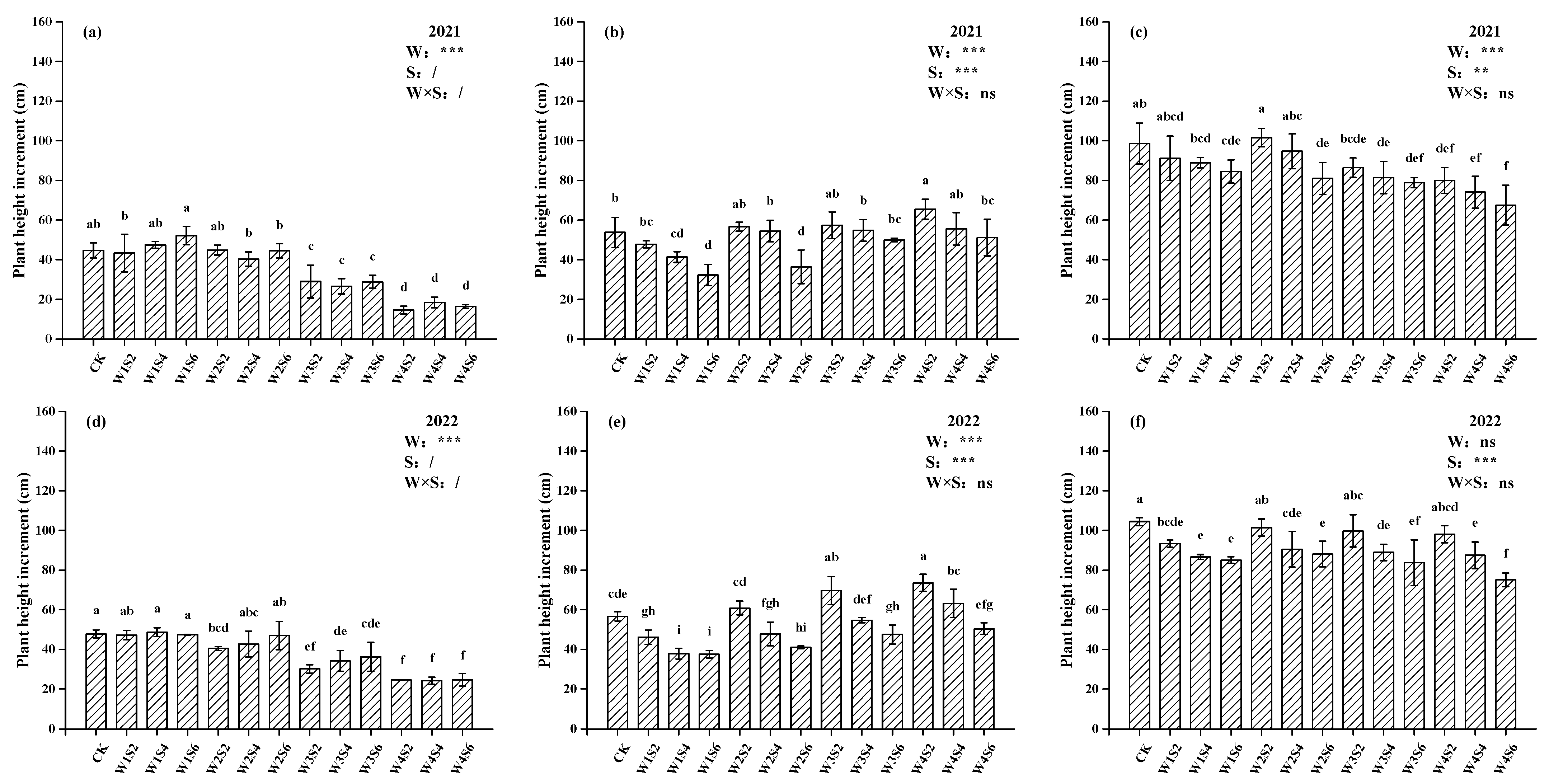
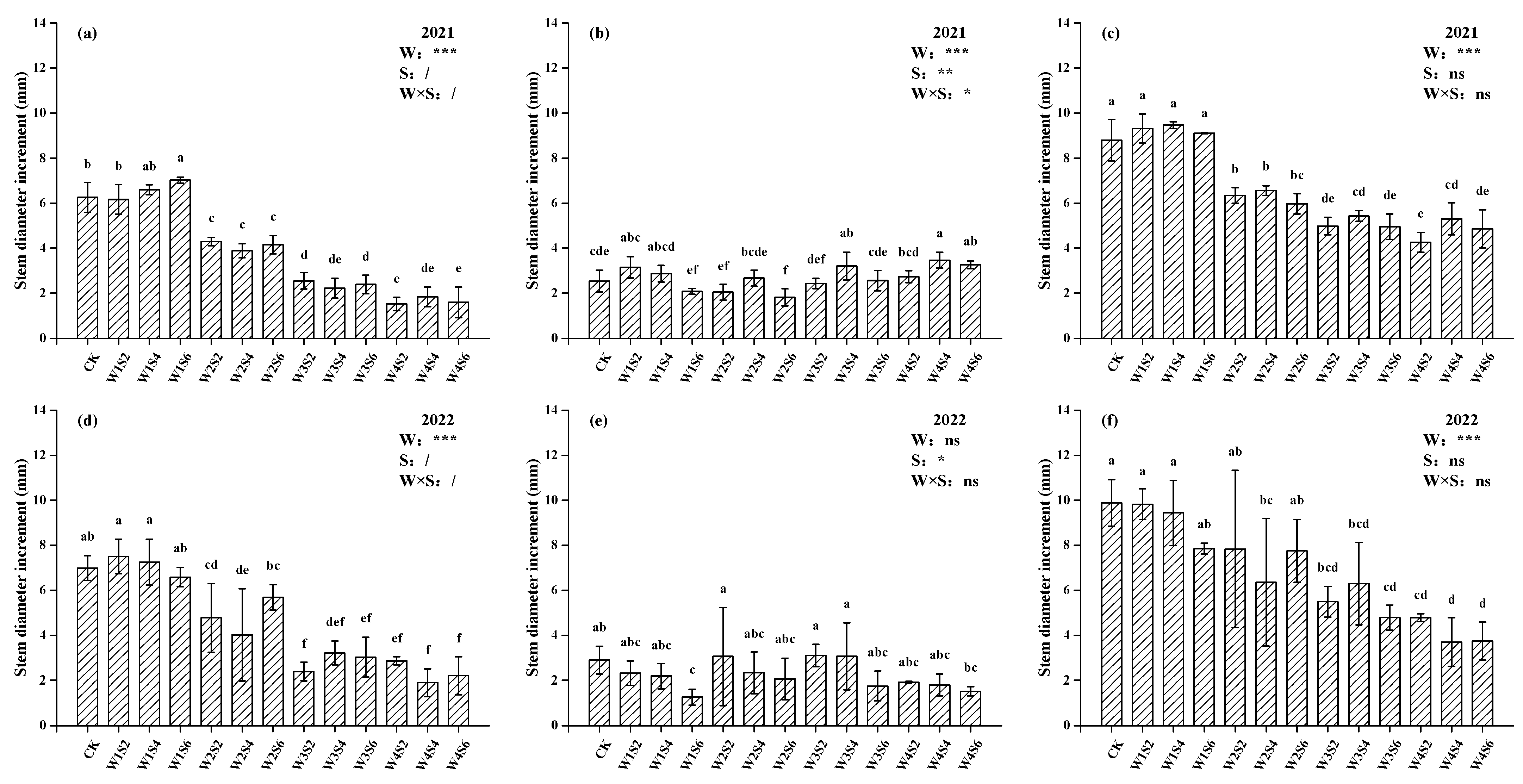
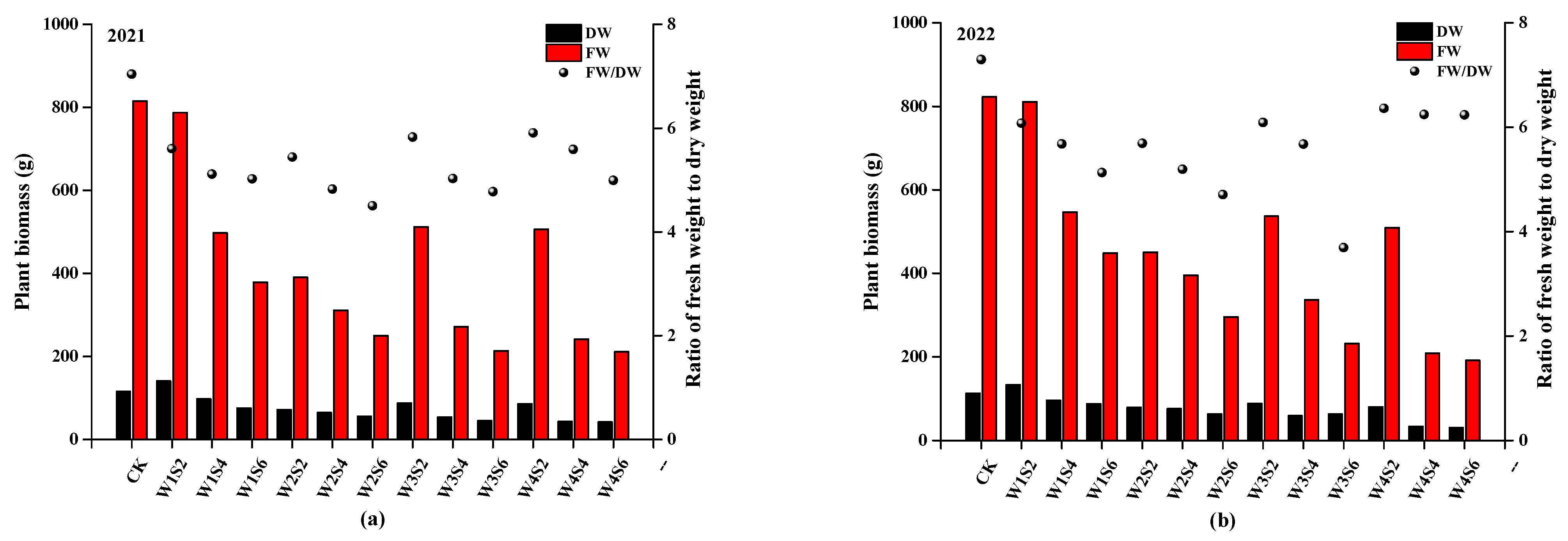
3.2. Growth Parameters
3.3. Physiological Properties
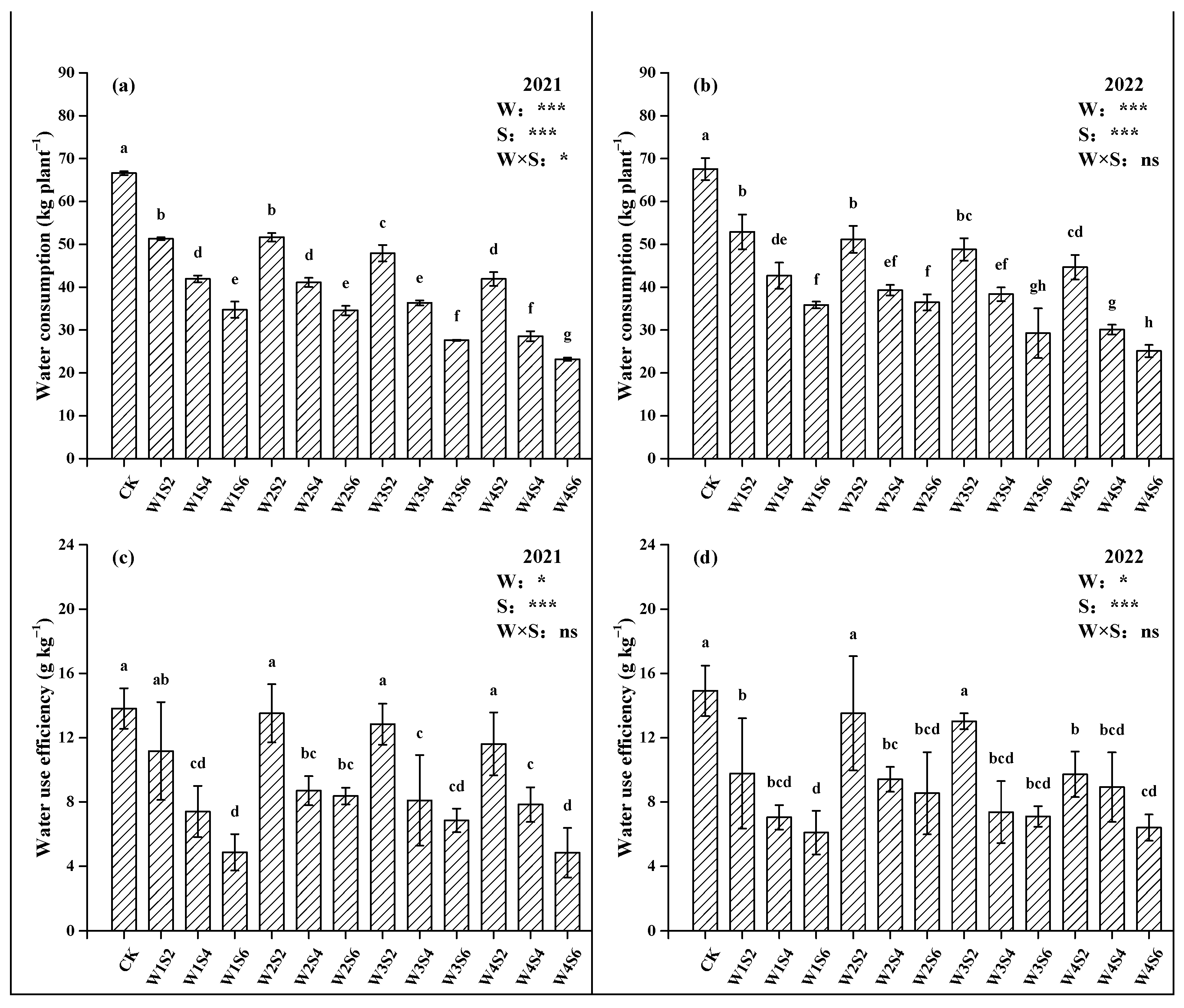
3.4. Yield Components and Water Use Efficiency (WUE)
3.5. Fruit Quality
4. Discussion
4.1. Effects of Drought Hardening on Tomato Seedlings
4.2. Effects of Saline Water Irrigation
4.3. Combined Effects of Drought Hardening and Saline Water Irrigation
4.4. Future Research Plan
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Shao, G.C.; Lu, J.; Zhang, K.; Wu, S.Q.; Wang, Z.Y. Effects of biochar application on crop water use efficiency depend on experimental conditions: A meta-analysis. Field Crops Res. 2020, 249, 107763. [Google Scholar] [CrossRef]
- Du, Y.D.; Niu, W.Q.; Gu, X.B.; Zhang, Q.; Cui, B.J.; Zhao, Y. Crop yield and water use efficiency under aerated irrigation: A meta-analysis. Agric. Water Manag. 2018, 210, 158–164. [Google Scholar] [CrossRef]
- Bañón, D.; Lorente, B.; Ortuño, M.F.; Bañón, S.; Sánchez-Blanco, M.J.; Alarcón, J.J. Effects of saline irrigation on the physiology and ornamental quality of Euphorbia Ascot Rainbow and its relationship with salinity indexes based on the bulk electrical conductivity. Sci. Hortic. 2022, 305, 111406. [Google Scholar] [CrossRef]
- Jafari, S.; Garmdareh, S.E.H. Effects of salinity on morpho-physiological, and biochemical characteristics of stock plant (Matthiola incana L.). Sci. Hortic. 2019, 257, 108731. [Google Scholar] [CrossRef]
- Su, H.; Sun, H.Y.; Dong, X.L.; Chen, P.; Zhang, X.J.; Tian, L.; Liu, X.J.; Wang, J.T. Did manure improve saline water irrigation threshold of winter wheat? A 3-year field investigation. Agric. Water Manag. 2021, 258, 107203. [Google Scholar] [CrossRef]
- Izadi, Y.; Moosavi, S.A.; Gharineh, M.H. Salinity affects eco-physiological aspects and biochemical compositions in chia (Salvia hispanica L.) during germination and seedling growth. Sci. Hortic. 2022, 306, 111461. [Google Scholar] [CrossRef]
- Loganathachetti, D.S.; Alhashmi, F.; Chandran, S.; Mundra, S. Irrigation water salinity structures the bacterial communities of date palm (Phoenix dactylifera)-associated bulk soil. Front. Plant Sci. 2022, 13, 944637. [Google Scholar] [CrossRef] [PubMed]
- Cássia Alves, R.; Medeiros, A.S.; Nicolau, M.C.M.; Assis Oliveira, F.; Lima, L.W.; Aroucha, E.M.M.; Gratão, P.L. Influence of partial root-zone saline irrigation management on tomato yield and fruit quality from a potted-plant study. Hortscience 2018, 53, 1326–1331. [Google Scholar] [CrossRef]
- Li, J.S.; Gao, Y.M.; Zhang, X.Y.; Tian, P.; Li, J.; Tian, Y.Q. Comprehensive comparison of different saline water irrigation strategies for tomato production: Soil properties, plant growth, fruit yield and fruit quality. Agric. Water Manag. 2019, 213, 521–533. [Google Scholar] [CrossRef]
