Silicon and Strigolecton Application Alleviates the Adversities of Cadmium Toxicity in Maize by Modulating Morpho-Physiological and Antioxidants Defense Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Treatments and Design
2.3. Morphological Traits
2.4. Photosynthetic Pigments
2.5. Determination of Oxidative Stress Indicators
2.6. Determination of Enzymatic Antioxidants Activities
2.7. Osmolytes Determination
2.8. Determination of the Cd and Si Contents
2.9. Statistical Analysis
3. Results
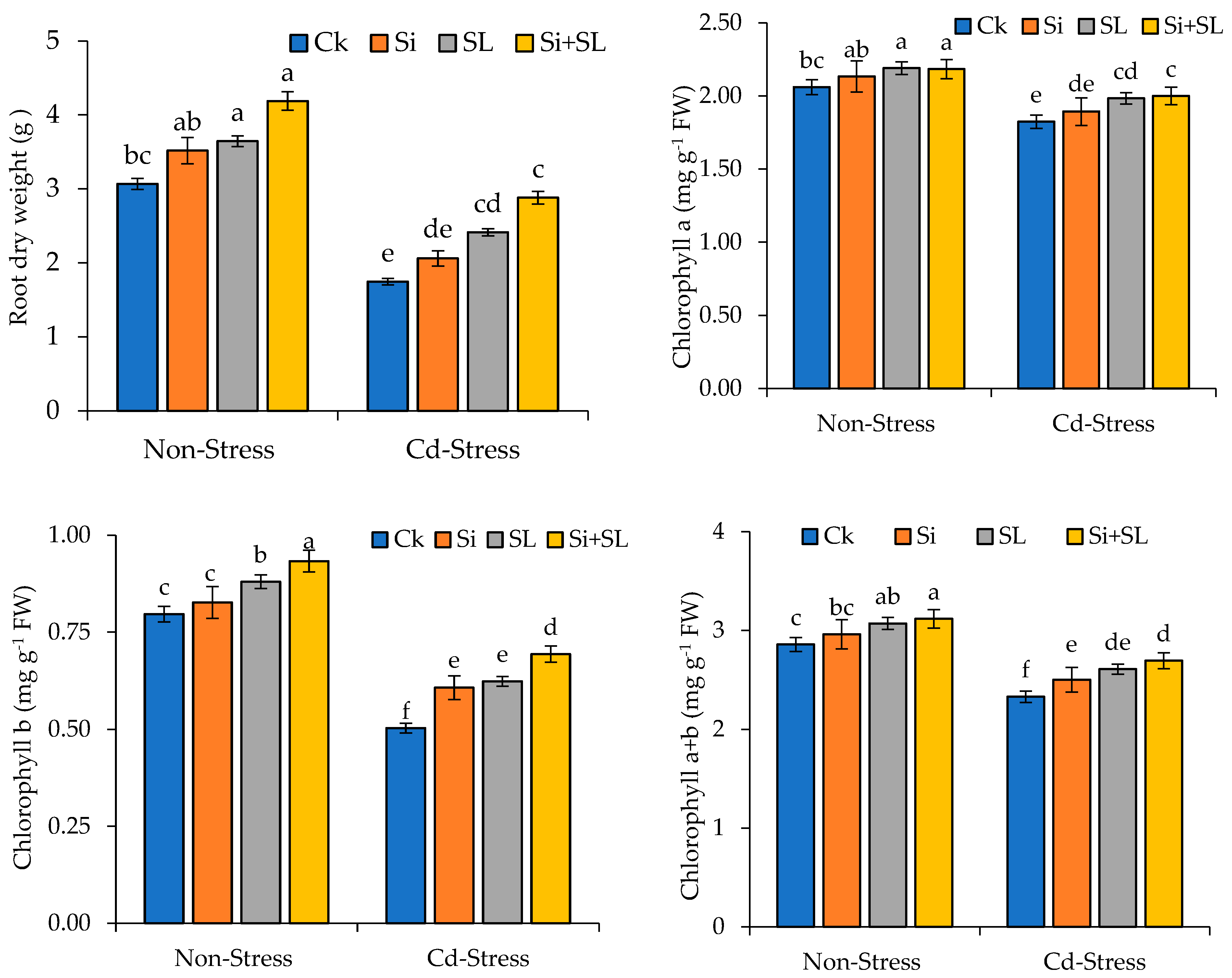
3.1. Morphological Attributes
3.2. Photosynthetic Pigments
3.3. Oxidative Stress Indicators
3.4. Enzymatic Antioxidants Activities
3.5. Concentration of Osmo-Protectants
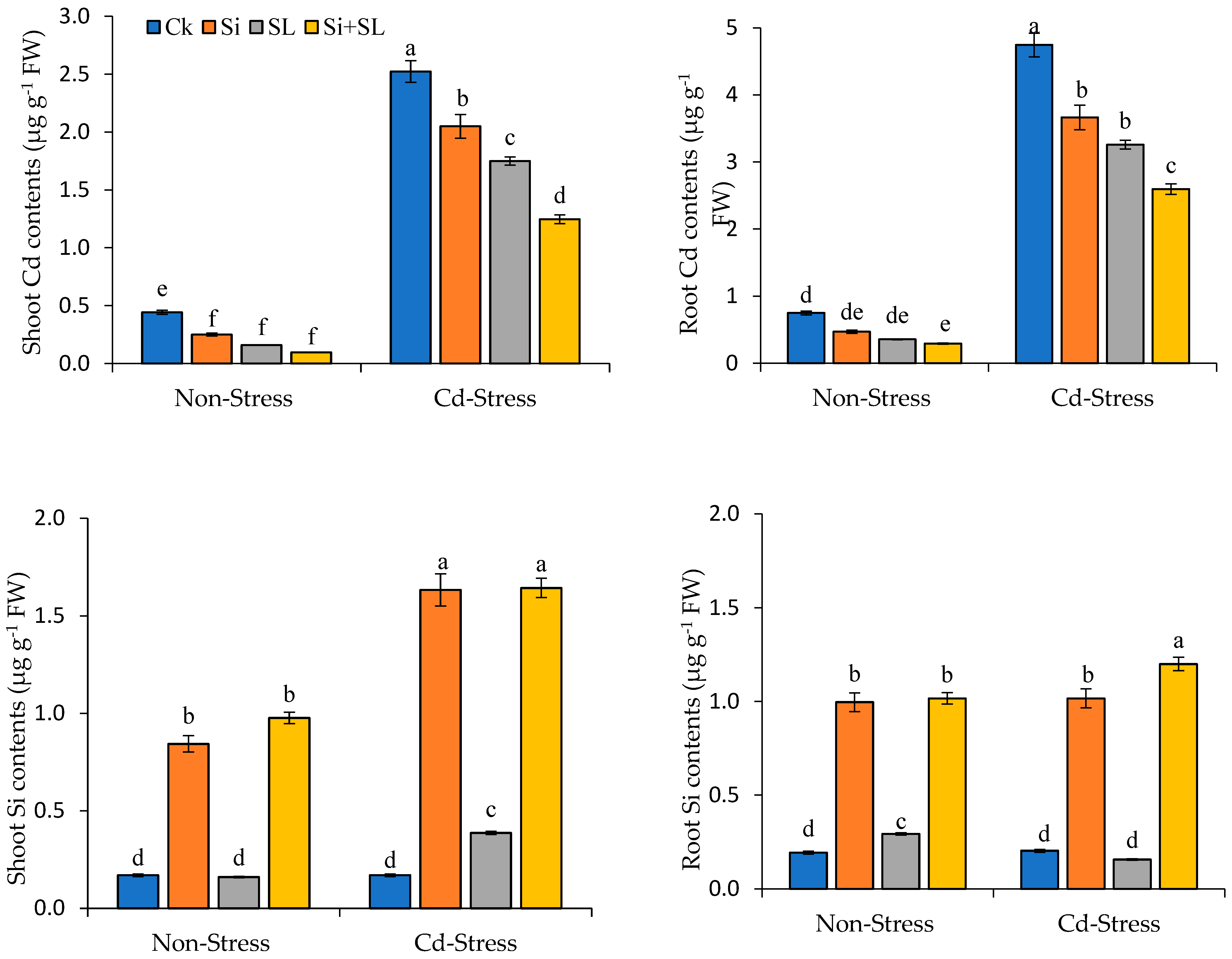
3.6. Concentration of Cadmium and Silicon in Maize Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.; Mfarrej, M.F.B.; El-Esawi, M.A.; Waseem, M.; Alatawi, A.; Nafees, M.; Saleem, M.H.; Rizwan, M.; Yasmeen, T.; Anayat, A.; et al. Chromium-resistant Staphylococcus aureus alleviates chromium toxicity by developing synergistic relationships with zinc oxide nanoparticles in wheat. Ecotoxicol. Environ. Saf. 2022, 230, 113142. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Majeed, S.; Naseem, Z.; Saeed, Q.; Khan, A.; Nawaz, A.; Baig, K.S.; Chen, J.T. Enhancing Cadmium Tolerance and Pea Plant Health through Enterobacter sp. MN17 Inoculation Together with Biochar and Gravel Sand. Plants 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; Rana, M.S.; Saleem, M.H.; Rasul, F.; Ali, K.H.; Potcho, M.P.; Pan, S.; Duan, M.; Tang, X. Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol. Environ. Saf. 2021, 211, 111911. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Ali, S.; Wen, X.; Khan, K.A.; Ghramh, H.A.; Zhang, Z.; Zhang, D. Impact of Cadmium Stress on Growth and Physio-Biochemical Attributes of Eruca sativa Mill. Plants 2022, 11, 2981. [Google Scholar] [CrossRef] [PubMed]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J. Effects of Zeolite on Aggregation, Nutrient Availability, and Growth Characteristics of Corn (Zea mays L.) in Cadmium-Contaminated Soils. Water Air Soil Pollut. 2022, 233, 436. [Google Scholar] [CrossRef]
