Predation Efficiency and Biological Control Potential of Micromus angulatus Against Aphis craccivora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Collection and Preparation
2.2. Predation Rate Measurement of Micromus angulatus on Aphis craccivora
2.3. Functional Response of Micromus angulatus to Aphis craccivora
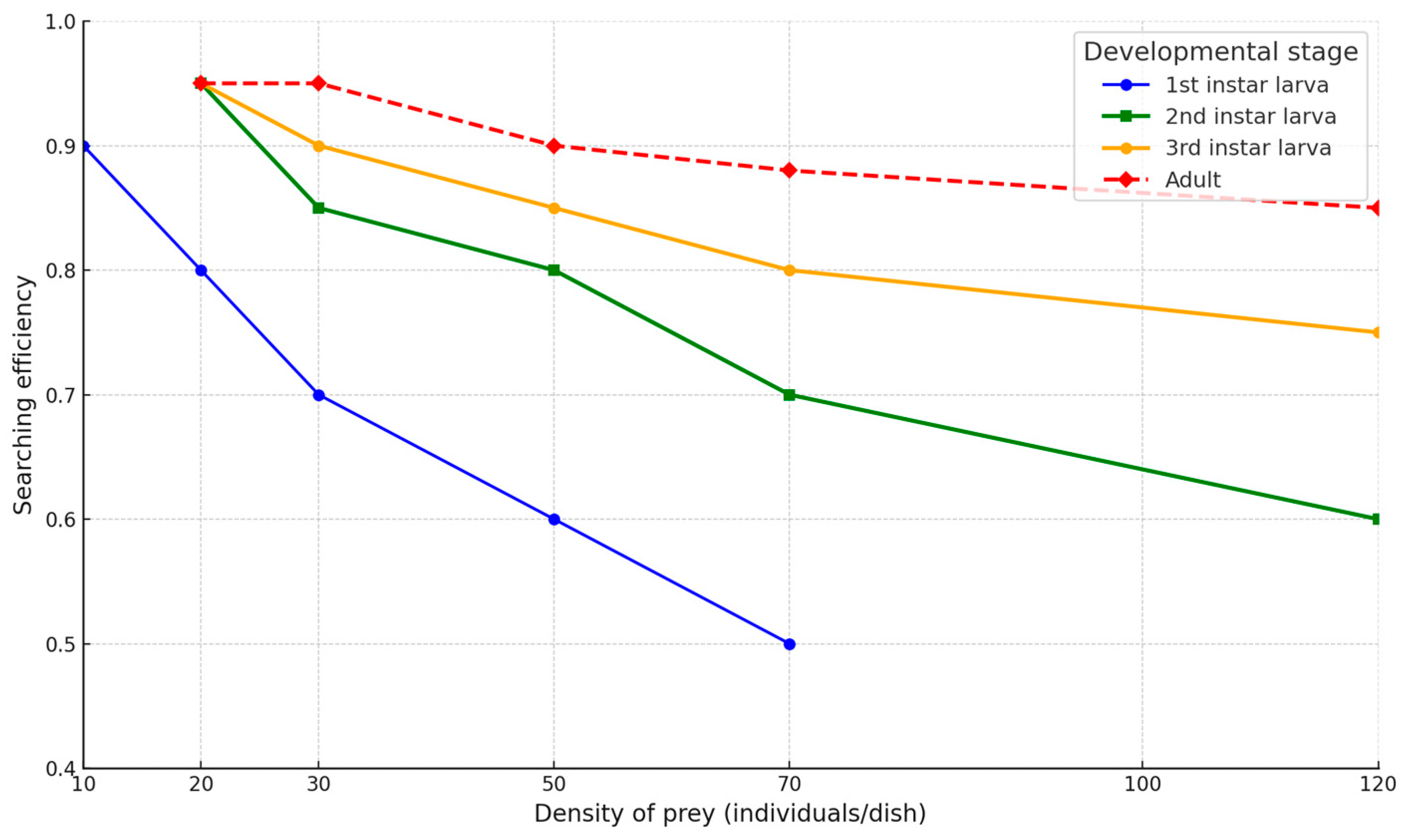
2.4. Searching Efficiency of Micromus angulatus on Aphis craccivora
2.5. Effects of Micromus angulatus Density on Predatory Activity
3. Results
3.1. Predation Rate Measurement of Micromus angulatus on Aphis craccivora
3.2. Functional Response of Micromus angulatus to Aphis craccivora
3.3. Searching Efficiency of Micromus angulatus on Aphis craccivora
3.4. Effects of Micromus angulatus Density on Predatory Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wongsa, K.; Duangphakdee, O.; Rattanawannee, A. Genetic Structure of the Aphis craccivora (Hemiptera: Aphididae) From Thailand Inferred from Mitochondrial COI Gene Sequence. J. Insect Sci. 2017, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Lin, R.H.; Li, Z.; Wang, A.Y.; Xue, C.; Duan, A.L.; Zhao, M.; Zhang, J.H. Function analysis of P450 and GST genes to imidacloprid in Aphis craccivora (Koch). Front. Physiol. 2021, 11, 624287. [Google Scholar] [CrossRef]
- Liu, S.K.; Chen, C.L.; Shen, Y.Y.; Li, J.H.; Tan, Z.Q.; Jin, P.F. Identification of Lecanicillium araneicola HK-1 and its biocontrol potential against Aphis craccivora (Hemiptera: Aphididae). Acta Entomol. Sin. 2023, 66, 486–500. [Google Scholar] [CrossRef]
- Wei, L.; Liu, H.L.; Wu, X.L.; Chen, H.Z.; Peng, Y.L.; Xiao, K.J.; Cai, P.; Fang, C.; Li, Y.J.; Pu, D.Q. Study on the predation characteristics of Megalocaria dilatata on aphid. Chin. Agric. Sci. Bull. 2024, 40, 105–109. [Google Scholar] [CrossRef]
- Rashed, A.; Feng, X.; Prager, S.M.; Porter, L.D.; Knodel, J.J.; Karasev, A.; Eigenbrode, S.D. Vector-Borne Viruses of Pulse Crops, With a Particular Emphasis on North American Cropping System. Ann. Entomol. Soc. Am. 2018, 111, 205–227. [Google Scholar] [CrossRef]
- Batra, S.W. Biological control in agroecosystems. Science 1982, 215, 134–139. [Google Scholar] [CrossRef]
- Pekas, A.; De Smedt, L.; Verachtert, N.; Boonen, S. The brown lacewing Micromus angulatus: A new predator for the augmentative biological control of aphids. Biol. Control 2023, 186, 105324. [Google Scholar] [CrossRef]
- Rocca, M.; Messelink, G.J. Combining lacewings and parasitoids for biological control of foxglove aphids in sweet pepper. J. Appl. Entomol. 2017, 141, 402–410. [Google Scholar] [CrossRef]
