Organic vs. Conventional Farming of Lavender: Effect on Yield, Phytochemicals and Essential Oil Composition
Abstract
:1. Introduction
2. Materials and Methods
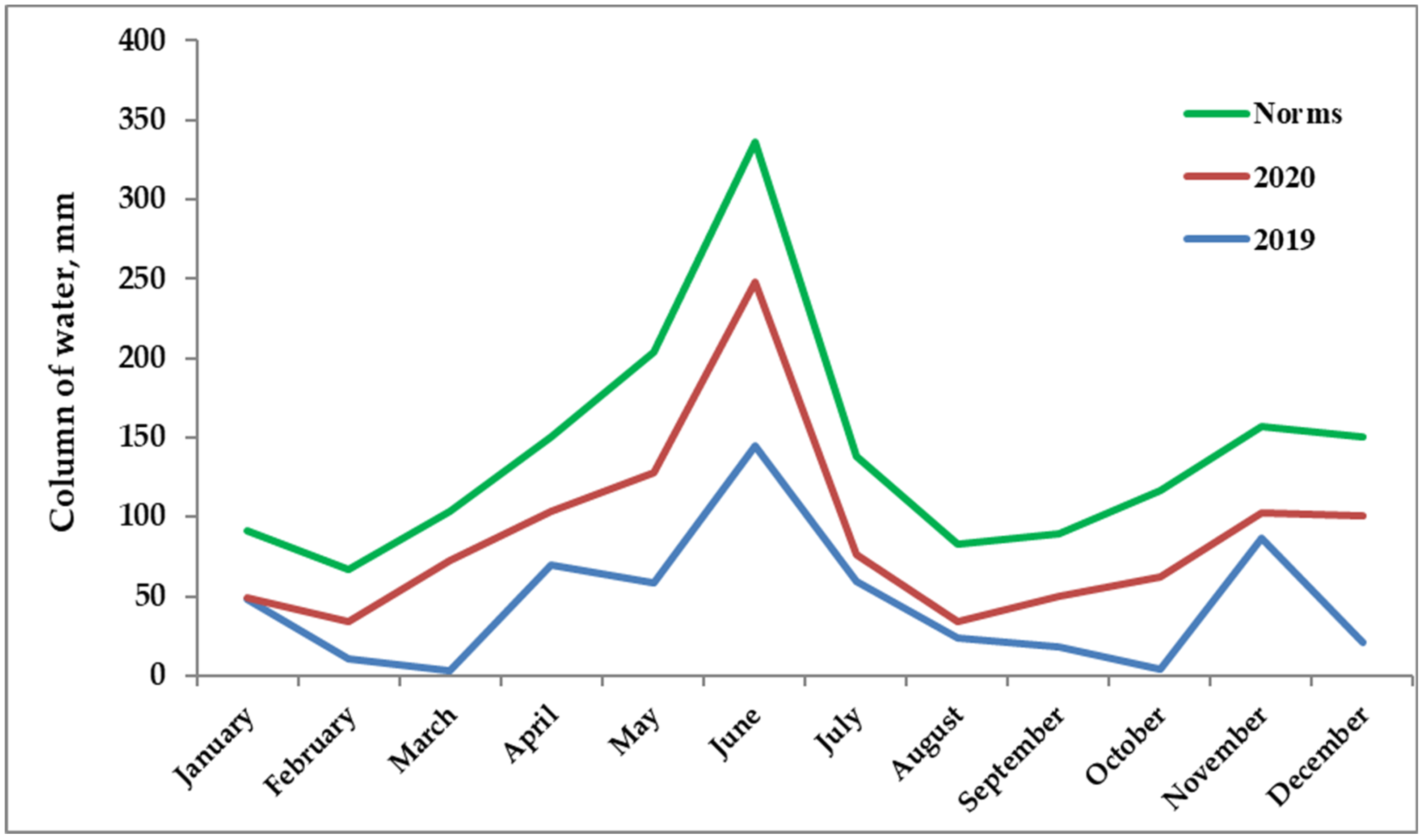
2.1. Plant Material and Processing
2.2. Chemical Analysis
2.2.1. Plant Pigments
2.2.2. GC/FID and GC/MS
2.3. Statistics
3. Results and Discussion
3.1. Content of the Plant Pigments
3.2. Yield and Chemical Composition of the Essential Oil (EO)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boelens, M.H. Chemical and sensory evaluation of Lavandula oils. Flavour Fragr. J. 1995, 20, 23–51. [Google Scholar]
- Giray, F. An Analysis of World Lavender Oil Markets and Lessons for Turkey. J. Essent. Oil Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Stanev, S.; Zagorcheva, T.; Atanassov, I. Lavender cultivation in Bulgaria—21th century developments, breeding challenges and opportunities. Bulg. J. Agric. Sci. 2016, 22, 584–590. [Google Scholar]
- Seidler-Lozykowska, K.; Mordalski, R.; Kucharski, W.; Kedzia, B.; Bocianowski, J. Yielding and quality of lavender flowers (Lavandula angustifolia Mill.) from organic cultivation. Acta Sci. Pol. Hortorum Cultus 2014, 13, 173–183. [Google Scholar]
- Lawrence, B. Progress in Essential Oils. Lavender Oil. Perfum. Flavorist 2015, 40, 42–52. [Google Scholar]
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, V.; Bechmann, I.E.; Behrens, A.; Stürup, S. Comparative Investigation of Concentrations of Major and Trace Elements in Organic and Conventional Danish Agricultural Crops. 1. Onions (Allium cepa Hysam) and Peas (Pisum sativum Ping Pong). J. Agric. Food Chem. 2000, 48, 6094–6102. [Google Scholar] [CrossRef]
- Tsvetkov, I.; Atanassov, A.; Vlahova, M.; Carlier, L.; Christov, N.; Lefort, F.; Rusanov, K.; Badjakov, I.; Dincheva, I.; Tchamitchian, M.; et al. Plant organic farming research—Current status and opportunities for future development. Biotechnol. Biotechnol. Equip. 2018, 32, 241–260. [Google Scholar] [CrossRef]