- Javed, M.T.; Saleem, M.H.; Aslam, S.; Rehman, M.; Iqbal, N.; Begum, R.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N.; Wijaya, L. Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol. Biochem. 2020, 157, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Su, J.; Yang, F.; Wu, Y.; Ye, J.; Huang, K.; Yang, Y. Effect of lossy thin-walled cylindrical food containers on microwave heating performance. J. Food Eng. 2023, 337, 111232. [Google Scholar] [CrossRef]
- Alam, P.; Kaur Kohli, S.; Al Balawi, T.; Altalayan, F.H.; Alam, P.; Ashraf, M.; Bhardwaj, R.; Ahmad, P. Foliar Application of 24-Epibrassinolide Improves Growth, Ascorbate-Glutathione Cycle, and Glyoxalase System in Brown Mustard (Brassica juncea (L.) Czern.) under Cadmium Toxicity. Plants 2020, 9, 1487. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Saleem, M.H.; Parveen, A.; Khan, S.U.; Hussain, I.; Wang, X.; Alshaya, H.; El-Sheikh, M.A.; Ali, S. Silicon Fertigation Regimes Attenuate Cadmium Toxicity and Phytoremediation Potential in Two Maize (Zea mays L.) Cultivars by Minimizing Its Uptake and Oxidative Stress. Sustainability 2022, 14, 1462. [Google Scholar] [CrossRef]
- Hussain, S.; Irfan, M.; Sattar, A.; Hussain, S.; Ullah, S.; Abbas, T.; Ur-Rehman, H.; Nawaz, F.; Al-Hashimi, A.; Elshikh, M.S.; et al. Alleviation of Cadmium Stress in Wheat through the Combined Application of Boron and Biochar via Regulating Morpho-Physiological and Antioxidant Defense Mechanisms. Agronomy 2022, 12, 434. [Google Scholar] [CrossRef]
- Batool, T.; Javied, S.; Ashraf, K.; Sultan, K.; Zaman, Q.U.; Haider, F.U. Alleviation of Cadmium Stress by Silicon Supplementation in Peas by the Modulation of Morpho-physio-biochemical Variables and Health Risk Assessment. Life 2022, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Lin, K.; Williams, D.V.; Liu, Y.; Dai, H.; Cao, F. Cadmium Accumulation in Cereal Crops and Tobacco: A Review. Agronomy 2022, 12, 1952. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef] [PubMed]
- Heile, A.O.; Zaman, Q.U.; Aslam, Z.; Hussain, A.; Aslam, M.; Saleem, M.H.; Abualreesh, M.H.; Alatawi, A.; Ali, S. Alleviation of Cadmium Phytotoxicity Using Silicon Fertilization in Wheat by Altering Antioxidant Metabolism and Osmotic Adjustment. Sustainability 2021, 13, 11317. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, D. Fascinating regulatory mechanism of silicon for alleviating drought stress in plants. Plant Physiol. Biochem. 