- Ntalia, P.; Broufas, G.D.; Wäckers, F.; Pekas, A.; Pappas, M.L. Overlooked lacewings in biological control: The brown lacewing Micromus angulatus and the green lacewing Chrysopa formosa suppress aphid populations in pepper. J. Appl. Entomol. 2022, 146, 796–800. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y. Hemerobiidae. In The Color Atlas of Neuropterida from China; Yang, D., Liu, X.Y., Yang, X.K., Eds.; Henan Science and Technology Press: Zhengzhou, China, 2023; pp. 449–565. [Google Scholar]
- Koutsoula, G.; Stamkopoulou, A.; Pekas, A.; Wäckers, F.; Broufas, G.; Pappas, M.L. Predation efficiency of the green lacewings Chrysoperla agilis and C. mutata against aphids and mealybugs in sweet pepper. Bull. Entomol. Res. 2023, 113, 162–168. [Google Scholar] [CrossRef]
- López Carretero, P.; Pekas, A.; Stubsgaard, L.; Sancho Blanco, G.; Lütken, H.; Sigsgaard, L. Glandular trichomes affect mobility and predatory behavior of two aphid predators on medicinal cannabis. Biol. Control 2022, 170, 104932. [Google Scholar] [CrossRef]
- Zhao, Y. Systematics of family Hemerobiidae from China (Insecta: Neuroptera, Hemerobiidae). Ph.D. Thesis, China Agricultural University, Beijing, China, 2016. [Google Scholar]
- Chen, B.; Zhang, W.; Liu, X.W.; Huang, Y.; Wang, L.; Li, G.H.; Peng, X.L. Predation ability of Chrysopa pallens (Rambur) on Myzus persicae. J. Environ. Entomol. 2022, 44, 830–837. [Google Scholar]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Williams, H.; Demiris, N.; Preston, S.P.; Milonas, P.G.; Kypraios, T. Bayesian inference and model choice for Holling’s disc equation: A case study on an insect predator-prey system. Community Ecol. 2016, 17, 71–78. [Google Scholar] [CrossRef]
- Ding, Y.Q. Insect Mathematical Ecology; Science Press: Beijing, China, 1994; pp. 257–258, 303–304. [Google Scholar]
- Hassell, M.P.; Varley, G.C. New inductive population model for insect parasites and its bearing on biological control. Nature 1969, 223, 1133–1137. [Google Scholar] [CrossRef]
- Du, Y.M.; Chen, H.X.; Cheng, G.Q.; Ouyang, Z.G.; Yu, H.Z.; Lu, Z.J. Functional response and prey preference of beautiful lacewing Chrysopa Formosa to adult of Asian citrus psyllid Diaphorina citri. J. Plant Prot. 2023, 50, 1025–1032. [Google Scholar]
- Udiarto, B.K.; Murtiningsih, R.; Muharam, A. Preferences and functional response of Coccinellidae to Bemisia tabaci (Hemiptera: Aleyrodidae). Chil. J. Agric. Res. 2023, 83, 715–726. [Google Scholar] [CrossRef]
