Agronomic and Phytochemical Characterization of Chickpea Local Genetic Resources for the Agroecological Transition and Sustainable Food Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Setup and Site Characteristics
2.2. Sampling and Measurements
2.3. Phytochemical Analyses
2.3.1. Chemicals
2.3.2. Protein, Oil Content, and Fatty Acids Composition
2.3.3. Sample Extraction and Total Phenolic and Antioxidant Activity Analyses
2.4. Color Characterization
2.5. Statistical Analysis
3. Results and Discussion
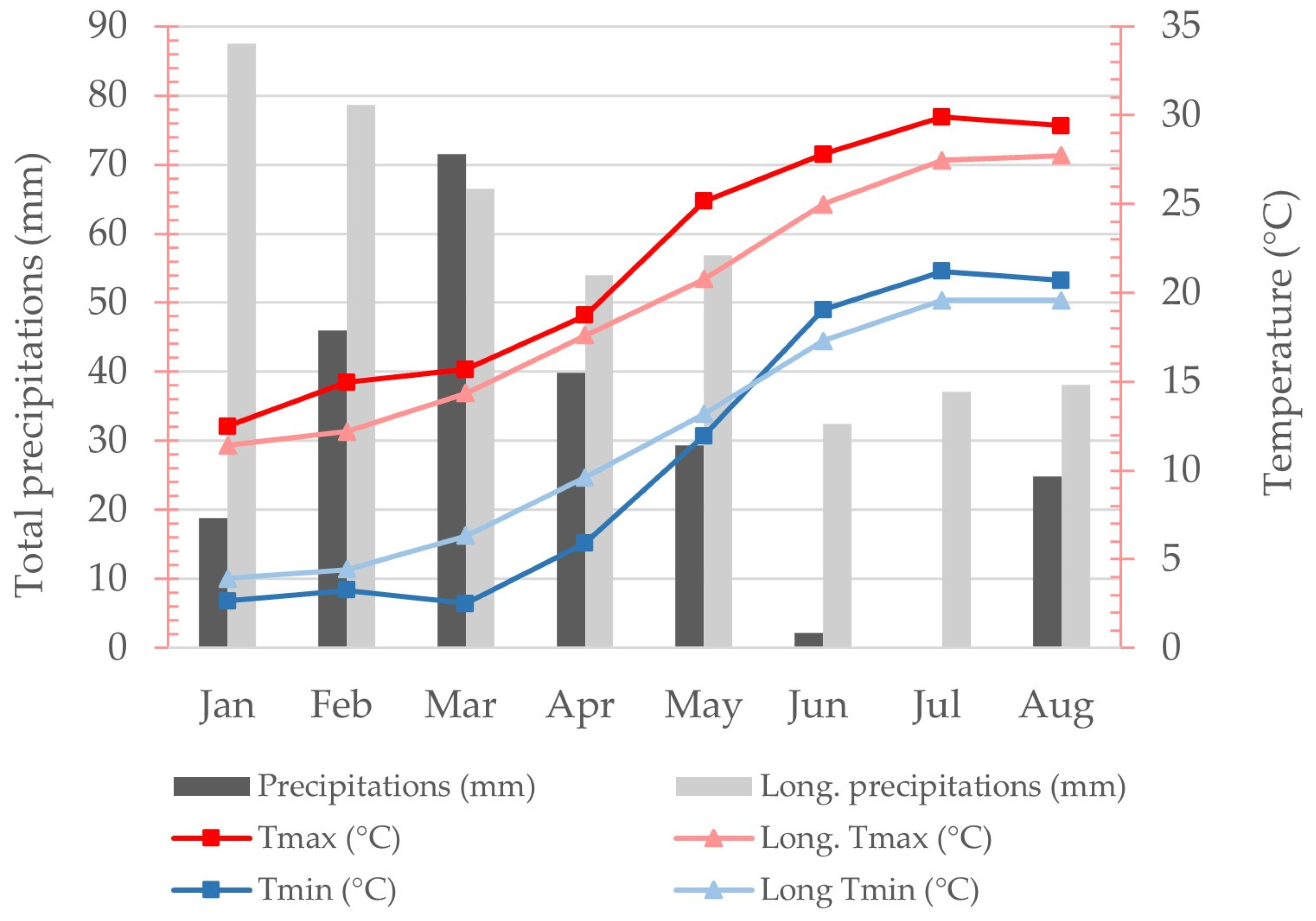
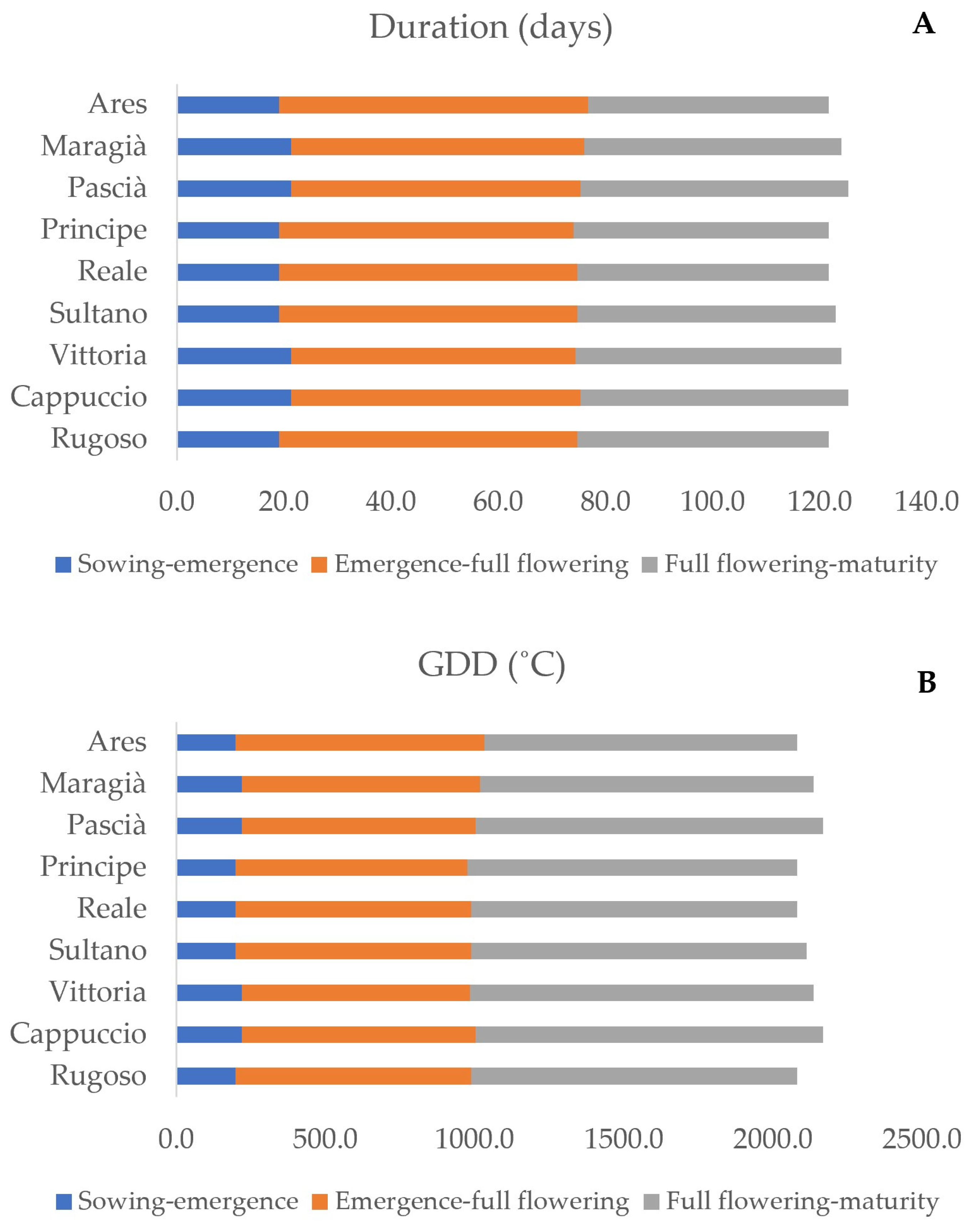
3.1. Weather and Plant Phenology
3.2. Yield and Yield Components
3.3. Phytochemical Evaluation and Antioxidant Activity
3.3.1. Seed Quality Analyses
3.3.2. Bioactive Compounds and Antioxidant Activity
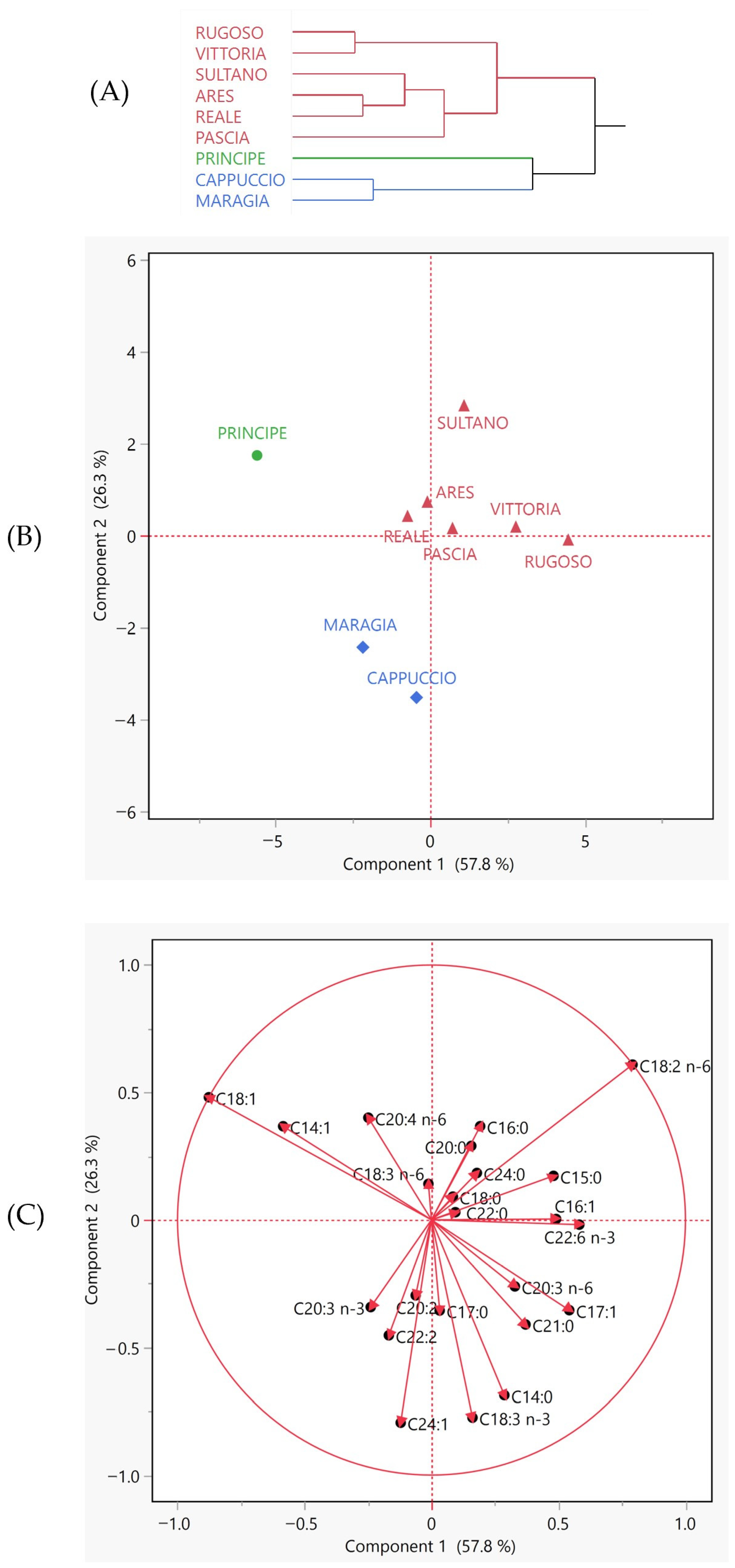
3.4. Colorimetric Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a Sustainable Source of Protein in Human Diets. Glob. Food Sec. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and Supply of High-quality Food Protein for Human Consumption: Sustainability, Challenges, and Innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A Systematic Review on Consumer Acceptance of Alternative Proteins: Pulses, Algae, Insects, Plant-Based Meat Alternatives, and Cultured Meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef]