- Chalova, V.; Manolov, I.; Manolova, V. Challenges for commercial organic production of oil-bearing rose in Bulgaria. Biol. Agric. Hortic. 2017, 33, 183–194. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural organic compounds for application in organic farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Renaud, E.; Charles, D.; Simon, J. Essential Oil Quantity and Composition from 10 Cultivars of Organically Grown Lavender and Lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Stanev, S.; Zheljazkov, V. Organic vs conventional production of peppermint, lemonbalm and lavender: Effect on yield and chemical composition. Facta Univ. Phys. Chem. Technol. 2018, 16, 15. [Google Scholar]
- Todorova, M.; Dobreva, A.; Petkova, N.; Grozeva, N.; Gerdzhikova, M.; Veleva, P. Organic vs conventional farming of oil-bearing rose: Effect on essential oil and antioxidant activity. BioRisk 2022, 17, 271–285. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Yassemin, S.; Ozkaya, A.; Koksal, N.; Gok, B. The effect of nitrogen on growth and physiological features of lavender. In Proceedings of the International Congress on Medicinal and Aromatic Plants—“Natural and Healthy Life”, Konya, Turkey, 10–12 May 2017; pp. 746–754. [Google Scholar]
- Angelova, D.; Dobreva, A.; Baeva, G. The impact of soil herbicides on the yield and quality of lavender (Lavandula angustifolia Mill.) essential oil. Facta Univ. Ser. Phys. Chem. Technol. 2018, 16, 70. [Google Scholar] [CrossRef]
- Silva, S.; Lus, J.; Nogueira, P.; Blank, A.; Sampaio, T.; Pinto, J.; Junior, A. Organo-mineral fertilization effect on biomass and essential oil of Lavender (Lavandula dentata L.). Ind. Crops Prod. 2017, 103, 133–140. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Maganga, A. Influence of Variety and Organic Cultural Practices on Yield and Essential Oil Content of Lavender and Rosemary in Interior BC. South Thomson Organic Producers Association (STOPA). 2004. Available online: http://www.certifiedorganic.bc.ca/programs/osdp/OSDP-FinalReport_I-016.pdf (accessed on 30 December 2020).
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- ISO 3515; Oil of Lavender (Lavandula angustifolia Mill.). Organization for Standardization: Geneva, Switzerland. Available online: https://www.iso.org/standard/36253.html (accessed on 30 December 2020).
