Soil Organic Carbon Sequestration after 20-Year Afforestation of Mangrove Plantations on Qi’ao Island, Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Sampling
2.3. Soil Properties Measurements
2.4. Density Fractionations
2.5. Calculation of Autochthonous and Allochthonous Sources
2.6. Calculations of Soil Organic Carbon Stock
2.7. Statistical Analyses
3. Results
3.1. Soil Properties
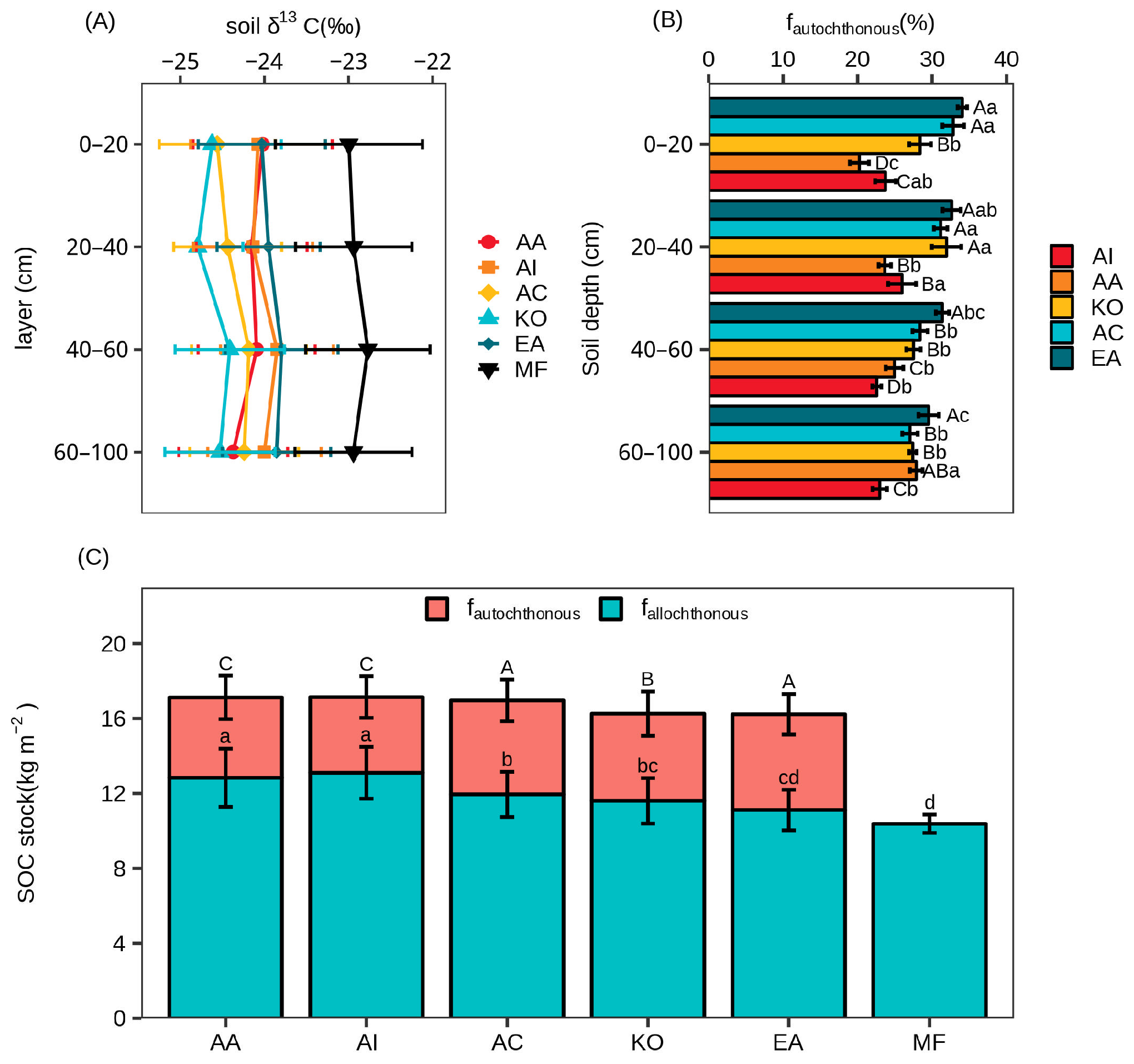
3.2. Soil Organic Carbon Concentration and Stock
3.3. Soil Organic Carbon Sources
3.4. Soil Organic Carbon Fractions
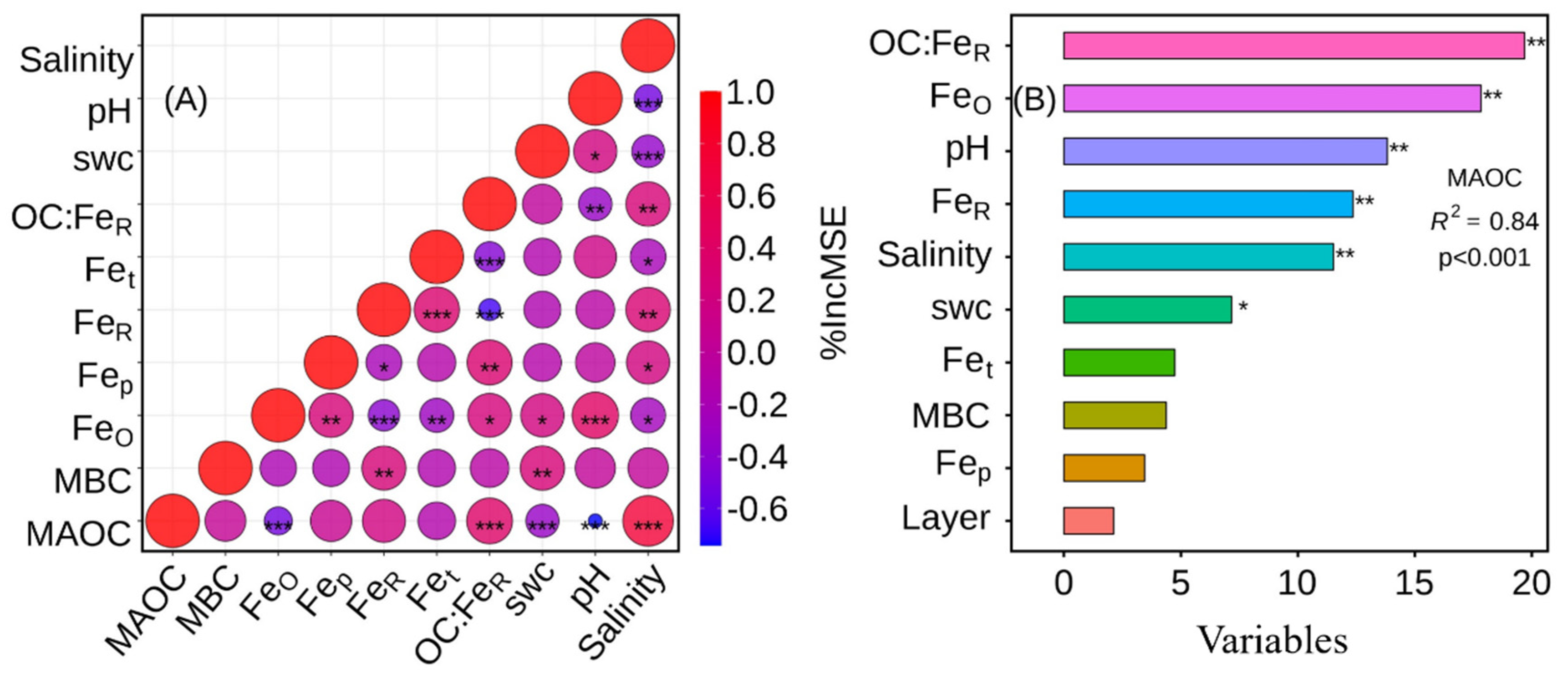
3.5. Soil Iron Phases
3.6. Drivers of MAOC in Afforestation Mangrove
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hamilton, S.E.; Friess, D.A. Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat. Clim. Chang. 2018, 8, 240–244. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Taillardat, P.; Clendenning, J.N.; Cameron, C.; Friess, D.A.; Murdiyarso, D.; Hutley, L.B. Effect of land-use and land-cover change on mangrove blue carbon: A systematic review. Glob. Chang. Biol. 2019, 25, 4291–4302. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Liao, B.; Wu, Z.; Wu, H.; Bao, D.; Dan, W.; Liu, S. Resources, Conservation Status and Main Threats of Mangrove Wetlands in China. Ecol. Environ Sci. 2016, 25, 1237–1243. [Google Scholar]
- Song, S.; Ding, Y.; Li, W.; Meng, Y.; Zhou, J.; Gou, R.; Zhang, C.; Ye, S.; Saintilan, N.; Krauss, K.W.; et al. Mangrove reforestation provides greater blue carbon benefit than afforestation for mitigating global climate change. Nat. Commun. 2023, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.P.; Kominoski, J.S.; Armitage, A.R.; Guo, H.; Weaver, C.A.; Pennings, S.C. Quantifying how changing mangrove cover affects ecosystem carbon storage in coastal wetlands. Ecology 2020, 101, e02916. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 2008, 11, 342–354. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis. J. Mar. Sci. Eng. 2020, 8, 767. [Google Scholar] [CrossRef]
- Twilley, R.R.; Chen, R.H.; Hargis, T. Carbon sinks in mangroves and their implications to carbon budget of tropical coastal ecosystems. Water Air Soil Pollut. 1992, 64, 265–288. [Google Scholar] [CrossRef]
