Transcriptome Analysis Revealed the Response Mechanism of Pomegranate to Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Physiological Indexes Measurement
2.3. Transcriptome Analysis
2.3.1. RNA Extraction and cDNA Library Sequence
2.3.2. Gene Annotation and Expression Analysis
2.3.3. Co-Expression Network Analysis
2.3.4. Hub Gene Screening
2.3.5. Gene Expression Validation
2.4. Statistical Analyses
3. Results
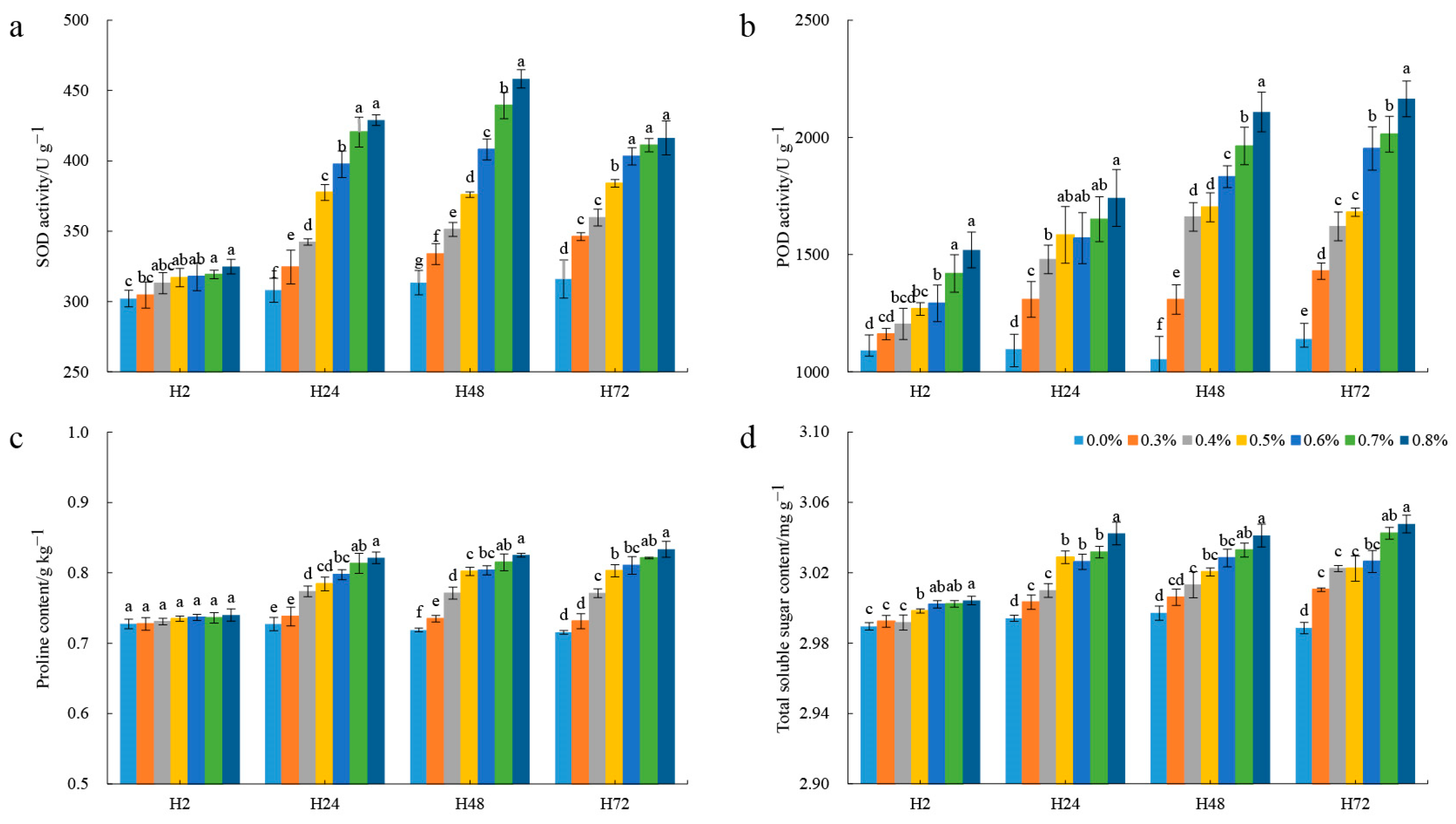
3.1. Changes in the Physiological Indexes after Salt Treatment
3.2. Transcriptome Sequencing and Gene Response Analysis
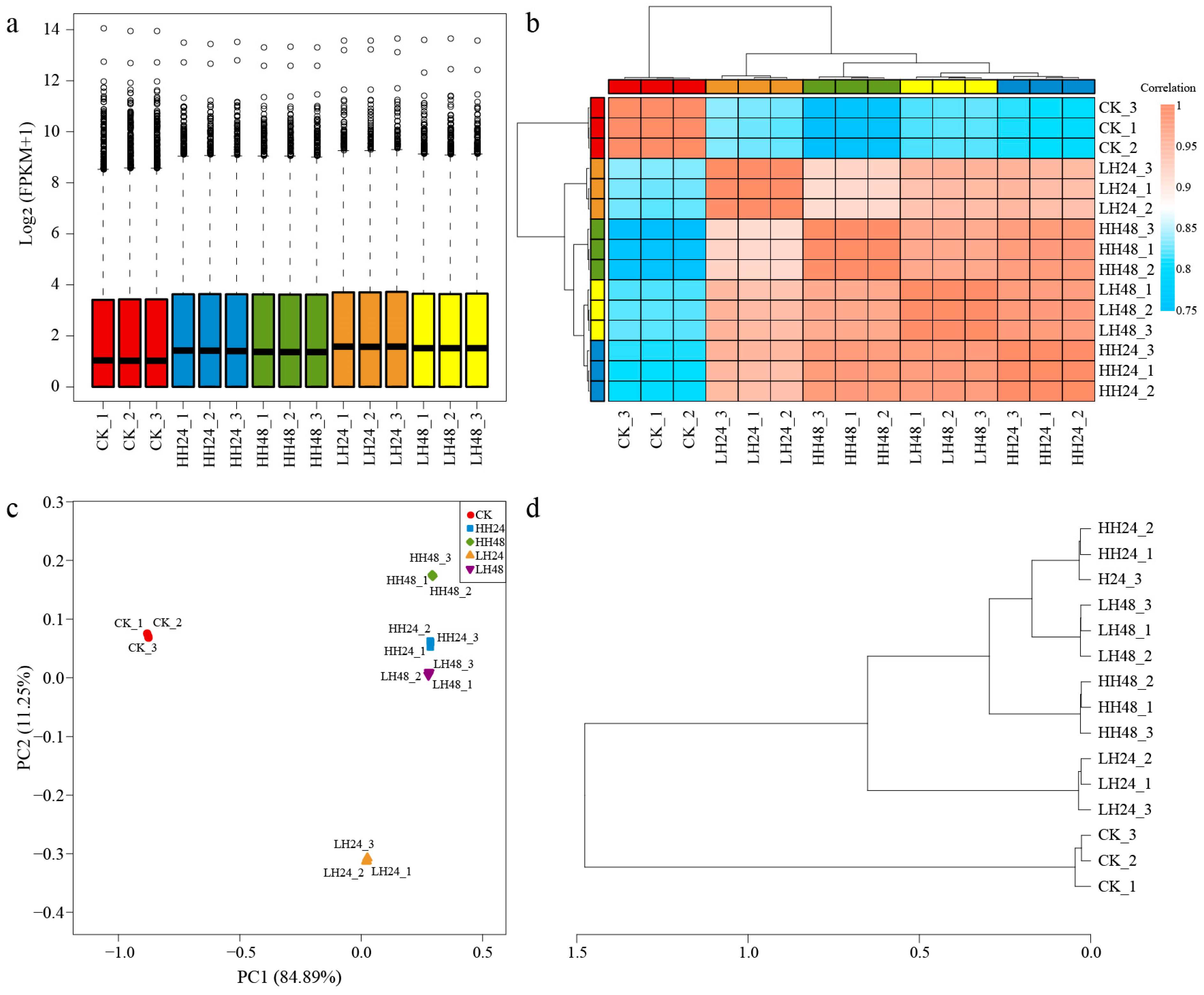