- Li, J.G.; Chen, J.; He, P.R.; Chen, D.; Dai, X.P.; Jin, Q.; Su, X.Y. The optimal irrigation water salinity and salt component for high-yield and good-quality of tomato in Ningxia. Agric. Water Manag. 2022, 274, 107940. [Google Scholar] [CrossRef]
- Han, X.Y.; Kang, Y.H.; Wan, S.Q.; Li, X.B. Effect of salinity on oleic sunflower (Helianthus annuus Linn.) under drip irrigation in arid area of Northwest China. Agric. Water Manag. 2022, 259, 107267. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, X.Y.; Šimůnek, J.; Shi, H.B.; Chen, N.; Hu, Q. Optimizing drip irrigation with alternate use of fresh and brackish waters by analyzing salt stress: The experimental and simulation approaches. Soil Tillage Res. 2022, 219, 105355. [Google Scholar] [CrossRef]
- Zhang, W.D.; Du, T.S. Fresh/brackish watering at growth period provided a trade-off between lettuce growth and resistance to NaCl-induced damage. Sci. Hortic. 2022, 304, 111283. [Google Scholar] [CrossRef]
- Song, J.S.; Zhang, H.Y.; Chang, F.D.; Yu, R.; Wang, J.; Wang, X.Q.; Li, Y.Y. If the combination of straw interlayer and irrigation water reduction maintained sunflower yield by boosting soil fertility and improving bacterial community in arid and saline areas. Agric. Water Manag. 2022, 262, 107424. [Google Scholar] [CrossRef]
- Li, X.B.; Kang, Y.H.; Wan, S.Q.; Chen, X.L.; Xu, J.C. Effect of drip-irrigation with saline water on Chinese rose (Rosa chinensis) during reclamation of very heavy coastal saline soil in a field trial. Sci. Hortic. 2015, 186, 163–171. [Google Scholar] [CrossRef]
- Zhu, M.J.; Wang, Q.J.; Sun, Y.; Zhang, J.H. Effects of oxygenated brackish water on germination and growth characteristics of wheat. Agric. Water Manag. 2021, 245, 106520. [Google Scholar] [CrossRef]
- Zhou, B.B.; Liang, C.F.; Chen, X.P.; Ye, S.T.; Peng, Y.; Yang, L.; Duan, M.L.; Wang, X.P. Magnetically-treated brackish water affects soil water-salt distribution and the growth of cotton with film mulch drip irrigation in Xinjiang, China. Agric. Water Manag. 2022, 263, 107487. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhou, B.B.; Mu, Y.; Wang, Y.H.; Liu, Y.Q.; Wang, L. Irrigation with activated water promotes root growth and improves water use of winter wheat. Agronomy 2021, 11, 2459. [Google Scholar] [CrossRef]
- Ghanbari, F.; Kordi, S. Hardening pretreatment by drought and low temperature enhanced chilling stress tolerance of cucumber seedlings. Acta Sci. Pol. Hortorum Cultus 2019, 18, 29–37. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.H.; Shah, S.; Wu, X.Y.; Shaheen, A.; Xiao, L.X.; Wu, Y.H.; Wang, S.S. Drought-hardening improves drought tolerance in Nicotiana tabacum at physiological, biochemical, and molecular levels. BMC Plant Biol. 2020, 20, 486. [Google Scholar] [CrossRef]