- Minev, N.; Matev, N.; Yordanova, N.; Milanov, I.; Sabeva, M.; Almaliev, M. Effect of foliar products on the inflorescence yield of lavender and essential oil. Agron. Res. 2022, 20, 660–671. [Google Scholar]
- Zielewicz, W.; Wróbel, B.; Niedbała, G. Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables. Forests 2020, 11, 1197. [Google Scholar] [CrossRef]
- Ponder, A.; Hallman, E. Phenolics and Carotenoid Contents in the Leaves of Different Organic and Conventional Raspberry (Rubus idaeus L.) Cultivars and Their In Vitro Activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Biesiada, A.; Kucharska, A. The effect of nitrogen fertilization on yielding and antioxidant activity of lavender (Lavandula angustifolia Mill.). Acta Sci. Pol. Hortorum Cultus 2008, 72, 33–40. [Google Scholar]
- Fritschi, F.; Ray, J. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 2008, 45, 92–98. [Google Scholar] [CrossRef]
- Gonçalves, F.; Gonçalves, J.C.; Ferrão, A.C.; Correia, P.; Guiné, R.P.F. Evaluation of phenolic compounds and antioxidant activity in some edible flowers. Open Agric. 2020, 5, 857–870. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Borowski, B. Changes in the content and chemical composition of sweet basil essential oil under the influence of fertilization of plants with nitrogen and potassium. Ann. UMCS Pharm. 2011, 3, 133–145. [Google Scholar]
- Blazeković, B.; Vladimir-Knezević, S.; Brantner, A.; Stefan, M.B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef]
- Costea, T.; Străinu, A.M.; Gîrd, C.E. 2019. Botanical characterization, chemical composition and antioxidant activity of Romanian lavander (Lavandula angustifolia Mill.) flowers. Studia Universitatis “Vasile Goldiş”. Ser. Ştiinţele Vieţii 2019, 29, 159–167. [Google Scholar]
- Konakchiev, A. Essential oils of Lavandula angustifolia Mill. varieties and Achillea L. species. Ph.D. Thesis, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2015. [Google Scholar]
- Kara, N.; Baydar, H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turk. J. Field Crops 2013, 18, 58–65. [Google Scholar]
- Lakušić, B.; Lakušić, D.; Ristić, M.; Marčetić, M.; Slavkovska, V. Seasonal Variations in the Composition of the Essential Oils of Lavandula angustifolia (Lamiacae). Nat. Prod. Comm. 2014, 9, 859–862. [Google Scholar] [CrossRef]