3.2.1. Overview of Transcriptome Profiles
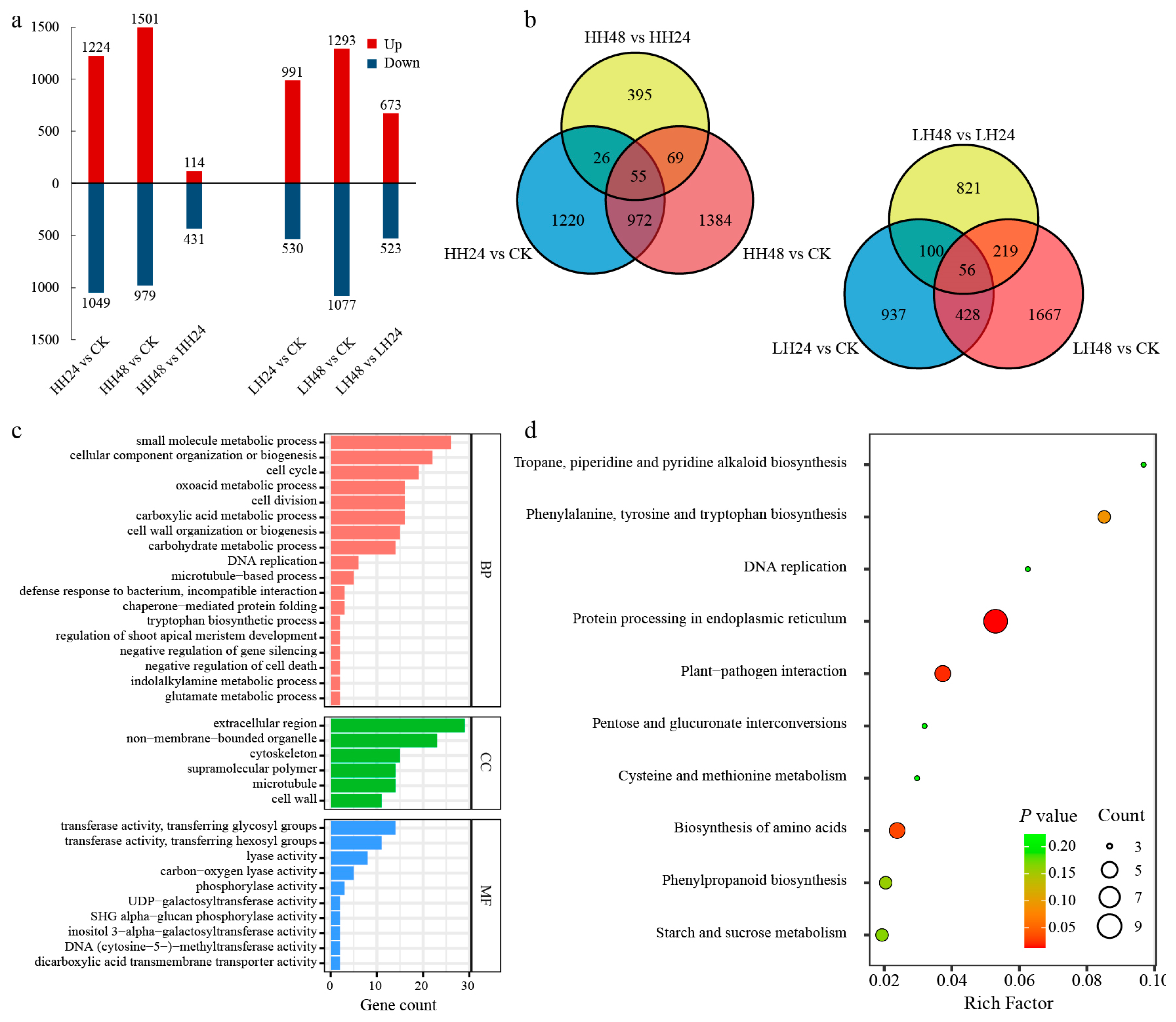
3.2.2. DEG Analysis under Different Salt Treatments
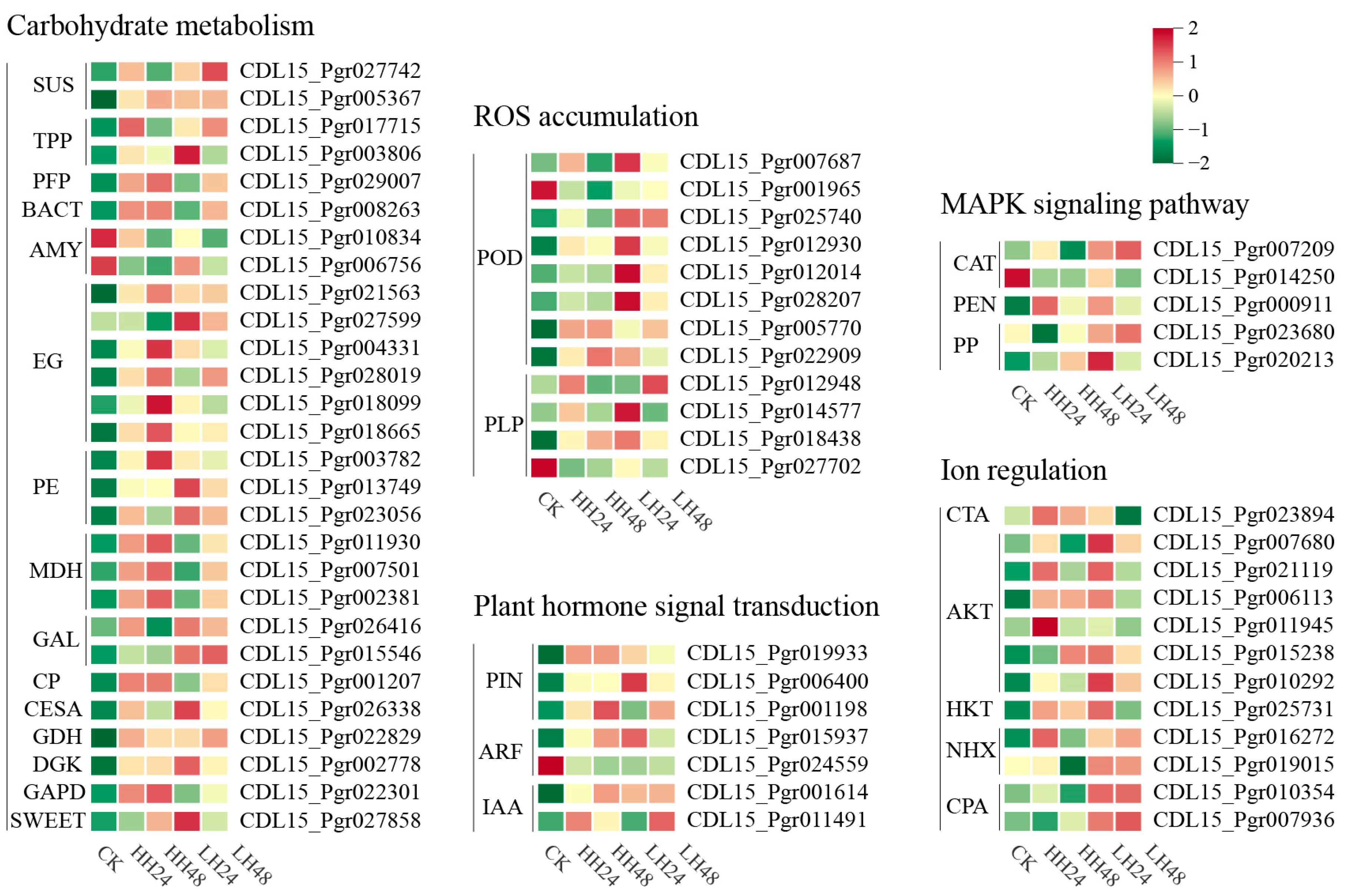
3.2.3. Expression Analysis of DEGs Involved in Salt Tolerance