- Kida, M.; Fujitake, N. Organic Carbon Stabilization Mechanisms in Mangrove Soils: A Review. Forests 2020, 11, 981. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Kuzyakov, Y.; Lubis, A.A.; Murdiyarso, D.; Hutley, L.B.; Bachri, S.; Friess, D.A.; Martius, C.; Borchard, N. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Tanaka, N.; Sasaki, Y.; Mowjood, M.I.M.; Jinadasa, K.B.S.N.; Homchuen, S. Coastal vegetation structures and their functions in tsunami protection: Experience of the recent Indian Ocean tsunami. Landsc. Ecol. Eng. 2007, 3, 33–45. [Google Scholar] [CrossRef]
- Furukawa, K.; Wolanski, E.; Mueller, H. Currents and sediment transport in mangrove forests. Estuar. Coast Shelf Sci. 1997, 44, 301–310. [Google Scholar] [CrossRef]
- Bouillon, S.; Borges, A.V.; Castaneda-Moya, E.; Diele, K.; Dittmar, T.; Duke, N.C.; Kristensen, E.; Lee, S.Y.; Marchand, C.; Middelburg, J.J.; et al. Mangrove production and carbon sinks: A revision of global budget estimates. Global Biogeochem. Cycles 2008, 22, GB2013. [Google Scholar] [CrossRef]
- He, S.; Lin, J.; Liu, X.; Jia, S.; Chen, S. Cordgrass Spartina alterniflora acts as a key carbon source to support macrozoobenthos in the salt marsh and nearby mudflat communities. Ecol. Indic. 2023, 148, 110052. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Routh, J.; Ramanathan, A.L.; Klump, J.V. Elemental and stable isotope records of organic matter input and its fate in the Pichavaram mangrove-estuarine sediments (Tamil Nadu, India). Mar. Chem. 2011, 126, 163–172. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Guan, D.; Xiao, L.; Singh, M. The potential of mature Sonneratia apetala plantations to enhance carbon stocks in the Zhanjiang Mangrove National Nature Reserve. Ecol. Indic. 2021, 133, 108415. [Google Scholar] [CrossRef]
- Sreelekshmi, S.; Harikrishnan, M.; Nandan, S.B.; Kaimal, V.S.; Hershey, N.R. Ecosystem Carbon Stock and Stable Isotopic Signatures of Soil Organic Carbon Sources Across the Mangrove Ecosystems of Kerala, Southern India. Wetlands 2022, 42, 29. [Google Scholar] [CrossRef]
- Suello, R.H.; Hernandez, S.L.; Bouillon, S.; Belliard, J.-P.; Dominguez-Granda, L.; Van de Broek, M.; Rosado Moncayo, A.M.; Ramos Veliz, J.; Pollette Ramirez, K.; Govers, G.; et al. Mangrove sediment organic carbon storage and sources in relation to forest age and position along a deltaic salinity gradient. Biogeosciences 2022, 19, 1571–1585. [Google Scholar] [CrossRef]