- Fu, X.T.; Cao, Y.Z.; Dong, X.T.; Chang, J.; Huo, Z.J. Functional responses of two species of predatory mites (Acari: Phytoseiidae) to eggs and first-instar nymphs of Bactericera Gobica Logniova (Homoptera: Psyllidae). Exp. Appl. Acarol. 2024, 93, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Zhang, J.; Yang, N.; Yang, D.; Zou, K. The inappropriate application of imidacloprid destroys the ability of predatory natural enemies to control pests in the food chain: A case study of the feeding behavior of Harmonia axyridis and Propylea japonica. Ecotoxicol. Environ. Saf. 2024, 248, 114631. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Wang, E.; Zhang, Z.; Wu, S.; Lei, Z. Laboratory and glasshouse evaluation of the green lacewing, Chrysopa pallens (Neuroptera: Chrysopidae) against the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2019, 54, 115–121. [Google Scholar] [CrossRef]
- Clark, T.L.; Messina, F.J. Foraging Behavior of Lacewing Larvae (Neuroptera: Chrysopidae) on Plants with Divergent Architectures. J. Insect Behav. 1998, 11, 303–317. [Google Scholar] [CrossRef]
- Shrestha, G.; Enkegaard, A. The green lacewing, Chrysoperla carnea: Preference between lettuce aphids, Nasonovia ribisnigri, and western flower thrips, Frankliniella occidentalis. J. Insect Sci. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Uiterwaal, S.F.; DeLong, J.P. Multiple factors, including arena size, shape the functional response of aphid predators. J. Appl. Ecol. 2018, 55, 2429–2438. [Google Scholar] [CrossRef]
- Mutz, J.; Thaler, J.S.; Ugine, T.A.; Inouye, B.D. Predator densities alter the influence of non-consumptive effects on the population dynamics of an agricultural pest. Ecol. Entomol. 2024, 49, 306–318. [Google Scholar] [CrossRef]
- Khan, M.H.; Yoldaş, Z.; Madahi, K. High Prey Density Affects the Functional Response of Variegated Ladybird Beetles Against Pea Aphids. Gesunde Pflanz. 2023, 75, 2293–2300. [Google Scholar] [CrossRef]
- Rostami, E.; Huang, D.-L.; Shi, M.-Z.; Zheng, L.-Z.; Li, J.-Y.; Madadi, H.; Fu, J.-W. Functional response and predation rate of Cryptolaemus montrouzieri (Coleoptera: Coccinellidae) to Paracoccus marginatus (Hemiptera: Pseudococcidae) at different temperatures. J. Econ. Entomol. 2024, 117, 1406–1417. [Google Scholar] [CrossRef]
- Mahzoum, A.M.; Villa, M.; Benhadi-Marín, J.; Pereira, J.A. Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control. Agronomy 2020, 10, 1511. [Google Scholar] [CrossRef]
- Zarikian, N.H.; Vardanyan, M.V.; Rukhkyan, M.Y.; Hovhannisyan, R.L.; Barseghyan, R.E.; Dudukchyan, Z.M.; Akopyan, K.V.; Harutyunova, L.J. The potential of Araneae as biological control agents against honey-wax pests (Pyralidae). Int. J. Agric. Biosci. 2024, 13, 288–294. [Google Scholar] [CrossRef]


| 1st-Instar | 2nd-Instar | 3rd-Instar | Adult | ||||
|---|---|---|---|---|---|---|---|