- Zheljazkov, V.; Astatkie, T.; Hristov, A. Lavender and hyssop productivity, oil content, and bioactivity as function of harvest time and drying. Ind. Crops Prod. 2012, 36, 222–228. [Google Scholar] [CrossRef]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Organic Fennel, Parsley, and Lavender from Spain. Food 2016, 5, 18. [Google Scholar] [CrossRef]
- Ognyanov, I. Bulgarian Lavender and Bulgarian Lavender oil. Perfum. Flavorist 1983, 8, 29–41. [Google Scholar]
- Bovill, G. The future of Lavender. Agricultural challenges meet growing consumer demand. Perfum. Flavorist 2013, 83, 34–35. [Google Scholar]
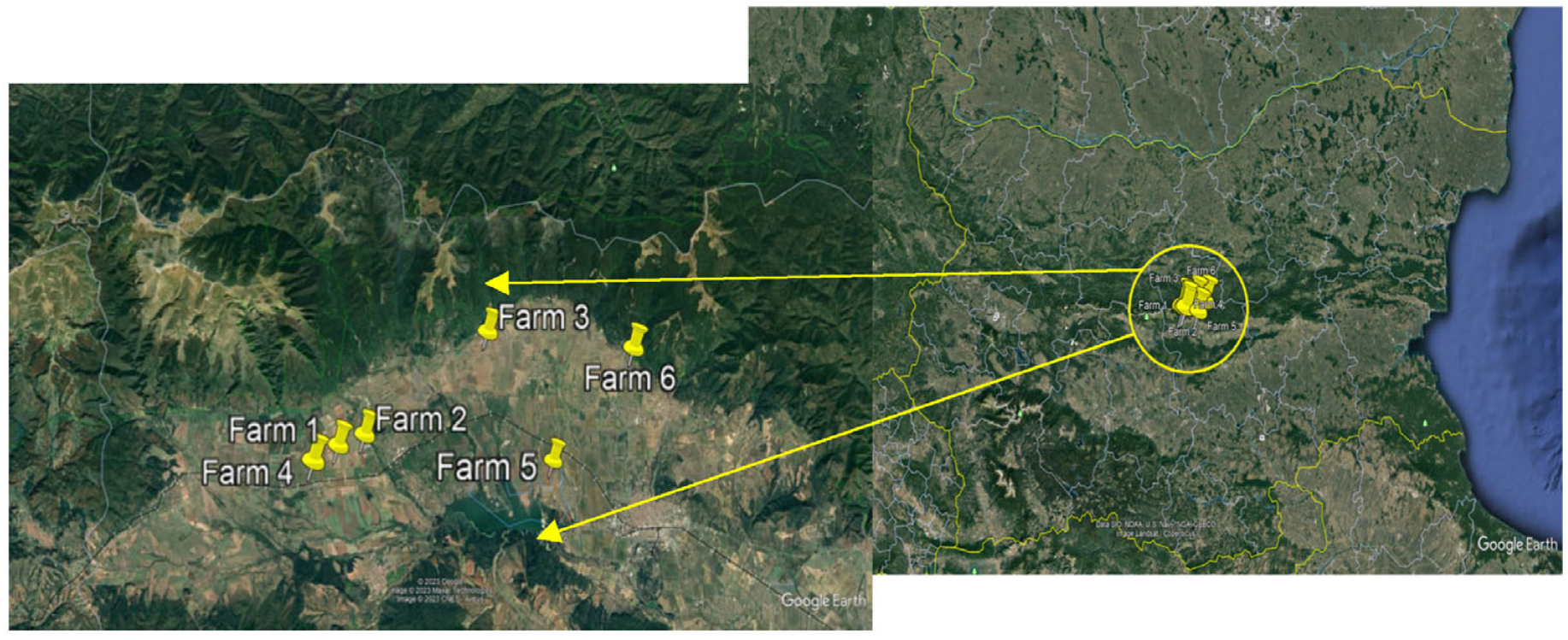



| Farms | Location | Localization (Garmin) | Farming Type Since | Altitude, m | Agricultural Practices during Vegetation | Fertilization/Plant Protection/Irrigation |
|---|---|---|---|---|---|---|
| Farm 1 (OF) | Asen | 42°38.548′ N 25°10.590′ E | Organic, 2016 | 483 | Soil tillage: 3–4 hoeing with a cultivator between the rows. Hand hoeing in the row. | 25 t/ha cattle manure was applied No plant protection was applied. No irrigation. |
| Farm 2 (OF) | Asen | 42°38.831′ N 25°11.675′ E | Organic, 2016 | 482 | Soil tillage: 3–4 hoeing with a cultivator between the rows. Hand hoeing in the row. | 25 t/ha cattle manure was applied No plant protection was applied. No irrigation. |
| Farm 3 (OF) | Yasenovo | 42°41.536′ N 25°16.786′ E | Organic, 2011 | 500 | Soil tillage: 3–4 hoeing with a cultivator between the rows and into the rows. | 30 t/ha cattle manure was applied. Lime melioration 3 t/ha No plant protection was applied. No irrigation. |
| Farm 4 (CF) | Gabarevo | 42°38.091′ N 25°09.531′ E | Conventional, 2016 | 423 | Soil tillage: 3–4 hoeing with a cultivator between the rows. | Spring—combined NPK April—foliar fertilizer NPK + micro elements. No plant protection was applied. No irrigation. Devrinol 4 F 400 mL/da (napropamide) was applied. |
| Farm 5 (CF) | Koprinka | 42°38.264′ N 25°19.506′ E | Conventional, 2016 | 395 | Soil tillage: 3–4 hoeing with a ”cultivator with suns” between the rows. | Spring—combined NPK April—foliar fertilizer NPK + micro elements. No plant protection was applied. No irrigation. Devrinol 4 F 400 mL/da (napropamide) was applied. |