- Dias Rodrigues, C.I.; Brito, L.M.; Nunes, L.J.R. Soil carbon sequestration in the context of climate change mitigation: A review. Soil Syst. 2023, 7, 64. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Yuttitham, M.; Hatano, R. Variations of soil properties and soil surface loss after fire in rotational shifting cultivation in Northern Thailand. Front. Environ. Sci. 2023, 7, 1213181. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, J.; Cui, L.; Feng, W.; Wang, Y.; Zhang, J. Soil organic carbon stabilization mechanisms in a subtropical mangrove and salt marsh ecosystems. Sci. Total Environ. 2019, 673, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.; Kelleway, J.J.; Saintilan, N.; Megonigal, J.P.; Adams, J.B.; Holmquist, J.R.; Lu, M.; Schile-Beers, L.; Zawadzki, A.; Mazumder, D.; et al. Wetland carbon storage controlled by millennial-scale variation in relative sea-level rise. Nature 2019, 567, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Swales, A.; Bentley, S.J., Sr.; Lovelock, C.E. Mangrove-forest evolution in a sediment-rich estuarine system: Opportunists or agents of geomorphic change? Earth Sur. Process Landf. 2015, 40, 1672–1687. [Google Scholar] [CrossRef]
- Conrad, S.R.; Santos, I.R.; White, S.A.; Holloway, C.J.; Brown, D.R.; Wadnerkar, P.D.; Correa, R.E.; Woodrow, R.L.; Sanders, C.J. Land use change increases contaminant sequestration in blue carbon sediments. Sci. Total Environ. 2023, 873, 162175. [Google Scholar] [CrossRef] [PubMed]
- Jupin, J.L.J.; Ruiz-Fernandez, A.C.; Sifeddine, A.; Sanchez-Cabeza, J.A.; Perez-Bernal, L.H.; Cardoso-Mohedano, J.G.; Gomez-Ponce, M.A.; Flores-Trujillo, J.G. Anthropogenic drivers of increasing sediment accumulation in contrasting Mexican mangrove ecosystems. Catena 2023, 226, 107037. [Google Scholar] [CrossRef]
- Kristensen, E.; Bouillon, S.; Dittmar, T.; Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 2008, 89, 201–219. [Google Scholar] [CrossRef]
- Nobrega, M.S.; Silva, B.S.; Tschoeke, D.A.; Appolinario, L.R.; Calegario, G.; Venas, T.M.; Macedo, L.; Asp, N.; Cherene, B.; Marques, J.S.J.; et al. Mangrove microbiome reveals importance of sulfur metabolism in tropical coastal waters. Sci. Total Environ. 2022, 813, 151889. [Google Scholar] [CrossRef]
- Pupin, B.; Nahas, E. Microbial populations and activities of mangrove, restinga and Atlantic forest soils from Cardoso Island, Brazil. J. Appl. Microbiol. 2014, 116, 851–864. [Google Scholar] [CrossRef]