2021, 166, 1044–1053. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of Silicon in Mitigation of Heavy Metal Stresses in Crop Plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Deng, X.; Gong, D.; Du, S.; Wang, S.; Zhang, Z. Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J. Hazard. Mater. 2020, 395, 122679. [Google Scholar] [CrossRef]
- Lu, L.; Zhai, X.; Li, X.; Wang, S.; Zhang, L.; Wang, L.; Wang, F. Met1-specific motifs conserved in OTUB subfamily of green plants enable rice OTUB1 to hydrolyze Met1 ubiquitin chains. Nat. Commun. 2022, 13, 4672. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Belanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Hussain, I.; Ashraf, M.A.; Rasheed, R.; Asghar, A.; Sajid, M.A.; Iqbal, M. Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum L.) grains. Braz. J. Bot. 2015, 38, 223–234. [Google Scholar] [CrossRef]
- Shi, G.R.; Zhang, Z.; Liu, C.F. Silicon influences cadmium translocation by altering sub-cellular distribution and chemical forms of cadmium in peanut roots. Arch. Agron. Soil Sci. 2017, 63, 117–123. [Google Scholar] [CrossRef]
- Wu, J.; Mock, H.P.; Giehl, R.F.H.; Pitann, B.; Muhling, K.H. Silicon decreases cadmium concentrations by modulating root endodermal suberin development in wheat plants. J. Hazard. Mater. 2019, 364, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, M.; Guo, L.; Yang, D.; He, N.; Ying, B.; Wang, Y. Influence of silicon on cadmium availability and cadmium uptake by rice in acid and alkaline paddy soils. J. Soils Sediments 2020, 20, 2343–2353. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Rashid, M.; Nawaz, R.; Hussain, A.; Ashraf, K.; Latif, M.; Heile, A.O.; Mehmood, F.; Salahuddin, S.; Chen, Y. Silicon Fertilization: A Step towards Cadmium-Free Fragrant Rice. Plants 2021, 10, 2440. [Google Scholar] [CrossRef]
- Xiong, H.; Lu, D.; Li, Z.; Wu, J.; Ning, X.; Lin, W.; Bai, Z.; Zheng, C.; Sun, Y.; Chi, W.; et al. The DELLA-ABI4-HY5 module integrates light and gibberellin signals to regulate hypocotyl elongation. Plant Commun. 2023, 31, 100597. [Google Scholar] [CrossRef]