- Dixon, R.A.; Sumner, L.W. Legume Natural Products: Understanding and Manipulating Complex Pathways for Human and Animal Health. Plant Physiol. 2003, 131, 878–885. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol Composition and Antioxidant Activity of Different Grass Pea (Lathyrus sativus), Lentils (Lens culinaris), and Chickpea (Cicer arietinum) Ecotypes of the Campania Region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Casini, P. Field Evaluation of New Kabuli Chickpeas Lines for the Production of Canned Seeds. Adv. Hortic. Sci. 2018, 32, 569–573. [Google Scholar] [CrossRef]
- Voisin, A.-S.; Guéguen, J.; Huyghe, C.; Jeuffroy, M.-H.; Magrini, M.-B.; Meynard, J.-M.; Mougel, C.; Pellerin, S.; Pelzer, E. Legumes for Feed, Food, Biomaterials and Bioenergy in Europe: A Review. Agron. Sustain. Dev. 2014, 34, 361–380. [Google Scholar] [CrossRef]
- Abbate, V. Aspetti Della Tecnica Colturale Del Cece. Agric. Ric. 1994, 155, 105–120. [Google Scholar]
- Rossini, F. Soluzione Ai Problemi Della Coltura Del Cece. L’informatore Agrar. 2008, 17, 45–47. [Google Scholar]
- Kumar, S.; Meena, R.S.; Datta, R.; Verma, S.K.; Yadav, G.S.; Pradhan, G.; Molaei, A.; Rahman, G.K.M.M.; Mashuk, H.A. Legumes for Carbon and Nitrogen Cycling: An Organic Approach. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 337–375. ISBN 9789811372643. [Google Scholar]
- European Commission. The European Green Deal. COM (2019) 640 Final. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 27 January 2024).
- Balázs, B.; Kelemen, E.; Centofanti, T.; Vasconcelos, M.W.; Iannetta, P.P.M. Integrated Policy Analysis to Identify Transformation Paths to More Sustainable Legume-Based Food and Feed Value-Chains in Europe. Agroecol. Sustain. Food Syst. 2021, 45, 931–953. [Google Scholar] [CrossRef]
- Wijerathna-Yapa, A.; Pathirana, R. Sustainable Agro-Food Systems for Addressing Climate Change and Food Security. Agriculture 2022, 12, 1554. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J. Economic Importance of Chickpea: Production, Value, and World Trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Cece-Cicer arietinum, L., Atlante Delle Coltivazioni Erbacee—Leguminose Da Granella. Available online: www.agraria.org (accessed on 4 July 2023).