- Kinjo, K.; Tokashiki, Y.; Sato, K.; Kitou, M.; Shimo, M. Characteristics of surface sediments along a creek in a mangrove forest. Soil Sci. Plant Nutr. 2005, 51, 809–817. [Google Scholar] [CrossRef]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 2019, 570, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Osland, M.J.; Spivak, A.C.; Nestlerode, J.A.; Lessmann, J.M.; Almario, A.E.; Heitmuller, P.T.; Russell, M.J.; Krauss, K.W.; Alvarez, F.; Dantin, D.D.; et al. Ecosystem Development After Mangrove Wetland Creation: Plant-Soil Change Across a 20-Year Chronosequence. Ecosystems 2012, 15, 848–866. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, H.; Ren, D.; Gao, H.; Li, J. Processes controlling the seasonal and spatial variations in sulfate profiles in the pore water of the sediments surrounding Qi’ao Island, Pearl River Estuary, Southern China. Cont. Shelf Res. 2015, 98, 26–35. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Liu, L.; Wang, S.; Liu, H. Retrieval of Mangrove Aboveground Biomass at the Individual Species Level with WorldView-2 Images. Remote Sens. 2015, 7, 12192–12214. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, S.; Feng, Z.; Liu, G.; Gan, Q.; Peng, S. Use of exotic plants to control Spartina alterniflora invasion and promote mangrove restoration. Sci. Rep. 2015, 5, 12980. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tuzen, M. Determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem. J. 2003, 74, 289–297. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Hall, S.J.; Hui, D.; Li, J.; Chen, G.; Sun, L.; Zhang, D.; Deng, Q. Increased interactions between iron oxides and organic carbon under acid deposition drive large increases in soil organic carbon in a tropical forest in southern China. Biogeochemistry 2022, 158, 287–301. [Google Scholar] [CrossRef]
- Dicen, G.P.; Navarrete, I.A.; Rallos, R.V.; Salmo, S.G., III; Garcia, M.C.A. Garcia MCA (2019) The role of reactive iron in long-term carbon sequestration in mangrove sediments. J. Soils Sediments 2019, 19, 501–510. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil Tillage Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Ye, F.; Guo, W.; Wei, G.; Jia, G. The Sources and Transformations of Dissolved Organic Matter in the Pearl River Estuary, China, as Revealed by Stable Isotopes. J. Geophys. Res. Oceans 2018, 123, 6893–6908. [Google Scholar] [CrossRef]
- Chmura, G.L.; Aharon, P. Stable carbon-isotope signatures of sedimentary carbon in coastal wetlands as indicators of salinity regime. J. Coastal Res. 1995, 11, 124–135. [Google Scholar]
- Chen, J.; Huang, Y.; Chen, G.; Ye, Y. Effects of simulated sea level rise on stocks and sources of soil organic carbon in Kandelia obovata mangrove forests. For. Ecol. Manag. 2020, 460, 117898. [Google Scholar] [CrossRef]