- Sattar, A.; Ul-Allah, S.; Ijaz, M.; Sher, A.; Butt, M.; Abbas, T.; Irfan, M.; Fatima, T.; Alfarraj, S.; Alharbi, S.A. Exogenous application of strigolactone alleviates drought stress in maize seedlings by regulating the physiological and antioxidants defense mechanisms. Cereal Res. Commun. 2022, 50, 263–272. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Gao, F.; Liu, Y.; Lang, S.; Wang, C.; Zhang, D. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 2023, 94, 106311. [Google Scholar] [CrossRef]
- Tai, Z.; Yin, X.; Fang, Z.; Shi, G.; Lou, L.; Cai, Q. Exogenous GR24 Alleviates Cadmium Toxicity by Reducing Cadmium Uptake in Switchgrass (Panicum virgatum) Seedlings. Int. J. Environ. Res. Public Health 2017, 14, 852. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Insights into strigolactone (GR24) mediated regulation of cadmium-induced changes and ROS metabolism in Artemisia annua. J. Hazard. Mater. 2023, 448, 130899. [Google Scholar] [CrossRef]
- Takaichi, S.; Tsuji, K.; Matsuura, K.; Shimada, K. A monocyclic carotenoid glucoside ester is a major carotenoid in the green filamentous bacterium Chloroflexus aurantiacus. Plant Cell Physiol. 1995, 36, 773–778. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice; IRRI: Los Banos, Philippines, 1976. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain treated bean plants. Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Agarie, S.; Hanaoka, N.; Kubota, F.; Agata, W.; Kaufman, P.B. Measurement of cell membrane stability evaluated by electrolyte leakage as a drought and heat tolerance test in rice (Oryza sativa L.). J. Fac. Agric. Kyushu Univ. 1995, 40, 233–240. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soybean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of Catalase and Peroxidase. In Methods of Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981, 22, 67–80. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Simaei, M.; Khavarinejada, R.A.; Saadatmanda, S.; Bernardb, F.; Fahimia, H. Interactive effects of salicylic acid and nitric oxide on soybean plants under NaCl salinity. Russ. J. Plant Physiol. 2011, 58, 783–790. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Julkunen-Titto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Ma, J.F.; Tamai, K.; Ichii, M.; Wu, K. A rice mutant defective in active Si uptake. Plant Physiol. 2002, 130, 2111–2117. [Google Scholar] [CrossRef]