- Faridy, J.-C.M.; Stephanie, C.-G.M.; Gabriela, M.-M.O.; Cristian, J.-M. Biological Activities of Chickpea in Human Health (Cicer arietinum L.). A Review. Plant Foods Hum. Nutr. 2020, 75, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Prasad, K. Technological, Processing and Nutritional Aspects of Chickpea (Cicer arietinum)—A Review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Li, A.; Liu, R.H.; Gao, X.; Li, D.; Kou, X.; Xue, Z. Nutritional Constituent and Health Benefits of Chickpea (Cicer arietinum L.): A Review. Food Res. Int. 2021, 150, 110790. [Google Scholar] [CrossRef] [PubMed]
- McLean, E.O. Soil PH and Lime Requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen Total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Monograph 9; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Mehlich, A. Determination of Cation- and Anion-Exchange Properties of Soils. Soil Sci. 1948, 66, 429–446. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; ISTA: Wallisellen, Switzerland, 2024; ISBN 978-3-906549-41-5. [Google Scholar]
- Rocchetti, L.; Bellucci, E.; Cortinovis, G.; Di Vittori, V.; Lanzavecchia, G.; Frascarelli, G.; Nanni, L.; Del Gatto, A.; Pieri, S.; Mangoni, L.; et al. The Development of a European and Mediterranean Chickpea Association Panel (EMCAP). Agronomy 2020, 10, 1417. [Google Scholar] [CrossRef]
- Clemente, C.; Ercolini, L.; Rossi, A.; Foschi, L.; Grossi, N.; Angelini, L.G.; Tavarini, S.; Silvestri, N. Spectral Response of Camelina (Camelina sativa (L.) Crantz) to Different Nitrogen Fertilization Regimes under Mediterranean Conditions. Agronomy 2023, 13, 1539. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of Lipid Extracts from Tissues. In Advances in Lipid Methodology-Two; Christie, W.W., Ed.; Oily Press: Dundee, UK, 1993; pp. 195–213. [Google Scholar]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed Sweet Corn Has Higher Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- De Santis, M.A.; Satriani, A.; De Santis, F.; Flagella, Z. Water Use Efficiency, Spectral Phenotyping and Protein Composition of Two Chickpea Genotypes Grown in Mediterranean Environments under Different Water and Nitrogen Supply. Agriculture 2022, 12, 2026. [Google Scholar] [CrossRef]
- Fotiadis, S.; Koutroubas, S.D.; Damalas, C.A. Sowing Date and Cultivar Effects on Assimilate Translocation in Spring Mediterranean Chickpea. Agron. J. 2017, 109, 2011–2024. [Google Scholar] [CrossRef]
- Ruggeri, R.; Primi, R.; Danieli, P.P.; Ronchi, B.; Rossini, F. Effects of Seeding Date and Seeding Rate on Yield, Proximate Composition and Total Tannins Content of Two Kabuli Chickpea Cultivars. Ital. J. Agron. 2017, 12, 201–207. [Google Scholar] [CrossRef]
- Richards, M.F.; Maphosa, L.; Preston, A.L. Impact of Sowing Time on Chickpea (Cicer arietinum L.) Biomass Accumulation and Yield. Agronomy 2022, 12, 160. [Google Scholar] [CrossRef]
- Chauhan, Y.; Allard, S.; Williams, R.; Williams, B.; Mundree, S.; Chenu, K.; Rachaputi, N.C. Characterisation of Chickpea Cropping Systems in Australia for Major Abiotic Production Constraints. Field Crops Res. 2017, 204, 120–134. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional Quality and Health Benefits of Chickpea (Cicer arietinum L.): A Review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, M.; Iqbal, S.; Ahmad, S.; Ali, H. Characterization and Compositional Studies of Oil from Seeds of Desi Chickpea (Cicer arietinum L.) Cultivars Grown in Pakistan. J. Am. Oil Chem. Soc. 2007, 84, 1143–1148. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Zhang, X.; Wang, M.; Liu, H.; Zhu, Y. Nutritional Components, Volatile Constituents and Antioxidant Activities of 6 Chickpea Species. Food Biosci. 2021, 41, 100964. [Google Scholar] [CrossRef]