- Xiong, Y.; Liao, B.; Wang, F. Mangrove vegetation enhances soil carbon storage primarily through in situ inputs rather than increasing allochthonous sediments. Mar. Pollut. Bull. 2018, 131, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cui, X.; Zhou, J.; Wang, L.; Zhu, X.; Lin, G. Effects of exotic and native mangrove forests plantation on soil organic carbon, nitrogen, and phosphorus contents and pools in Leizhou, China. Catena 2019, 180, 1–7. [Google Scholar] [CrossRef]
- He, Z.; Peng, Y.; Guan, D.; Hu, Z.; Chen, Y.; Lee, S.Y. Appearance can be deceptive: Shrubby native mangrove species contributes more to soil carbon sequestration than fast-growing exotic species. Plant Soil 2018, 432, 425–436. [Google Scholar] [CrossRef]
- Yu, C.; Feng, J.; Yue, W.; Wei, L.; Ma, Y.; Huang, X.; Ling, J.; Dong, J. The role of blue carbon stocks becomes more labile with mangrove development. Ecol. Indic. 2023, 154, 110634. [Google Scholar] [CrossRef]
- Hoque, M.M.; Kamal, A.H.M.; Idris, M.H.; Ahmed, O.H.; Hoque, A.T.M.R.; Billah, M.M. Litterfall production in a tropical mangrove of Sarawak, Malaysia. Zool. Ecol. 2015, 25, 157–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Guan, D.; Chen, Y.; Motelica-Heino, M.; Peng, Y.; Lee, S.Y. The role of mangrove fine root production and decomposition on soil organic carbon component ratios. Ecol. Indic. 2021, 125, 107525. [Google Scholar] [CrossRef]
- Li, S.-B.; Chen, P.-H.; Huang, J.-S.; Hsueh, M.-L.; Hsieh, L.-Y.; Lee, C.-L.; Lin, H.-J. Factors regulating carbon sinks in mangrove ecosystems. Glob. Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gao, M.; Pang, B.; Chen, S.; Ye, Y. Top-meter soil organic carbon stocks and sources in restored mangrove forests of different ages. For. Ecol. Manag. 2018, 422, 87–94. [Google Scholar] [CrossRef]
- Tian, Y.; Yan, C.; Wang, Q.; Ma, W.; Yang, D.; Liu, J.; Lu, H. Glomalin-related soil protein enriched in δ13C and δ15N excels at storing blue carbon in mangrove wetlands. Sci. Total Environ. 2020, 732, 138327. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Loh, P.S.; Pradit, S.; Le, T.P.Q.; Oeurng, C.; Mohamed, C.A.R.; Lee, C.W.; Lu, X.; Anshari, G.Z.; Kandasamy, S.; et al. Assessing the Effect of Age and Geomorphic Setting on Organic Carbon Accumulation in High-Latitude Human-Planted Mangroves. Forests 2022, 13, 105. [Google Scholar] [CrossRef]
- Qiu, N.; Xu, S.; Qiu, P.; Yang, W.; Yang, X.; Yang, Q. Community Distribution and Landscape Pattern of the Mangrove on the Qiao Island, Zhuhai. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar]