- Yang, S.L.; Chen, K.; Wang, S.S.; Gong, M. Osmoregulation as a key factor in drought hardening-induced drought tolerance in Jatropha curcas. Biol. Plant. 2015, 59, 529–536. [Google Scholar] [CrossRef]
- Khan, R.; Ma, X.H.; Zhang, J.; Wu, X.Y.; Iqbal, A.; Wu, Y.H.; Zhou, L.; Wang, S.S. Circular drought-hardening confers drought tolerance via modulation of the antioxidant defense system, osmoregulation, and gene expression in tobacco. Physiol. Plant. 2021, 172, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Lou, R.T.; Li, D.X.; Li, Y.B.; Bian, Z.P.; Zhu, Y.N. Effect of pre-anthesis drought hardening on post-anthesis physiological characteristics, yield and WUE in winter wheat. Phyton-Int. J. Exp. Bot. 2021, 90, 245–257. [Google Scholar] [CrossRef]
- Rajashekar, C.B.; Panda, M. Water stress is a component of cold acclimation process essential for inducing full freezing tolerance in strawberry. Sci. Hortic. 2014, 174, 54–59. [Google Scholar] [CrossRef]
- Ghanbari, F.; Sayyari, M. Controlled drought stress affects the chilling-hardening capacity of tomato seedlings as indicated by changes in phenol metabolisms, antioxidant enzymes activity, osmolytes concentration and abscisic acid accumulation. Sci. Hortic. 2018, 229, 167–174. [Google Scholar] [CrossRef]
- Álvarez, S.; Gómez-Bellot, M.J.; Acosta-Motos, J.R.; Sánchez-Blanco, M.J. Application of deficit irrigation in Phillyrea angustifolia for landscaping purposes. Agric. Water Manag. 2019, 218, 193–202. [Google Scholar] [CrossRef]
- Shao, G.C.; Cheng, X.; Liu, N.; Zhang, Z.H. Effect of drought pretreatment before anthesis and post-anthesis waterlogging on water relation, photosynthesis, and growth of tomatoes. Arch. Agron. Soil Sci. 2016, 62, 935–946. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Li, X.; Li, N.; Jameson, P.E.; Zhou, W.B. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021, 186, 677–695. [Google Scholar] [CrossRef]
- Ors, S.; Suarez, D.L. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric. Water Manag. 2017, 190, 31–41. [Google Scholar] [CrossRef]
- Caliskan, O.; Radusiene, J.; Temizel, K.E.; Staunis, Z.; Cirak, C.; Kurt, D.; Odabas, M.S. The effects of salt and drought stress on phenolic accumulation in greenhouse-grown Hypericum pruinatum. Ital. J. Agron. 2017, 12, 271–275. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U.; Turan, M.; Dursun, A. Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. S. Afr. J. Bot. 2021, 137, 335–339. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.M.; Bertin, N.; Ding, R.S.; Du, T.S. Quantitative responses of tomato yield, fruit quality and water use efficiency to soil salinity under different water regimes in Northwest China. Agric. Water Manag. 2023, 277, 108134. [Google Scholar] [CrossRef]