- Dragičević, V.; Kratovalieva, S.; Dumanović, Z.; Dimov, Z.; Kravić, N. Variations in Level of Oil, Protein, and Some Antioxidants in Chickpea and Peanut Seeds. Chem. Biol. Technol. Agric. 2015, 2, 2. [Google Scholar] [CrossRef]
- Summo, C.; De Angelis, D.; Ricciardi, L.; Caponio, F.; Lotti, C.; Pavan, S.; Pasqualone, A. Nutritional, Physico-Chemical and Functional Characterization of a Global Chickpea Collection. J. Food Compos. Anal. 2019, 84, 103306. [Google Scholar] [CrossRef]
- Shah, T.M.; Iqbal, Z.; Asi, M.R.; Atta, B.M. Induced Genetic Variability for Fatty Acids and Oil Contents in Chickpea (Cicer arietinum). Int. J. Agric. Biol. 2013, 15, 419–426. [Google Scholar]
- Xiao, S.; Li, Z.; Zhou, K.; Fu, Y. Chemical Composition of Kabuli and Desi Chickpea (Cicer arietinum L.) Cultivars Grown in Xinjiang, China. Food Sci. Nutr. 2023, 11, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Vivar-Quintana, A.M.; Absi, Y.; Hernández-Jiménez, M.; Revilla, I. Nutritional Value, Mineral Composition, Fatty Acid Profile and Bioactive Compounds of Commercial Plant-Based Gluten-Free Flours. Appl. Sci. 2023, 13, 2309. [Google Scholar] [CrossRef]
- Madurapperumage, A.; Tang, L.; Thavarajah, P.; Bridges, W.; Shipe, E.; Vandemark, G.; Thavarajah, D. Chickpea (Cicer arietinum L.) as a Source of Essential Fatty Acids—A Biofortification Approach. Front. Plant Sci. 2021, 12, 734980. [Google Scholar] [CrossRef]
- Gül, M.K.; Egesel, C.Ö.; Turhan, H. The Effects of Planting Time on Fatty Acids and Tocopherols in Chickpea. Eur. Food Res. Technol. 2008, 226, 517–522. [Google Scholar] [CrossRef]
- Jahreis, G.; Brese, M.; Leiterer, M.; Schäfer, U.; Böhm, V. Legume Flours: Nutritionally Important Sources of Protein and Dietary Fiber. Ernahrungs Umschau 2016, 63, 36–42. [Google Scholar] [CrossRef]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total Polyphenol Content, Carotenoid, Tocopherol and Fatty Acid Composition of Commonly Consumed Canadian Pulses and Their Contribution to Antioxidant Activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Grasso, N.; Lynch, N.L.; Arendt, E.K.; O’Mahony, J.A. Chickpea Protein Ingredients: A Review of Composition, Functionality, and Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 435–452. [Google Scholar] [CrossRef]
- Grewal, S.K.; Sharma, K.P.; Bharadwaj, R.D.; Hegde, V.; Sidhu, S.K.; Singh, S.; Jain, P.K.; Rasool, S.; Arya, D.K.; Agrawal, P.K.; et al. Characterization of Chickpea Cultivars and Trait Specific Germplasm for Grain Protein Content and Amino Acids Composition and Identification of Potential Donors for Genetic Improvement of Its Nutritional Quality. Plant Genet. Resour. Charact. Util. 2022, 20, 383–393. [Google Scholar] [CrossRef]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional Composition, Health Benefits and Bio-Active Compounds of Chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef]
- Kaur, K.; Grewal, S.K.; Gill, P.S.; Singh, S. Comparison of Cultivated and Wild Chickpea Genotypes for Nutritional Quality and Antioxidant Potential. J. Food Sci. Technol. 2019, 56, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M. Variability in the Distribution of Phenolic Compounds in Milled Fractions of Chickpea and Horse Gram: Evaluation of Their Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 8322–8330. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Soto, M.F.; Saracho-Peña, A.G.; Chavez-Ontiveros, J.; Garzon-Tiznado, J.A.; Pineda-Hidalgo, K.V.; Delgado-Vargas, F.; Lopez-Valenzuela, J.A. Phenolic Profiles and Their Contribution to the Antioxidant Activity of Selected Chickpea Genotypes from Mexico and ICRISAT Collections. Plant Foods Hum. Nutr. 2018, 73, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Badani, H.; Kapulnik, Y.; Shomer, I.; Oren-Shamir, M.; Galili, S. Determination of Polyphenols, Flavonoids, and Antioxidant Capacity in Colored Chickpea (Cicer arietinum L.). J. Food Sci. 2010, 75, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Milán-Carrillo, J.; Reyes-Moreno, C.; Armienta-Rodelo, E.; Carábez-Trejo, A.; Mora-Escobedo, R. Physicochemical and Nutritional Characteristics of Extruded Flours from Fresh and Hardened Chickpeas (Cicer arietinum L.). LWT-Food Sci. Technol. 2000, 33, 117–123. [Google Scholar] [CrossRef]
- Teterycz, D.; Sobota, A. Legume Flour as a Natural Colouring Component in Pasta Production. J. Food Sci. Technol. 2020, 57, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, K.; Tar’an, B.; Diapari, M.; Arganosa, G.; Warkentin, T.D. Effect of Cultivar and Environment on Carotenoid Profile of Pea and Chickpea. Crop Sci. 2014, 54, 2225–2235. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Diapari, M.; Jha, A.B.; Tar’an, B.; Arganosa, G.; Warkentin, T.D. Genetic Diversity of Nutritionally Important Carotenoids in 94 Pea and 121 Chickpea Accessions. J. Food Compos. Anal. 2015, 43, 49–60. [Google Scholar] [CrossRef]
- Kazemzadeh Pournaki, S.; Biswas, A.; Hall, C. Effects of Storage Conditions on Chemistry and Technological Properties of Different Cultivars of Chickpea. J. Agric. Food Res. 2024, 16, 101066. [Google Scholar] [CrossRef]
| Type | Genotype | Provider | Origin |
|---|---|---|---|
| Commercial | Ares | SAIS 1 | Italy |
| Maragià | ISEA 2 S.r.l. | Italy | |
| Pascià | ISEA S.r.l. | Italy | |
| Principe | SAIS | Italy | |
| Reale | ISEA S.r.l. | Italy | |
| Sultano | ISEA S.r.l. | Italy | |
| Vittoria | SAIS | Italy | |
| Landrace | Cappuccio della Valtiberina | Germplasm bank of the DAFE 3 | Italy |
| Rugoso della Maremma | Germplasm bank of the DAFE | Italy |
| Sand (%) | 58.70 |
| Silt (%) | 27.50 |
| Clay (%) | 13.80 |
| pH | 7.85 |
| Electric conductivity (mS cm−1) | 0.14 |
| Total N (g kg−1) | 0.89 |
| NO3-N (mg kg−1) | 50.70 |
| Available P Olsen (mg kg−1) | 4.5 |
| Exchangeable K (mg kg−1) | 141.5 |
| Organic matter (%) | 2.15 |
| Active CaCO3 (%) | 2.83 |
| Cation exchange capacity (meq 100 g−1) | 7.24 |
| Genotype | Plant Height ‡ | Height to the First Pod | Branches | Pods per Plant ‡ | No. Seeds per Pod | 1000-Seed Weight | Seed Production | Plant Density | Seed Yield | Harvest Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary | Secondary | ||||||||||
| cm | cm | No. Plant−1 | No. Plant−1 | No. Plant−1 | No. Pod−1 | g DW Plant−1 | g DW Plant−1 | No. Plant m−2 | g m−2 | ||
| Commercial | |||||||||||
| Ares | 54.40 ± 1.82 | 33.20 ± 2.47 | 2.30 ± 0.28 | 10.37 ± 0.87 a | 38.36 ± 5.64 | 0.83 ± 0.09 | 280.67 ± 13.49 c | 8.75 ± 1.85 | 27.55 ± 3.36 | 241.68 ± 26.19 | 0.51 ± 0.01 ab |
| Maragià | 45.97 ± 1.82 | 27.43 ± 2.47 | 2.40 ± 0.28 | 7.29 ± 0.66 b | 21.77 ± 3.25 | 1.09 ± 0.13 | 432.92 ± 13.49 a | 9.90 ± 1.85 | 27.22 ± 3.33 | 261.24 ± 26.19 | 0.53 ± 0.01 ab |
| Pascià | 45.85 ± 1.81 | 25.59 ± 2.46 | 2.12 ± 0.26 | 7.29 ± 0.66 b | 27.01 ± 4.02 | 0.93 ± 0.11 | 377.48 ± 13.49 ab | 10.72 ± 1.85 | 26.22 ± 3.26 | 219.40 ± 26.19 | 0.53 ± 0.01 ab |
| Principe | 50.27 ± 1.82 | 32.00 ± 2.47 | 2.53 ± 0.29 | 6.33 ± 0.60 bc | 22.84 ± 3.40 | 0.85 ± 0.09 | 392.32 ± 13.49 ab | 7.51 ± 1.85 | 32.53 ± 3.71 | 240.74 ± 26.19 | 0.52 ± 0.01 ab |