- Horstman, E.M.; Dohmen-Janssen, C.M.; Narra, P.M.F.; van den Berg, N.J.F.; Siemerink, M.; Hulscher, S.J.M.H. Wave attenuation in mangroves: A quantitative approach to field observations. Coasta. Eng. 2014, 94, 47–62. [Google Scholar] [CrossRef]
- Lee, W.K.; Tay, S.H.X.; Ooi, S.K.; Friess, D.A. Potential short wave attenuation function of disturbed mangroves. Estuar. Coast Shelf Sci. 2021, 248, 106747. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, R.; Cui, B.; Zhang, K.; Wang, Q.; Liu, X.; Gao, H.; Huang, L. Assessment: Of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River Estuary, South China. Environ. Pollut. 2011, 159, 817–824. [Google Scholar] [CrossRef]
- Qiao, Y.; Yang, Y.; Gu, J.; Zhao, J. Distribution and geochemical speciation of heavy metals in sediments from coastal area suffered rapid urbanization, a case study of Shantou Bay, China. Mar. Pollut. Bull. 2013, 68, 140–146. [Google Scholar] [CrossRef]
- Poulton, S.W.; Raiswell, R. The low-temperature geochemical cycle of iron: From continental fluxes to marine sediment deposition. Am. J. Sci. 2002, 302, 774–805. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Hao, X.-C.; Shi, X.-N.; Yang, G.-P.; Li, T. Speciation and spatial distribution of solid-phase iron in surface sediments of the East China Sea continental shelf. Appl. Geochem. 2012, 27, 892–905. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Campbell, A.N.; Tfaily, M.M.; Lin, Y.; Kukkadapu, R.K.; Silver, W.L.; Nico, P.S.; Pett-Ridge, J. Redox Fluctuations Control the Coupled Cycling of Iron and Carbon in Tropical Forest Soils. Environ. Sci. Technol. 2018, 52, 14129–14139. [Google Scholar] [CrossRef]
- Liu, J.; Lai, D.Y.F. Subtropical mangrove wetland is a stronger carbon dioxide sink in the dry than wet seasons. Agric. For. Meteorol. 2019, 278, 107644. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]



| Layer | Species | Bulk Density (g cm−3) | SWC (%) | pH | Salinity (‰) | MBC (mg kg−1) |
|---|---|---|---|---|---|---|
| 0–20 | AA | 0.9 ± 0.0 Dc | 39.7 ± 1.1 Bab | 6.6 ± 0.1 Bb | 11.9 ± 0.1 Ca | 781.9 ± 88.6 Dd |
| AI | 1.0 ± 0.0 Cc | 50.3 ± 1.1 Aa | 6.6 ± 0.3 Ba | 12.6 ± 0.2 Aa | 782.3 ± 59.6 Dd | |
| AC | 0.9 ± 0.0 Dc | 34.3 ± 2.1 Dc | 6.5 ± 0.1 Ba | 12.8 ± 0.1 Aa | 768.4 ± 95.6 Dd | |
| KO | 1.2 ± 0.0 Ab | 35.2 ± 0.4 CDd | 6.8 ± 0.2 Bb | 8.9 ± 0.1 Ec | 733.3 ± 51.4 Dd | |