- Yang, H.; Du, T.S.; Mao, X.M.; Ding, R.S.; Shukla, M.K. A comprehensive method of evaluating the impact of drought and salt stress on tomato growth and fruit quality based on EPIC growth model. Agric. Water Manag. 2019, 213, 116–127. [Google Scholar] [CrossRef]
- Agbna, G.H.D.; She, D.L.; Liu, Z.P.; Elshaikh, N.A.; Shao, G.C.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Cao, Y.E.; Gao, Y.M.; Li, J.S.; Tian, Y.Q. Straw composts, gypsum and their mixtures enhance tomato yields under continuous saline water irrigation. Agric. Water Manag. 2019, 223, 105721. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Shalan, A.M.; El-Boray, M.S.; Vincent, C.I.; El-Kady, M.E.; Grosser, J.W.; Dutt, M. Application of silicon nanoparticles enhances oxidative stress tolerance in salt stressed ‘Valencia’ sweet orange plants. Sci. Hortic. 2022, 295, 110856. [Google Scholar] [CrossRef]
- Shao, G.C.; Gao, Y.; Lin, J.; Liu, Z.J.; Huang, D.D. Effects of biochar on water requirement regulation and yield of tomato under different groundwater tables. Trans. Chin. Soc. Agric. Mach. 2019, 50, 250–258. [Google Scholar]
- She, D.L.; Sun, X.Q.; Gamareldawla, A.H.D.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S.E. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci. Rep. 2018, 8, 14743. [Google Scholar] [CrossRef]
- Li, J.G.; Chen, J.; Qu, Z.Y.; Wang, S.L.; He, P.R.; Zhang, N. Effects of alternating irrigation with fresh and saline water on the soil salt, soil nutrients, and yield of tomatoes. Water 2019, 11, 1693. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Li, B.; Shukla, M.K.; Du, T.S. Combined environmental stresses induced by drip irrigation positively affect most solar greenhouse grown tomato fruit quality. Sci. Hortic. 2021, 288, 110334. [Google Scholar] [CrossRef]
- Ors, S.; Ekinci, M.; Yildirim, E.; Sahin, U. Changes in gas exchange capacity and selected physiological properties of squash seedlings (Cucurbita pepo L.) under well-watered and drought stress conditions. Arch. Agron. Soil Sci. 2016, 62, 1700–1710. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Kiziloglu, F.M.; Yildirim, E.; Turan, M.; Kotan, R.; Ors, S. Ameliorative effects of plant growth promoting bacteria on water-yield relationships, growth, and nutrient uptake of lettuce plants under different irrigation levels. Hortscience 2015, 50, 1379–1386. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Dai, H.X.; Zheng, W.T.; Cao, F.B.; Zhang, G.P.; Sun, D.F.; Wu, F.B. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2013, 63, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Huo, Z.L.; Zhang, L.D.; Wang, J.H.; Zhao, Y. Impact of saline water irrigation on water use efficiency and soil salt accumulation for spring maize in arid regions of China. Agric. Water Manag. 2016, 163, 125–138. [Google Scholar] [CrossRef]