| Reale | 46.70 ± 1.82 | 28.67 ± 2.47 | 2.70 ± 0.30 | 4.74 ± 0.49 c | 21.55 ± 3.22 | 0.90 ± 0.10 | 382.79 ± 13.49 ab | 7.15 ± 1.85 | 30.87 ± 3.59 | 220.63 ± 26.19 | 0.55 ± 0.01 a |
| Sultano | 53.83 ± 1.82 | 33.80 ± 2.47 | 2.20 ± 0.27 | 8.48 ± 0.74 ab | 28.19 ± 4.18 | 0.81 ± 0.09 | 283.44 ± 13.49 c | 6.48 ± 1.85 | 35.85 ± 3.94 | 237.23 ± 26.19 | 0.50 ± 0.01 ab |
| Vittoria | 49.73 ± 1.82 | 30.47 ± 2.47 | 2.87 ± 0.31 | 6.76 ± 0.63 b | 30.41 ± 4.50 | 0.88 ± 0.10 | 331.57 ± 13.49 bc | 8.79 ± 1.85 | 29.87 ± 3.52 | 245.15 ± 26.19 | 0.53 ± 0.01 ab |
| Local | |||||||||||
| Cappuccio | 47.07 ± 1.82 | 27.13 ± 2.47 | 2.50 ± 0.29 | 6.53 ± 0.61 bc | 30.50 ± 4.51 | 0.78 ± 0.08 | 425.50 ± 13.49 a | 10.49 ± 1.85 | 33.86 ± 3.80 | 292.83 ± 26.19 | 0.55 ± 0.01 a |
| Rugoso | 47.23 ± 1.82 | 28.83 ± 2.47 | 2.83 ± 0.31 | 8.15 ± 0.72 ab | 29.06 ± 4.30 | 0.96 ± 0.11 | 271.27 ± 13.49 c | 7.40 ± 1.85 | 36.51 ± 3.98 | 272.45 ± 26.19 | 0.50 ± 0.01 b |
| Significance | * | n.s. | n.s. | *** | * | n.s. | *** | n.s. | n.s. | n.s. | * |
| Commercial | 49.49 ± 1.53 | 30.11 ± 1.84 | 2.44 ± 0.11 | 7.07 ± 0.50 | 26.65 ± 2.29 | 0.90 ± 0.03 | 354.46 ± 14.16 | 8.47 ± 1.17 | 30.01 ± 1.98 | 238.01 ± 8.88 b | 0.52 ± 0.01 |
| Local | 47.15 ± 1.64 | 27.98 ± 1.94 | 2.67 ± 0.21 | 7.21 ± 0.89 | 29.73 ± 3.94 | 0.87 ± 0.07 | 348.39 ± 26.49 | 8.95 ± 1.50 | 35.19 ± 3.05 | 282.64 ± 16.62 a | 0.52 ± 0.01 |
| Significance | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | * | n.s. |
| Plant Height | First Pod Height | No. Primary Branches ‡ | No. Secondary Branches ‡ | No. Pods per Plant | No. Seeds per Pod | 1000-Seed Weight | Seed Production ‡ | Plant Density | Yield | Harvest Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | - | - | - | - | - | - | - | - | - | - |
| First pod height | 0.84 *** | 1 | - | - | - | - | - | - | - | - | - |
| No. primary branches‡ | n.s. | n.s. | 1 | - | - | - | - | - | - | - | - |
| No. secondary branches‡ | n.s. | n.s. | n.s. | 1 | - | - | - | - | - | - | - |
| No. pods per plant | n.s. | −0.39 * | n.s. | 0.76 *** | 1 | - | - | - | - | - | - |
| No. seeds per pod | n.s. | n.s. | n.s. | n.s. | n.s. | 1 | - | - | - | - | - |
| 1000-seed weight | n.s. | n.s. | n.s. | −0.44 * | n.s. | n.s. | 1 | - | - | - | - |
| Seed production ‡ | −0.46 * | −0.77 *** | n.s. | 0.42 * | 0.71 *** | n.s. | n.s. | 1 | - | - | - |
| Plant density | 0.48 * | 0.69 *** | n.s. | n.s. | −0.47 * | −0.48 * | n.s. | −0.79 *** | 1 | - | - |
| Yield | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | 0.51 * | 1 | - |
| Harvest index | −0.39 * | −0.42 * | n.s. | n.s. | n.s. | n.s. | 0.51 ** | 0.43 * | −0.41 * | n.s. | 1 |
| Genotype | Protein Content | Oil Content | C16:0 | C18:1 | C18:2 | C18:3 | ω-6/ω-3 |
|---|---|---|---|---|---|---|---|
| % | |||||||
| Commercial | |||||||
| Ares | 18.87 ± 0.99 | 6.6 ± 0.09 abc | 10.99 ± 0.09 d | 31.04 ± 0.25 bc | 50.50 ± 0.41 b | 3.39 ± 0.03 c | 14.90 ± 0.01 f |
| Maragià | 18.24 ± 1.65 | 6.9 ± 0.18 ab | 10.66 ± 0.08 e | 30.71 ± 0.22 cd | 46.89 ± 0.41 c | 3.98 ± 0.03 a | 11.78 ± 0.01 i |
| Pascià | 19.66 ± 1.49 | 7.2 ± 0.14 a | 9.98 ± 0.07 f | 29.97 ± 0.21 d | 50.38 ± 0.41 b | 2.97 ± 0.01 f | 16.96 ± 0.08 b |
| Principe | 20.48 ± 1.26 | 6.9 ± 0.23 ab | 11.25 ± 0.10 cd | 35.92 ± 0.30 a | 47.78 ± 0.41 c | 3.08 ± 0.02 e | 15.51 ± 0.03 e |
| Reale | 19.10 ± 0.14 | 6.2 ± 0.32 bc | 11.52 ± 0.07 b | 31.37 ± 0.27 bc | 49.92 ± 0.46 b | 3.06 ± 0.02 e | 16.31 ± 0.04 c |
| Sultano | 17.45 ± 1.58 | 6.5 ± 0.24 abc | 11.07 ± 0.09 d | 31.50 ± 0.28 b | 52.96 ± 0.50 a | 2.98 ± 0.02 f | 17.77 ± 0.05 a |
| Vittoria | 19.08 ± 2.14 | 6.1 ± 0.26 c | 11.74 ± 0.11 a | 28.55 ± 0.20 e | 51.98 ± 0.47 a | 3.26 ± 0.01 d | 15.94 ± 0.10 d |
| Local | |||||||