| EA | 1.2 ± 0.0 Ba | 38.4 ± 1.0 BCb | 6.8 ± 0.2 Bb | 12.2 ± 0.1 Ba | 1706.2 ± 45.2 Aa | |
| MF | 1.2 ± 0.0 ABbc | 47.7 ± 2.6 Aa | 7.7 ± 0.1 Aa | 9.2 ± 0.0 Da | 1538.6 ± 64.8 Aa | |
| 20–40 | AA | 1.0 ± 0.0 Eb | 41.1 ± 0.9 Ba | 7.1 ± 0.1 Ba | 11.4 ± 0.4 Ba | 1282.3 ± 76.6 Aa |
| AI | 1.1 ± 0.0 Da | 42.3 ± 0.6 Bc | 6.7 ± 0.1 CDa | 12.7 ± 0.2 Aa | 1179.4 ± 55.3 Bb | |
| AC | 1.0 ± 0.1 Fb | 47.7 ± 1.9 Aa | 6.4 ± 0.3 Da | 10.8 ± 0.1 Cc | 667.8 ± 69.4 Dd | |
| KO | 1.2 ± 0.0 Bb | 51.3 ± 0.8 Aa | 6.8 ± 0.1 BCb | 9.2 ± 0.0 Db | 675.1 ± 82.6 Dd | |
| EA | 1.1 ± 0.0 Cb | 41.7 ± 1.2 Ba | 6.8 ± 0.1 Cb | 10.7 ± 0.1 Cd | 900.7 ± 76.7 CDcd | |
| MF | 1.3 ± 0.0 Aa | 47.9 ± 3.3 Aa | 7.5 ± 0.2 Aa | 8.9 ± 0.0 Db | 1435.6 ± 96.8 Aa | |
| 40–60 | AA | 1.1 ± 0.1 Cb | 37.2 ± 0.7 Bb | 6.72 ± 0.1 Bb | 11.8 ± 0.1 Aa | 1338.8 ± 92.7 Bb |
| AI | 1.1 ± 0.0 Cb | 47.3 ± 0.7 Ab | 6.88 ± 0.1 Ba | 11.8 ± 0.1 Ab | 1090.1 ± 91.5 Cc | |
| AC | 0.9 ± 0.0 Dd | 37.0 ± 0.7 Bc | 6.22 ± 0.3 Ca | 11.3 ± 0.1 Bb | 1221.4 ± 63.2 ABab | |
| KO | 1.2 ± 0.0 Aa | 39.3 ± 0.3 Bc | 6.64 ± 0.1 BCb | 9.3 ± 0.1 Cb | 1109.7 ± 80.2 BCbc | |
| EA | 1.2 ± 0.0 Ba | 39.6 ± 0.8 Bb | 6.83 ± 0.3 Bb | 11.2 ± 0.0 Bc | 1417.2 ± 87.4 Bb | |
| MF | 1.2 ± 0.0 ABc | 46.5 ± 3.2 Aa | 7.52 ± 0.2 Aa | 8.7 ± 0.0 Dc | 1263.7 ± 73.5 Bb | |
| 60–100 | AA | 1.2 ± 0.0 Ba | 30.3 ± 1.8 Cc | 6.69 ± 0.0 BCb | 11.7 ± 0.3 Ba | 1058.6 ± 80.0 BCbc |
| AI | 1.0 ± 0.0 Dd | 39.1 ± 0.8 Bd | 6.91 ± 0.2 Ba | 12.0 ± 0.1 Ab | 1180.5 ± 74.2 Bb | |
| AC | 1.1 ± 0.0 Ca | 43.2 ± 2.7 Ab | 6.32 ± 0.2 Ca | 11.4 ± 0.1 Cb | 1161.6 ± 48.6 Cc | |
| KO | 1.2 ± 0.1 Bc | 42.1 ± 0.8 ABb | 7.52 ± 0.2 Aa | 10.4 ± 0.1 Da | 993.9 ± 41.5 Cc | |
| EA | 1.1 ± 0.0 BCc | 33.2 ± 0.8 Cc | 7.34 ± 0.3 Aa | 11.5 ± 0.0 BCb | 1201.7 ± 57.6 ABab | |
| MF | 1.3 ± 0.1 Aab | 45.7 ± 2.2 Aa | 7.61 ± 0.2 Aa | 8.8 ± 0.0 Eb | 1017.5 ± 44.0 Cc |
| Layer | Species | Fet (g kg−1) | FeR (g kg−1) | Fep (g kg−1) | Feo (g kg−1) | OC:FeR (m:m) |
|---|---|---|---|---|---|---|
| 0–20 | AA | 61.5 ± 0.7 Aa | 37.3 ± 1.5 Aa | 1.1 ± 0.1 Aa | 12.2 ± 0.8 Ca | 2.0 ± 0.2 Cb |
| AI | 56.9 ± 1.1 BCb | 37.4 ± 1.5 Aa | 1.1 ± 0.1 Aa | 12.8 ± 0.3 Ca | 2.4 ± 0.1 ABa | |
| AC | 54.1 ± 1.4 Cb | 31.6 ± 1.0 BCa | 1.2 ± 0.1 Aa | 15.1 ± 0.6 Ba | 2.5 ± 0.2 Aab | |
| KO | 59.2 ± 0.9 ABb | 34.8 ± 1.0 ABa | 0.9 ± 0.2 Aa | 10.8 ± 0.3 Dab | 2.0 ± 0.1 Ca | |
| EA | 60.4 ± 2.4 Aa | 37.6 ± 2.1 Aa | 1.1 ± 0.0 Aab | 10.6 ± 0.5 Db | 2.1 ± 0.1 BCa | |
| MF | 60.3 ± 0.9 Aa | 31.2 ± 1.4 Cb | 1.1 ± 0.3 Aa | 16.4 ± 0.4 Abc | 2.2 ± 0.2 ABCa | |