- Gao, H.; Fu, T.G.; Tang, S.P.; Liu, J.T. Effects of saline water irrigation on winter wheat and its safe utilization under a subsurface drainage system in coastal saline-alkali land of Hebei Province, China. Irrig. Sci. 2023, 41, 251–260. [Google Scholar] [CrossRef]
- Wang, X.D.; Tian, W.; Zheng, W.D.; Shah, S.; Li, J.S.; Wang, X.Z.; Zhang, X.Y. Quantitative relationships between salty water irrigation and tomato yield, quality, and irrigation water use efficiency: A meta-analysis. Agric. Water Manag. 2023, 280, 108213. [Google Scholar] [CrossRef]
- Yan, S.H.; Gao, Y.M.; Tian, M.J.; Tian, Y.Q.; Li, J.S. Comprehensive evaluation of effects of various carbon-rich amendments on tomato production under continuous saline water irrigation: Overall soil quality, plant nutrient uptake, crop yields and fruit quality. Agric. Water Manag. 2021, 255, 106995. [Google Scholar] [CrossRef]
- Trabelsi, L.; Gargouri, K.; Ayadi, M.; Mbadra, C.; Nasr, M.B.; Mbarek, H.B.; Ghrab, M.; Ahmed, G.B.; Kammoun, Y.; Loukil, E.; et al. Impact of drought and salinity on olive potential yield, oil and fruit qualities (cv. Chemlali) in an arid climate. Agric. Water Manag. 2022, 269, 107726. [Google Scholar] [CrossRef]
- Zhai, Y.M.; Yang, Q.; Wu, Y.Y. Soil salt distribution and tomato response to saline water irrigation under straw mulching. PLoS ONE 2016, 11, e0165985. [Google Scholar] [CrossRef]
- Wang, T.Y.; Xu, Z.H.; Pang, G.B. Effects of irrigating with brackish water on soil moisture, soil salinity, and the agronomic response of winter wheat in the Yellow River Delta. Sustainability 2019, 11, 5801. [Google Scholar] [CrossRef]
- Culha, S.; Cakırlar, H. The effect of salinity on plants and salt tolerance mechanisms. Afyon Kocatepe Univ. J. Sci. 2011, 11, 11–34. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Sarker, A.; Hossain, M.I.; Kashem, M.A. Salinity (NaCl) tolerance of four vegetable crops during germination and early seedling growth. Int. J. Latest Res. Sci. Technol. 2014, 3, 91–95. [Google Scholar]
- Zhai, Y.M.; Yang, Q.; Hou, M.M. The effects of saline water drip irrigation on tomato yield, quality, and blossom-end rot incidence—A 3a case study in the south of China. PLoS ONE 2015, 10, e0142204. [Google Scholar] [CrossRef]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Sanoubar, R.; Cellini, A.; Veroni, A.M.; Spinelli, F.; Masia, A.; Antisari, L.V.; Orsini, F.; Gianquinto, G. Salinity thresholds and genotypic variability of cabbage (Brassica oleracea L.) grown under saline stress. J. Sci. Food Agric. 2016, 96, 319–330. [Google Scholar] [CrossRef]