- Azzi, V.S.; Kanso, A.; Kobeissi, A.; Kazpard, V.; Lartiges, B.; El Samrani, A. Effect of Cadmium on Lactuca sativa Grown in Hydroponic Culture Enriched with Phosphate Fertilizer. J. Environ. Prot. 2015, 6, 1337. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Hatamian, M.; Nejad, A.R.; Kafi, M.; Souri, M.K.; Shahbazi, K. Interaction of lead and cadmium on growth and leaf morphophysiological characteristics of European hackberry (Celtis australis) seedlings. Chem. Biol. Technol. Agric. 2020, 7, 9. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Sagardoy, R.; Solanas, M.; Abadía, A.; Abadía, J. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. J. Environ. Exp. Bot. 2009, 65, 376–385. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Xia, J.; Li, Y.; Zhang, L.; Feng, H.; Zhang, X. Flexible sensing enabled agri-food cold chain quality control: A review of mechanism analysis, emerging applications, and system integration. Trends Food Sci. Technol. 2023, 133, 189–204. [Google Scholar] [CrossRef]
- Abbas, T.; Fan, R.; Hussain, S.; Sattar, A.; Khalid, S.; Butt, M.; Shahzad, U.; Atif, H.M.; Batool, M.; Ullah, S.; et al. Protective effect of jasmonic acid and potassium against cadmium stress in peas (Pisum sativum L.). Saudi J. Biol. Sci. 2022, 29, 2626–2633. [Google Scholar] [CrossRef]
- Andresen, E.; Küpper, H. Cadmium toxicity in plants. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sige, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 395–413. [Google Scholar]
- Alcantara, E.; Romera, F.J.; Cafiete, M.; De La Guardia, M.D. Effects of heavy metals on both induction and function of root Fe(III) reductase in Fe-deficient cucumber (Cucumis sativus L.) plants. J. Exp. Bot. 1994, 45, 1893–1898. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Sen, A. Oxidative stress studies in plant tissue culture. In Antioxidant Enzyme; ElMissiry, M.A., Ed.; INTECH: London, UK, 2012; pp. 59–88. [Google Scholar]
- Gheshlaghpour, J.; Asghari, B.; Khademian, R.; Sedaghati, B. Silicon alleviates cadmium stress in basil (Ocimum basilicum L.) through alteration of phytochemical and physiological characteristics. Ind. Crops Prod. 2021, 163, 113338. [Google Scholar] [CrossRef]
- Khaliq, A.; Ali, S.; Hameed, A.; Farooq, M.A.; Farid, M.; Shakoor, M.B.; Mahmood, K.; Ishaque, W.; Rizwan, M. Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis and suppressing Ni uptake and oxidative stress. Arch. Agron. Soil Sci. 2016, 62, 633–647. [Google Scholar] [CrossRef]