| Cappuccio | 18.55 ± 1.55 | 7.1 ± 0.09 a | 10.57 ± 0.06 e | 28.72 ± 0.25 e | 47.31 ± 0.40 c | 3.80 ± 0.03 b | 12.45 ± 0.01 h |
| Rugoso | 19.40 ± 0.47 | 6.5 ± 0.10 abc | 11.42 ± 0.10 bc | 27.13 ± 0.19 f | 52.91 ± 0.49 a | 3.86 ± 0.03 b | 13.71 ± 0.02 g |
| Significance | n.s. | *** | *** | *** | *** | *** | *** |
| Genotype | TPC (mg GAE g−1 DW) | DPPH (μmol TE g−1 DW) | FRAP (μmol TE g−1 DW) |
|---|---|---|---|
| Commercial | |||
| Ares | 0.51 ± 0.01 b | 0.51 ± 0.02 cde | 0.94 ± 0.06 d |
| Maragià | 0.44 ± 0.01 d | 0.53 ± 0.04 bcd | 0.81 ± 0.03 e |
| Pascià | 0.46 ± 0.02 bcd | 0.39 ± 0.06 e | 0.88 ± 0.04 de |
| Principe | 0.50 ± 0.03 bc | 0.62 ± 0.03 ab | 1.23 ± 0.02 a |
| Reale | 0.50 ± 0.01 bc | 0.61 ± 0.03 abc | 0.98 ± 0.06 cd |
| Sultano | 0.50 ± 0.00 bc | 0.60 ± 0.03 abc | 1.12 ± 0.01 ab |
| Vittoria | 0.56 ± 0.01 a | 0.67 ± 0.05 a | 1.06 ± 0.00 bc |
| Local | |||
| Cappuccio | 0.45 ± 0.03 cd | 0.43 ± 0.03 de | 1.07 ± 0.02 bc |
| Rugoso | 0.48 ± 0.03 bcd | 0.54 ± 0.05 bc | 1.09 ± 0.06 bc |
| Significance | *** | *** | *** |
| Genotype | L* | a* | b* |
|---|---|---|---|
| Commercial | |||
| Ares | 85.61 ± 0.08 cd | 1.77 ± 0.03 ab | 26.52 ± 0.06 ab |
| Maragià | 86.40 ± 0.13 abc | 1.40 ± 0.08 abc | 23.73 ± 0.54 cd |
| Pascià | 86.33 ± 0.01 abc | 1.37 ± 0.03 abc | 24.08 ± 0.57 cd |
| Principe | 87.18 ± 0.07 ab | 1.27 ± 0.00 bc | 22.80 ± 0.10 d |
| Reale | 87.41 ± 0.03 a | 1.21 ± 0.02 c | 24.88 ± 0.13 bcd |
| Sultano | 86.10 ± 0.47 bc | 1.63 ± 0.23 abc | 25.72 ± 0.08 abc |
| Vittoria | 87.08 ± 0.01 ab | 1.17 ± 0.01 c | 24.46 ± 0.05 bcd |
| Local | |||
| Cappuccio | 86.37 ± 0.47 abc | 1.38 ± 0.09 abc | 23.73 ± 0.45 cd |
| Rugoso | 84.87 ± 0.5 d | 1.85 ± 0.28 a | 27.32 ± 1.35 a |
| Significance | ** | * | ** |
| Maragià | Pascià | Principe | Reale | Sultano | Vittoria | Cappuccio | Rugoso | |
|---|---|---|---|---|---|---|---|---|
| Ares | 2.92 | 2.58 | 4.07 | 2.5 | 0.95 | 2.6 | 2.92 | 1.09 |
| Maragià | - | 0.35 | 1.22 | 1.54 | 2.03 | 1.02 | 0.03 | 3.93 |
| Pascià | - | 1.54 | 1.18 | 1.68 | 0.86 | 0.35 | 6.08 | |
| Principe | - | 10.47 | 2.09 | 3.13 | 1.67 | 1.24 | ||
| Reale | - | 1.61 | 0.54 | 1.56 | 3.58 | |||
| Sultano | - | 1.66 | 2.02 | 2.03 | ||||
| Vittoria | - | 1.04 | 3.68 | |||||
| Cappuccio | - | 3.92 | ||||||
| Rugoso | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Chehade, L.; Tavarini, S.; Bozzini, M.F.; Koskey, G.; Caturegli, L.; Antichi, D.; Angelini, L.G. Agronomic and Phytochemical Characterization of Chickpea Local Genetic Resources for the Agroecological Transition and Sustainable Food Systems. Agronomy 2024, 14, 2229. https://doi.org/10.3390/agronomy14102229
Abou Chehade L, Tavarini S, Bozzini MF, Koskey G, Caturegli L, Antichi D, Angelini LG. Agronomic and Phytochemical Characterization of Chickpea Local Genetic Resources for the Agroecological Transition and Sustainable Food Systems. Agronomy. 2024; 14(10):2229. https://doi.org/10.3390/agronomy14102229
Chicago/Turabian StyleAbou Chehade, Lara, Silvia Tavarini, Maria Francesca Bozzini, Gilbert Koskey, Lisa Caturegli, Daniele Antichi, and Luciana G. Angelini. 2024. "Agronomic and Phytochemical Characterization of Chickpea Local Genetic Resources for the Agroecological Transition and Sustainable Food Systems" Agronomy 14, no. 10: 2229. https://doi.org/10.3390/agronomy14102229
APA StyleAbou Chehade, L., Tavarini, S., Bozzini, M. F., Koskey, G., Caturegli, L., Antichi, D., & Angelini, L. G. (2024). Agronomic and Phytochemical Characterization of Chickpea Local Genetic Resources for the Agroecological Transition and Sustainable Food Systems. Agronomy, 14(10), 2229. https://doi.org/10.3390/agronomy14102229