- Jiang, P.; Cai, F.; Zhao, Z.Q.; Meng, Y.; Gao, L.Y.; Zhao, T.H. Physiological and dry matter characteristics of spring maize in Northeast China under drought stress. Water 2018, 10, 1561. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Yamagishi, T.; Ishii, R. Leaf nitrogen distribution to maximize the canopy photosynthesis in rice. Field Crops Res. 2006, 95, 291–304. [Google Scholar] [CrossRef]
- Cheng, M.H.; Wang, H.D.; Fan, J.L.; Wang, X.K.; Sun, X.; Yang, L.; Zhang, S.H.; Xiang, Y.Z.; Zhang, F.C. Crop yield and water productivity under salty water irrigation: A global meta-analysis. Agric. Water Manag. 2021, 256, 107105. [Google Scholar] [CrossRef]
- Li, J.G.; He, P.R.; Chen, J.; Hamad, A.A.A.; Dai, X.P.; Jin, Q.; Ding, S.Y. Tomato performance and changes in soil chemistry in response to salinity and Na/Ca ratio of irrigation water. Agric. Water Manag. 2023, 285, 108363. [Google Scholar] [CrossRef]
- Yuan, M.; Shao, G.C.; Cui, J.T.; Guo, Y.X.; Zhu, M.R. Effect of pre-anthesis drought on fruit yield and quality of post-anthesis waterlogged tomato. J. Drain. Irrig. Mach. Eng. 2016, 34, 436–442. [Google Scholar]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Vignjevic, M.; Jiang, D.; Jacobsen, S.; Wollenweber, B. Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 2014, 65, 6441–6456. [Google Scholar] [CrossRef]
- Hou, X.M.; Zhang, W.D.; Du, T.S.; Kang, S.Z.; Davies, W.J. Responses of water accumulation and solute metabolism in tomato fruit to water scarcity and implications for main fruit quality variables. J. Exp. Bot. 2020, 71, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Day, B.L.H.; Carins-Murphy, M.R.; Brodribb, T.J. Reproductive water supply is prioritized during drought in tomato. Plant Cell Environ. 2022, 45, 69–79. [Google Scholar]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.X.; Yu, X.; You, C.C.; He, H.B.; Li, J.; Wu, L.Q. Adaptation physiological mechanism of rice under dual stress of drought and high temperature in booting stage. J. Gansu Agric. Univ. 2019, 54, 39–46. [Google Scholar]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Zhou, Q.; Dai, T.B.; Jiang, D. Physiological mechanisms of abiotic stress priming induced the crops stress tolerance: A review. Sci. Agric. Sin. 2021, 54, 2287–2301. [Google Scholar]



| Treatment | 2021 | ||||
|---|---|---|---|---|---|
| A (μmol m−2 s−1) | E (mmol m−2 s−1) | Gs (mmol m−2 s−1) | Ci (μmol mol−1) | SPAD | |
| CK | 14.01 ± 1.15 a | 11.22 ± 0.81 a | 415.79 ± 28.66 ab | 354.51 ± 6.18 c | 34.3 ± 3.6 ab |
| W1S2 | 12.25 ± 1.18 abc | 9.28 ± 0.77b cd | 389.85 ± 17.12 abcd | 370.33 ± 28.18 bc | 35.6 ± 3.8 ab |
| W1S4 | 10.11 ± 1.50 de | 8.52 ± 1.33 bcde | 350.36 ± 27.89 cde | 399.17 ± 52.22 abc | 34.0 ± 2.4 ab |
| W1S6 | 9.28 ± 0.35 e | 7.16 ± 0.05 e | 347.25 ± 24.05 cde | 443.91 ± 67.91 abc | 31.9 ± 1.7 b |
| W2S2 | 13.33 ± 2.08 ab | 9.48 ± 1.81 bcd | 405.76 ± 35.07 abc | 417.21 ± 109.43 abc | 36.0 ± 2.9 ab |