| 20–40 | AA | 62.9 ± 1.1 Aa | 33.5 ± 1.5 BCb | 1.2 ± 0.3 Aa | 10.8 ± 0.5 Bb | 2.4 ± 0.2 Aa |
| AI | 58.5 ± 1.1 Bab | 36.4 ± 0.9 ABa | 1.2 ± 0.2 ABa | 10.8 ± 0.5 Bb | 2.2 ± 0.1 Aab | |
| AC | 60.9 ± 1.2 ABa | 33.8 ± 0.9 BCa | 1.2 ± 0.1 Aa | 15.2 ± 0.4 Aa | 2.3 ± 0.2 Ab | |
| KO | 63.1 ± 2.5 Aa | 36.8 ± 2.3 ABa | 0.9 ± 0.2 ABa | 9.7 ± 0.8 Bbc | 1.5 ± 0.2 Bb | |
| EA | 63.4 ± 2.2 Aa | 39.1 ± 1.6 Aa | 0.7 ± 0.2 Bc | 10.2 ± 0.4 Bbc | 1.8 ± 0.1 Bb | |
| MF | 60.1 ± 2.2 ABa | 30.5 ± 1.5 Cb | 1.1 ± 0.2 ABa | 15.9 ± 0.5 Ac | 2.2 ± 0.3 Aa | |
| 40–60 | AA | 62.2 ± 1.8 Aa | 34.2 ± 0.9 Bab | 1.3 ± 0.1 Aa | 10.8 ± 0.3 Cb | 2.2 ± 0.1 Bab |
| AI | 57.7 ± 2.9 Bab | 36.0 ± 0.9 ABa | 1.2 ± 0.2 Aa | 12.1 ± 0.4 Ba | 2.1 ± 0.1 BCb | |
| AC | 61.0 ± 1.0 ABa | 33.0 ± 1.5 Ba | 1.2 ± 0.2 Aa | 12.8 ± 0.6 Bb | 2.6 ± 0.2 Aa | |
| KO | 62.3 ± 1.3 Aab | 34.0 ± 2.7 Ba | 1.2 ± 0.3 Aa | 8.8 ± 0.7 Dc | 1.9 ± 0.2 Ca | |
| EA | 63.4 ± 2.1 Aa | 39.8 ± 2.4 Aa | 0.9 ± 0.1 Abc | 9.3 ± 0.5 Dc | 1.5 ± 0.2 Db | |
| MF | 60.6 ± 1.9 ABa | 34.8 ± 1.2 Ba | 1.2 ± 0.1 Aa | 17.3 ± 0.4 Aa | 2.0 ± 0.1 BCa | |
| 60–100 | AA | 62.2 ± 3.0 Aa | 35.0 ± 2.0 BCab | 1.1 ± 0.1 ABa | 12.4 ± 0.7 CDa | 2.1 ± 0.1 Bab |
| AI | 61. ± 1.4 Aa | 37.0 ± 1.4 ABa | 1.3 ± 0.1 Aa | 12.8 ± 0.5 Ca | 1.9 ± 0.1 BCc | |
| AC | 62.1 ± 2.6 Aa | 32.9 ± 0.5 Ca | 1.1 ± 0.0 ABa | 13.3 ± 0.3 Cb | 2.5 ± 0.1 Aab | |
| KO | 64.0 ± 1.8 Aa | 37.2 ± 2.4 ABa | 1.0 ± 0.1 Ba | 11.4 ± 0.7 Da | 1.7 ± 0.2 CDab | |
| EA | 63.4 ± 2.4 Aa | 40.6 ± 2.4 Aa | 1.2 ± 0.1 ABa | 15.2 ± 0.6 Ba | 1.6 ± 0.1 Db | |
| MF | 59.1 ± 1.6 Aa | 32.1 ± 1.3 Cab | 1.1 ± 0.2 ABa | 17.1 ± 0.3 Aab | 2.1 ± 0.0 Ba |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Zhang, M.; Yao, X.; Zhu, Y.; Hu, Y.; Hui, D.; Li, J.; Chen, J.; Deng, Q. Soil Organic Carbon Sequestration after 20-Year Afforestation of Mangrove Plantations on Qi’ao Island, Southern China. Agronomy 2023, 13, 2389. https://doi.org/10.3390/agronomy13092389
Chen G, Zhang M, Yao X, Zhu Y, Hu Y, Hui D, Li J, Chen J, Deng Q. Soil Organic Carbon Sequestration after 20-Year Afforestation of Mangrove Plantations on Qi’ao Island, Southern China. Agronomy. 2023; 13(9):2389. https://doi.org/10.3390/agronomy13092389
Chicago/Turabian StyleChen, Guoyin, Meixia Zhang, Xianyu Yao, Yiren Zhu, Yuanliu Hu, Dafeng Hui, Jianling Li, Jingwen Chen, and Qi Deng. 2023. "Soil Organic Carbon Sequestration after 20-Year Afforestation of Mangrove Plantations on Qi’ao Island, Southern China" Agronomy 13, no. 9: 2389. https://doi.org/10.3390/agronomy13092389