| Farm 6 (CF) | Kran | 42°41.076′ N 25°22.820′ E | Conventional, 2017 | 440 | Soil tillage: 3–4 hoeing with a cultivator between the rows. Hand hoeing in the row. Devrinol 4 F 400 mL/da was applied. | Spring—combined NPK April—foliar fertilizer NPK + trace elements. No irrigation. Devrinol 4 F 400 mL/da, Deka EC 60 mL/da. |
| Farm/Pigments | Farm 1 (OF) | Farm 2 (OF) | Farm 3 (OF) | Farm 4 (CF) | Farm 5 (CF) | Farm 6 (CF) | |
|---|---|---|---|---|---|---|---|
| Chlorophyll a (Chla) | 2019 | 275.6 ± 59.1 f | 270.2 ± 32.0 f | 272.3 ± 17.0 f | 295.1 ± 31.5 f | 301.0 ± 14.0 f | 245.9 ± 24.0 f |
| 2020 | 274.9 ± 39.6 d | 275.7 ± 17.8 d | 251.3 ± 14.0 d | 356.1 ± 74.8 df | 377.5 ± 99.0 df | 292.8 ± 40.5 df | |
| Chlorophyll b (Chlb) | 2019 | 211.4 ± 54.2 b | 193.2 ± 31.3 b | 201.7 ± 6.2 b | 188.8 ± 50.6 e | 240.0 ± 3.8 e | 139.4 ± 12 e |
| 2020 | 100.5 ± 52.4 bde | 134.6 ± 14.4 bde | 137.3 ± 5.2 bde | 175.3 ± 25.0 d | 217.9 ± 33.9 d | 174.8 ± 21.3 d | |
| Total chlorophyll | 2019 | 487.0 ± 113.3 b | 463.4 ± 58.7 b | 474.0 ± 20.2 b | 483.9 ± 76.0 bd | 541.0 ± 13.2 bd | 385.3 ± 11.4 bd |
| 2020 | 375.3 ± 23.0 bd | 410.3 ± 26.3 bd | 388.5 ± 18.1 bd | 531.4 ± 99.4 d | 595.4 ± 131.8 d | 467.6 ± 61.8 d | |
| Total carotenoids | 2019 | 52.2 ± 15.5 abc | 36.9 ± 9.8 abc | 45.9 ± 19.8 abc | 61.5 ± 7.8 c | 63.1 ± 24.4 c | 55.5 ± 7.4 c |
| 2020 | 72.2 ± 32.0 b | 70.3 ± 7.7 b | 57.5 ± 2.6 b | 77.3 ± 15.6 a | 65.8 ± 20.0 a | 58.5 ± 4.8 a | |
| Total anthocyanins | 2019 | 780 ± 0.1 ns | 630 ± 0.5 ns | 410 ± 0.2 ns | 570 ± 0.2 ns | 330 ± 0.3 ns | 340 ± 0.0 ns |
| 2020 | 460 ± 0.3 ns | 420 ± 0.2 ns | 1240 ± 0.6 ns | 780 ± 0.1 ns | 200 ± 0.0 ns | 370 ± 0.0 ns | |
| No | Component, % | Farm 1 (OF) | Farm 2 (OF) | Farm 3 (OF) | Farm 4(CF) | Farm 5 (CF) | Farm 6 (CF) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| EO, % | 2.22 ± 0.4 ns | 1.94 ± 0.16 ns | 2.29 ± 0.28 ns | 1.43 ± 0.11 ns | 1.80 ± 0.41 ns | 2.75 ± 0.20 ns | 1.60 ± 0.46 ns | 1.75 ± 0.2 ns | 2.26 ± 0.30 ns | 1.75 ± 0.67 ns | 2.39 ± 0.31 ns | 2.78 ± 0.93 ns | |