| W2S4 | 11.21 ± 0.08 cd | 8.68 ± 1.13 bcde | 384.51 ± 44.24 abcd | 420.11 ± 59.69 abc | 35.4 ± 1.4 ab |
| W2S6 | 10.45 ± 0.50 cde | 7.85 ± 0.32 de | 363.14 ± 29.32 bcde | 453.79 ± 49.35 ab | 35.2 ± 2.8 ab |
| W3S2 | 13.69 ± 0.64 ab | 10.04 ± 1.51 ab | 435.92 ± 33.23 a | 434.43 ± 40.57 abc | 36.8 ± 2.1 a |
| W3S4 | 11.80 ± 1.83 bcd | 9.86 ± 0.22 abc | 400.35 ± 28.92 abc | 458.70 ± 39.39 ab | 34.9 ± 2.6 ab |
| W3S6 | 10.85 ± 0.20 cde | 8.42 ± 1.24 bcde | 334.63 ± 19.23 de | 484.70 ± 92.38 a | 32.2 ± 2.2 b |
| W4S2 | 12.04 ± 1.62 bc | 9.69 ± 1.07 abc | 416.18 ± 88.67 ab | 363.52 ± 7.00 bc | 33.9 ± 3.1 ab |
| W4S4 | 9.94 ± 0.47 de | 8.78 ± 0.22 bcde | 354.43 ± 4.48 cde | 398.01 ± 53.94 abc | 32.6 ± 2.5 ab |
| W4S6 | 9.23 ± 0.51 e | 8.23 ± 0.79 cde | 323.05 ± 0.79 e | 410.37 ± 40.26 abc | 31.6 ± 2.3 b |
| W | ** | ns | ns | ns | ns |
| S | *** | *** | *** | ns | ns |
| W × S | ns | ns | ns | ns | ns |
| Treatment | 2022 | ||||
|---|---|---|---|---|---|
| A (μmol m−2 s−1) | E (mmol m−2 s−1) | Gs (mmol m−2 s−1) | Ci (μmol mol−1) | SPAD | |
| CK | 12.99 ± 0.80 a | 9.87 ± 0.40 a | 385.72 ± 4.32 a | 311.08 ± 55.32 bc | 34.2 ± 0.6 ab |
| W1S2 | 10.26 ± 0.22 bc | 7.73 ± 0.28 de | 278.97 ± 13.07 cd | 344.11 ± 43.36 bc | 32.6 ± 0.6 abc |
| W1S4 | 10.07 ± 0.42 bc | 7.68 ± 0.22 de | 274.78 ± 8.68 de | 393.12 ± 61.63 bc | 30.4 ± 1.4 bc |
| W1S6 | 8.80 ± 0.88 e | 7.41 ± 0.12 e | 264.04 ± 4.49 e | 430.21 ± 53.63 bc | 29.6 ± 6.6 c |
| W2S2 | 10.40 ± 0.33 bc | 8.15 ± 0.18 bcd | 289.50 ± 9.66 bc | 373.62 ± 32.99 bc | 33.9 ± 1.9 abc |
| W2S4 | 10.26 ± 0.05 bc | 8.07 ± 0.10 cd | 281.39 ± 4.07 bcd | 422.12 ± 9.47 bc | 32.9 ± 2.8 abc |
| W2S6 | 8.99 ± 0.11 de | 7.47 ± 0.36 e | 264.11 ± 10.79 e | 442.63 ± 33.47 b | 30.0 ± 1.5 bc |
| W3S2 | 10.47 ± 0.23 bc | 8.40 ± 0.24 bc | 291.82 ± 7.80 bc | 392.71 ± 89.21 bc | 35.7 ± 2.2 a |
| W3S4 | 10.38 ± 0.84 bc | 8.13 ± 0.19 bcd | 284.32 ± 3.72 bcd | 593.63 ± 223.46 a | 33.3 ± 2.9 abc |
| W3S6 | 9.83 ± 0.40 cd | 8.12 ± 0.34 cd | 280.00 ± 9.28 bcd | 625.14 ± 30.97 a | 31.5 ± 2.3 abc |
| W4S2 | 10.80 ± 0.43 b | 8.62 ± 0.27 b | 294.09 ± 7.30 b | 293.66 ± 44.90 c | 33.5 ± 2.6 abc |
| W4S4 | 10.67 ± 0.47 bc | 8.54 ± 0.35 bc | 292.36 ± 9.09 bc | 353.94 ± 33.45 bc | 33.0 ± 0.2 abc |
| W4S6 | 10.52 ± 0.49 bc | 8.16 ± 0.47 bcd | 280.21 ± 12.58 bcd | 731.44 ± 160.89 a | 32.7 ± 0.7 abc |
| W | ** | *** | ** | ** | ns |
| S | *** | *** | *** | *** | * |
| W × S | ns | ns | ns | ** | ns |
| Treatment | 2021 | |||
|---|---|---|---|---|
| TSS (%) | TS (%) | TA (%) | VC (mg 100g−1) | |
| CK | 6.00 ± 0.43 j | 1.80 ± 0.39 f | 0.430 ± 0.030 h | 10.18 ± 0.71 f |
| W1S2 | 6.68 ± 0.26 i | 1.91 ± 0.02 f | 0.539 ± 0.071 g | 11.42 ± 0.47 def |
| W1S4 | 8.48 ± 0.05 e | 2.44 ± 0.32 de | 0.608 ± 0.035 e | 13.82 ± 0.48 abc |
| W1S6 | 9.96 ± 0.06 b | 2.75 ± 0.25 cd | 0.398 ± 0.029 h | 10.58 ± 0.69 ef |