| Number of Hosts (N0) | Number of Preyed Hosts (Na) ± SD | Number of Hosts (N0) | Number of Preyed Hosts (Na) ± SD | Number of Hosts (N0) | Number of Preyed Hosts (Na) ± SD | Number of Hosts (N0) | Number of Preyed Hosts (Na) ± SD |
| 20 | 20.00 ± 0.00 | 20 | 20.00 ± 0.00 | 20 | 20.00 ± 0.00 | ||
| 10 | 9.17 ± 1.33 | 30 | 30.00 ± 0.00 | 30 | 29.83 ± 0.41 | 30 | 30.00 ± 0.00 |
| 20 | 13.33 ± 2.66 | 50 | 48.17 ± 1.72 | 50 | 50.00 ± 0.00 | 50 | 49.83 ± 0.41 |
| 30 | 23.50 ± 2.35 | 70 | 53.83 ± 8.95 | 70 | 61.50 ± 8.14 | 70 | 69.17 ± 0.75 |
| 50 | 36.17 ± 3.76 | 100 | 72.17 ± 9.68 | 100 | 94.67 ± 2.73 | 100 | 94.33 ± 2.58 |
| 70 | 42.67 ± 2.66 | 120 | 84.50 ± 6.95 | 120 | 103.83 ± 6.68 | 120 | 102.50 ± 7.28 |
| Stage | Functional Response Equation | R2 | Instant Attack Rate (a) | Handling Time (Th)/d | Predation Capacity (a/Th) | Maximum Daily Consumption (1/Th) | X2 |
|---|---|---|---|---|---|---|---|
| 1st-instar | Na = 0.9983N0/(1 + 0.0158N0) | 0.9319 | 1.0017 | 0.0158 | 63.3989 | 63.2911 | 5.8093 |
| 2nd-instar | Na = 0.9571N0/(1 + 0.0053N0) | 0.9941 | 1.0448 | 0.0051 | 204.8672 | 196.0784 | 2.636 |
| 3rd-instar | Na = 1.0437N0/(1 + 0.0015N0) | 0.9938 | 0.9581 | 0.0016 | 598.8311 | 625 | 3.0853 |
| Adult | Na = 1.0518N0/(1 + 0.0010N0) | 0.9936 | 0.9508 | 0.0011 | 864.3192 | 909.0909 | 2.4112 |
| Density of M. angulatus (Individuals/Disk) | Number of Preyed Hosts (Na) | Average Number of Preyed Hosts | Theoretical Number of Consumed Preys | X2 | Intensity of Scramble Competition |
|---|---|---|---|---|---|
| 1 | 123.00 ± 6.708 | 123.00 ± 6.708 | 123.888 | 0.006 | 0 |
| 2 | 123.00 ± 6.708 | 61.50 ± 3.354 | 135.89 | 0.001 | 0.446 |
| 3 | 150.60 ± 14.673 | 50.20 ± 4.891 | 143.443 | 0.357 | 0.592 |
| 4 | 139.20 ± 5.630 | 34.80 ± 1.408 | 149.054 | 0.651 | 0.717 |
| 5 | 157.40 ± 10.784 | 31.48 ± 2.517 | 153.558 | 0.096 | 0.744 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Lou, T.; Cao, R.; Jiang, L.; Xu, Q.; Zhan, Q. Predation Efficiency and Biological Control Potential of Micromus angulatus Against Aphis craccivora. Agronomy 2024, 14, 2242. https://doi.org/10.3390/agronomy14102242
Zhao Y, Lou T, Cao R, Jiang L, Xu Q, Zhan Q. Predation Efficiency and Biological Control Potential of Micromus angulatus Against Aphis craccivora. Agronomy. 2024; 14(10):2242. https://doi.org/10.3390/agronomy14102242
Chicago/Turabian StyleZhao, Yang, Tiancheng Lou, Rongxiang Cao, Liben Jiang, Qiujing Xu, and Qingbin Zhan. 2024. "Predation Efficiency and Biological Control Potential of Micromus angulatus Against Aphis craccivora" Agronomy 14, no. 10: 2242. https://doi.org/10.3390/agronomy14102242