| 1 | α-pinene | 0.25 ± 0.02 ns | 0.62 ± 0.03 ns | 0.27 ± 0.04 ns | 0.24 ± 0.02 ns | 0.31 ± 0.07 ns | 0.19 ± 0.02 ns | 0.32 ± 0.02 ns | 0.33 ± 0.02 ns | 0.16 ± 0.0 ns | 0.23 ± 0.01 ns | 0.13 ± 0.02 ns | 0.14 ± 0.04 ns |
| 2 | Camphen | 0.14 ± 0.02 ns | 0.19 ± 0.02 ns | 0.14 ± 0.02 ns | 0.13 ± 0.00 ns | 0.17 ± 0.02 ns | 0.10 ± 0.00 ns | 0.17 ± 0.02 ns | 0.16 ± 0.00 ns | 0.16 ± 0.02 ns | 0.13 ± 0.02 ns | 0.15 ± 0.00 ns | 0.11 ± 0.02 ns |
| 3 | 3-octanone | 1.04 ± 0.05 ns | 0.98 ± 0.02 ns | 1.11 ± 0.03 ns | 0.86 ± 0.05 ns | 0.95 ± 0.00 ns | 1.48 ± 0.05 ns | 0.29 ± 0.05 ns | 0.29 ± 0.02 ns | 1.87 ± 0.05 ns | 1.18 ± 0.04 ns | 2.44 ± 0.02 ns | 2.91 ± 0.02 ns |
| 4 | Cis-β ocimene | 8.01 ± 0.09 ns | 8.81 ± 0.04 ns | 8.41 ± 0.02 ns | 9.66 ± 0.00 ns | 7.17 ± 0.10 ns | 4.30 ± 0.0 ns | 11.14 ± 0.09 ns | 12.46 ± 0.10 ns | 2.34 ± 0.02 ns | 6.66 ± 0.05 ns | 1.91 ± 0.04 ns | 2.48 ± 0.00 ns |
| 5 | Limonene + 1,8-cineole | 1.45 ± 0.02 ns | 0.91 ± 0.05 ns | 1.57 ± 0.04 ns | 0.92 ± 0.04 ns | 1.36 ± 0.08 ns | 1.30 ± 0.04 ns | 0.91 ± 0.02 ns | 0.47 ± 0.04 ns | 2.54 ± 0.04 ns | 1.09 ± 0.02 ns | 2.69 ± 0.07 ns | 1.84 ± 0.00 ns |
| 6 | Trans-β ocimene | 3.29 ± 0.03 ns | 3.67 ± 0.03 ns | 2.98 ± 0.07 ns | 3.64 ± 0.07 ns | 3.04 ± 0.10 ns | 2.85 ± 0.10 ns | 2.93 ± 0.02 ns | 3.72 ± 0.02 ns | 2.74 ± 0.06 ns | 2.92 ± 0.00 ns | 2.85 ± 0.04 ns | 3.20 ± 0.72 ns |
| 7 | Linalool | 23.65 ± 0.12 ns | 26.83 ± 0.09 ns | 26.62 ± 0.02 ns | 25.50 ± 0.12 ns | 23.16 ± 0.14 ns | 26.49 ± 0.2 ns | 20.01 ± 0.2 ns | 21.55 ± 0.00 ns | 29.64 ± 0.12 ns | 28.03 ± 0.05 ns | 31.04 ± 0.24 ns | 30.54 ± 0.15 ns |
| 8 | Camphor | 0.08 ± 0.00 ns | 0.36 ± 0.06 ns | 0.08 ± 0.02 ns | 0.18 ± 0.04 ns | 0.08 ± 0.01 ns | 0.15 ± 0.05 ns | 0.08 ± 0.01 ns | 0.18 ± 0.01 ns | 0.10 ± 0.02 ns | 0.11 ± 0.02 ns | 0.10 ± 0.02 ns | 0.09 ± 0.02 ns |
| 9 | Borneol | 0.55 ± 0.02 ns | 0.75 ± 0.02 ns | 0.63 ± 0.00 ns | 0.64 ± 0.00 ns | 0.68 ± 0.0 ns | 0.76 ± 0.00 ns | 0.45 ± 0.00 ns | 0.55 ± 0.04 ns | 1.00 ± 0.00 ns | 0.79 ± 0.05 ns | 0.26 ± 0.00 ns | 0.87 ± 0.0 ns |
| 10 | Lavandulol | 0.49 ± 0.15 ns | 0.71 ± 0.00 ns | 0.49 ± 0.00 ns | 0.45 ± 0.00 ns | 0.57 ± 0.00 ns | 0.47 ± 0.02 ns | 0.46 ± 0.02 ns | 0.44 ± 0.02 ns | 0.56 ± 0.00 ns | 0.45 ± 0.05 ns | 0.26 ± 0.02 ns | 0.47 ± 0.0 ns |