- Haider, F.U.; Farooq, M.; Naveed, M.; Cheema, S.A.; Ain, U.-N.; Salim, M.A.; Liqun, C.; Mustafa, A. Influence of biochar and microorganism co-application on the remediation and maize growth in cadmium-contaminated soil. Front. Plant Sci. 2022, 13, 983830. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Resnick, N.; Mayzlish-Gati, E.; Kaplan, Y.; Wininger, S.; Hershenhorn, J.; Koltai, H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011, 62, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2018, 135, 11037. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.-W.; Zhang, C.; Wang, N.-H.; Mao, W.; Wu, F. Strigolactone GR24 improves cadmium tolerance by regulating cadmium uptake, nitric oxide signaling and antioxidant metabolism in barley (Hordeum vulgare L.). Environ. Pollut. 2021, 273, 116486. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Ha, C.V.; Rahman, M.M.; Nguyen, K.H.; Keya, S.S.; Watanabe, Y.; Itouga, M.; Hashem, A.; Abd_Allah, E.F.; Fujita, M.; et al. Strigolactones modulate cellular antioxidant defense mechanisms to mitigate arsenate toxicity in rice shoots. Antioxidants 2021, 10, 1815. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Abbas, T.; Hussain, S.; Hussain, J.; Khalil, H.B.; Alharbi, B.M.; El-Yazied, A.A.; et al. Silicon and Strigolecton Application Alleviates the Adversities of Cadmium Toxicity in Maize by Modulating Morpho-Physiological and Antioxidants Defense Mechanisms. Agronomy 2023, 13, 2352. https://doi.org/10.3390/agronomy13092352
Sattar A, Sher A, Ijaz M, Ul-Allah S, Abbas T, Hussain S, Hussain J, Khalil HB, Alharbi BM, El-Yazied AA, et al. Silicon and Strigolecton Application Alleviates the Adversities of Cadmium Toxicity in Maize by Modulating Morpho-Physiological and Antioxidants Defense Mechanisms. Agronomy. 2023; 13(9):2352. https://doi.org/10.3390/agronomy13092352
Chicago/Turabian StyleSattar, Abdul, Ahmad Sher, Muhammad Ijaz, Sami Ul-Allah, Tahira Abbas, Sajjad Hussain, Jamshad Hussain, Hala Badr Khalil, Basmah M. Alharbi, Ahmed Abou El-Yazied, and et al. 2023. "Silicon and Strigolecton Application Alleviates the Adversities of Cadmium Toxicity in Maize by Modulating Morpho-Physiological and Antioxidants Defense Mechanisms" Agronomy 13, no. 9: 2352. https://doi.org/10.3390/agronomy13092352