| W2S2 | 7.15 ± 0.05 h | 2.14 ± 0.12 ef | 0.546 ± 0.009 g | 12.33 ± 0.73 cd |
| W2S4 | 9.39 ± 0.13 c | 2.54 ± 0.21 d | 0.753 ± 0.029 b | 15.02 ± 2.37 a |
| W2S6 | 11.20 ± 0.18 a | 2.90 ± 0.28 c | 0.696 ± 0.046 cd | 11.84 ± 1.05 de |
| W3S2 | 7.67 ± 0.18 g | 2.77 ± 0.12 cd | 0.602 ± 0.035 ef | 12.83 ± 0.89 bcd |
| W3S4 | 9.08 ± 0.09 d | 3.70 ± 0.04 ab | 0.868 ± 0.020 a | 14.78 ± 0.44 a |
| W3S6 | 10.01 ± 0.03 b | 3.81 ± 0.17 a | 0.652 ± 0.017 cde | 11.26 ± 1.20 def |
| W4S2 | 7.00 ± 0.02 h | 2.63 ± 0.09 cd | 0.549 ± 0.018 fg | 11.74 ± 0.47 def |
| W4S4 | 8.01 ± 0.05 f | 2.89 ± 0.07 c | 0.703 ± 0.018 bc | 14.37 ± 0.48 ab |
| W4S6 | 9.75 ± 0.05 b | 3.40 ± 0.17 b | 0.647 ± 0.022 de | 10.37 ± 0.53 ef |
| W | *** | *** | *** | * |
| S | *** | *** | *** | *** |
| W × S | *** | ns | *** | ns |
| Treatment | 2022 | |||
|---|---|---|---|---|
| TSS (%) | TS (%) | TA (%) | VC (mg 100g−1) | |
| CK | 5.85 ± 0.02 h | 1.40 ± 0.01 f | 0.468 ± 0.030 d | 11.84 ± 1.46 g |
| W1S2 | 6.44 ± 0.03 g | 1.72 ± 0.14 ef | 0.489 ± 0.202 cd | 15.01 ± 0.90 ef |
| W1S4 | 8.84 ± 0.27 d | 2.09 ± 0.07 de | 0.666 ± 0.009 abcd | 18.30 ± 0.34 ab |
| W1S6 | 11.85 ± 0.18 a | 2.84 ± 0.24 ab | 0.865 ± 0.062 abcd | 15.72 ± 0.43 de |
| W2S2 | 7.33 ± 0.17 f | 1.82 ± 0.06 ef | 0.692 ± 0.023 abcd | 15.61 ± 0.77 de |
| W2S4 | 10.23 ± 0.05 b | 2.50 ± 0.05 bcd | 0.874 ± 0.055 abc | 19.17 ± 0.40 a |
| W2S6 | 12.07 ± 0.32 a | 3.09 ± 0.50 a | 1.015 ± 0.823 a | 16.83 ± 0.85 cd |
| W3S2 | 7.90 ± 0.05 e | 1.93 ± 0.05 e | 0.586 ± 0.021 bcd | 14.67 ± 0.51 ef |
| W3S4 | 10.11 ± 0.03 b | 2.63 ± 0.49 abc | 0.779 ± 0.068 abcd | 17.50 ± 0.83 bc |
| W3S6 | 11.93 ± 0.10 a | 3.05 ± 0.67 a | 0.950 ± 0.037 ab | 15.71 ± 0.47 de |
| W4S2 | 7.50 ± 0.16 f | 1.85 ± 0.05 ef | 0.583 ± 0.032 bcd | 13.95 ± 1.30 f |
| W4S4 | 10.02 ± 0.01 b | 2.19 ± 0.08 cde | 0.694 ± 0.014 abcd | 15.84 ± 0.65 de |
| W4S6 | 11.87 ± 0.14 a | 2.70 ± 0.13 ab | 0.941 ± 0.056 ab | 12.33 ± 0.94 g |
| W | *** | ns | ns | *** |
| S | *** | *** | ** | *** |
| W × S | *** | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Shao, G.; Cui, J.; Lu, J.; Tian, L.; Song, E.; Zeng, Z. Effects of Drought Hardening and Saline Water Irrigation on the Growth, Yield, and Quality of Tomato. Agronomy 2023, 13, 2351. https://doi.org/10.3390/agronomy13092351
Gao Y, Shao G, Cui J, Lu J, Tian L, Song E, Zeng Z. Effects of Drought Hardening and Saline Water Irrigation on the Growth, Yield, and Quality of Tomato. Agronomy. 2023; 13(9):2351. https://doi.org/10.3390/agronomy13092351
Chicago/Turabian StyleGao, Yang, Guangcheng Shao, Jintao Cui, Jia Lu, Longjia Tian, Enze Song, and Zhongyi Zeng. 2023. "Effects of Drought Hardening and Saline Water Irrigation on the Growth, Yield, and Quality of Tomato" Agronomy 13, no. 9: 2351. https://doi.org/10.3390/agronomy13092351