| 11 | Terpinen-4-ol | 3.05 ± 0.07 ab | 3.78 ± 0.00 b | 3.83 ± 0.02 ab | 3.56 ± 0.04 b | 3.74 ± 0.02 ab | 3.15 ± 0.03 b | 3.67 ± 0.06 c | 4.46 ± 0.05 ac | 1.62 ± 0.04 c | 3.29 ± 0.09 ac | 1.62 ± 0.07 c | 1.98 ± 0.04 a |
| 12 | α-terpineol | 0.38 ± 0.00 ns | 0.46 ± 0.02 ns | 0.38 ± 0.04 ns | 0.58 ± 0.00 ns | 0.37 ± 0.02 ns | 0.56 ± 0.08 ns | 0.37 ± 0.07 ns | 0.48 ± 0.00 ns | 0.44 ± 0.04 ns | 0.59 ± 0.05 ns | 0.47 ± 0.02 ns | 0.56 ± 0.04 ns |
| 13 | Linalyl acetate | 34.90 ± 0.05 ns | 28.40 ± 0.00 ns | 31.77 ± 0.07 ns | 33.27 ± 0.12 ns | 31.64 ± 0.02 ns | 33.37 ± 0.09 ns | 38.23 ± 0.12 ns | 35.34 ± 0.07 ns | 28.69 ± 0.10 ns | 31.99 ± 0.16 ns | 28.42 ± 0.10 ns | 29.96 ± 0.12 ns |
| 14 | Lavandulyl acetate | 3.89 ± 0.02 ns | 2.89 ± 0.05 ns | 3.41 ± 0.09 ns | 3.68 ± 0.07 ns | 4.55 ± 0.01 ns | 3.71 ± 0.00 ns | 4.16 ± 0.04 ns | 3.70 ± 0.02 ns | 3.93 ± 0.04 ns | 3.52 ± 0.07 ns | 3.88 ± 0.05 ns | 3.44 ± 0.07 ns |
| 15 | β-caryophyllene | 11.43 ± 0.03 b | 8.12 ± 0.07 bd | 10.99 ± 0.10 b | 7.92 ± 0.14 bd | 11.71 ± 0.10 b | 10.73 ± 0.12 bd | 10.05 ± 0.14 cd | 7.95 ± 0.09 cd | 14.97 ± 0.10 cd | 9.40 ± 0.04 cd | 13.65 ± 0.70 cd | 11.19 ± 0.94 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobreva, A.; Petkova, N.; Todorova, M.; Gerdzhikova, M.; Zherkova, Z.; Grozeva, N. Organic vs. Conventional Farming of Lavender: Effect on Yield, Phytochemicals and Essential Oil Composition. Agronomy 2024, 14, 32. https://doi.org/10.3390/agronomy14010032
Dobreva A, Petkova N, Todorova M, Gerdzhikova M, Zherkova Z, Grozeva N. Organic vs. Conventional Farming of Lavender: Effect on Yield, Phytochemicals and Essential Oil Composition. Agronomy. 2024; 14(1):32. https://doi.org/10.3390/agronomy14010032
Chicago/Turabian StyleDobreva, Ana, Nadezhda Petkova, Mima Todorova, Mariya Gerdzhikova, Zornitza Zherkova, and Neli Grozeva. 2024. "Organic vs. Conventional Farming of Lavender: Effect on Yield, Phytochemicals and Essential Oil Composition" Agronomy 14, no. 1: 32. https://doi.org/10.3390/agronomy14010032
APA StyleDobreva, A., Petkova, N., Todorova, M., Gerdzhikova, M., Zherkova, Z., & Grozeva, N. (2024). Organic vs. Conventional Farming of Lavender: Effect on Yield, Phytochemicals and Essential Oil Composition. Agronomy, 14(1), 32. https://doi.org/10.3390/agronomy14010032