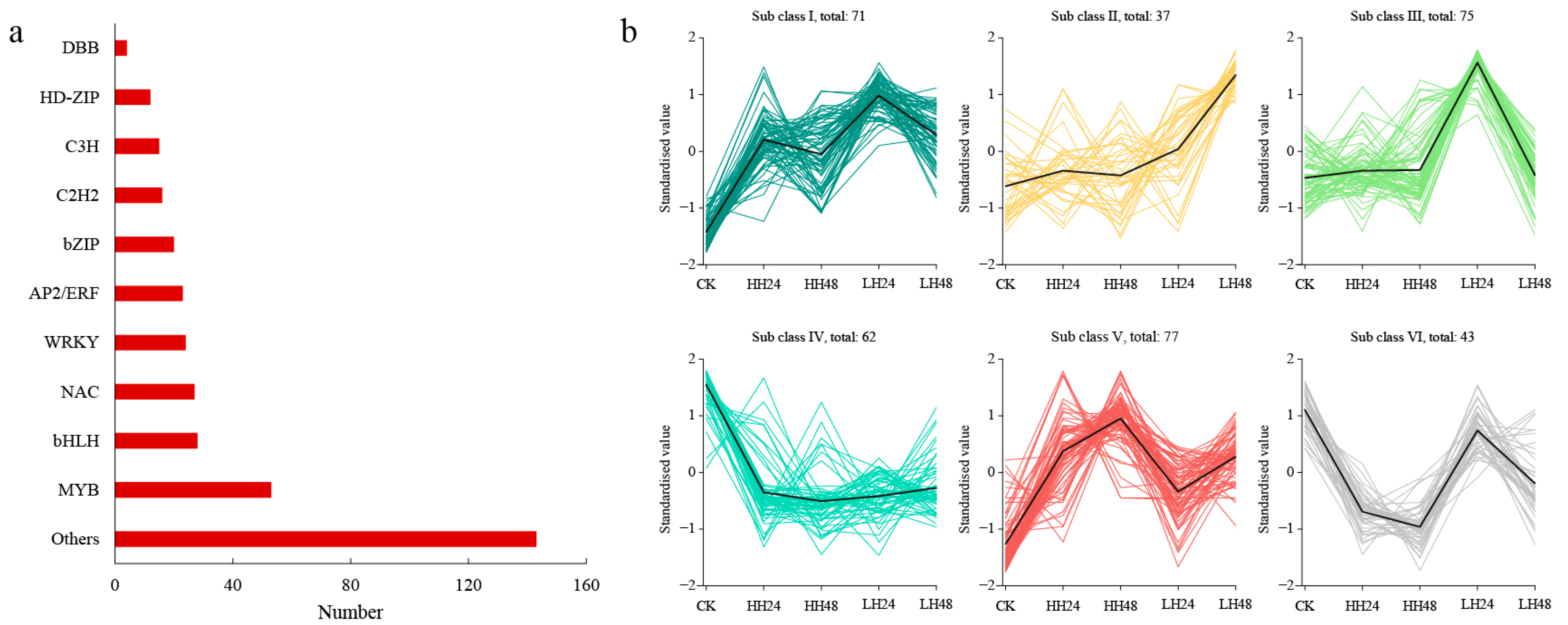
3.2.4. TF Analysis under Different Salt Treatments
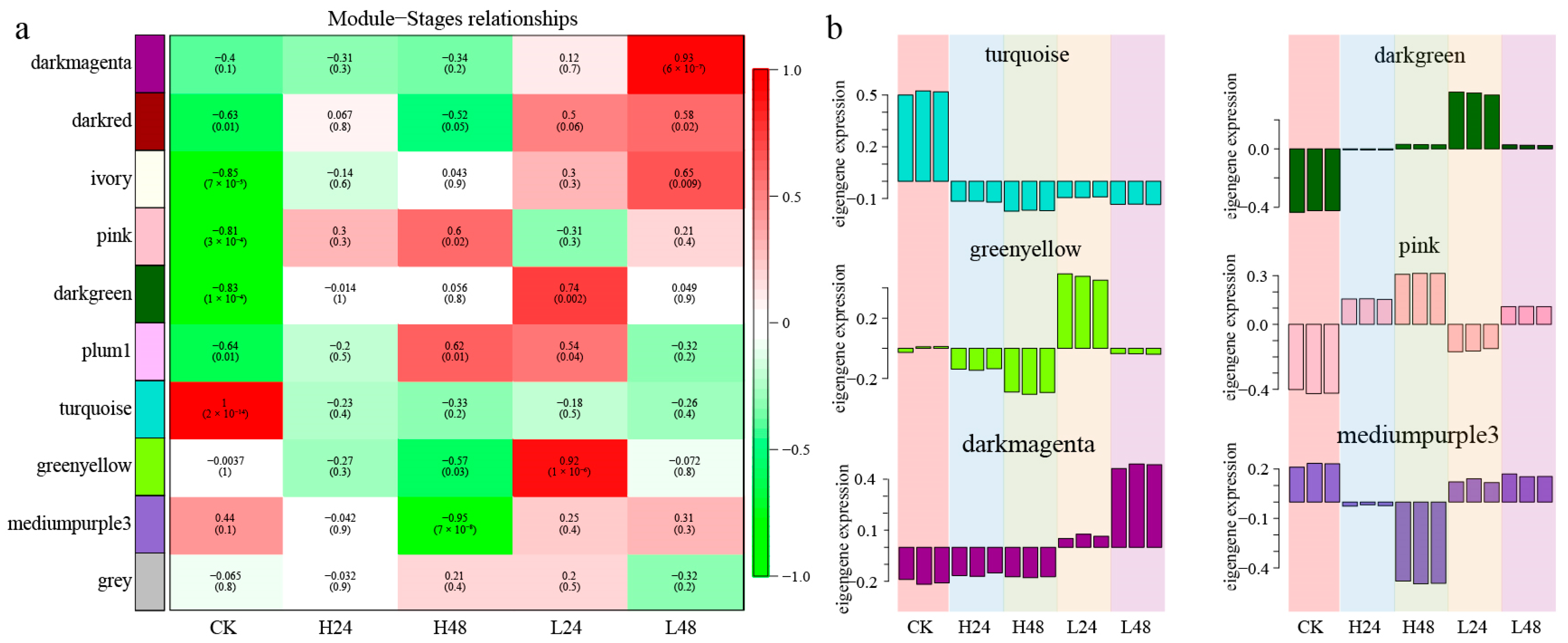
3.3. Co-Expression Network and WGCNA Module Analysis
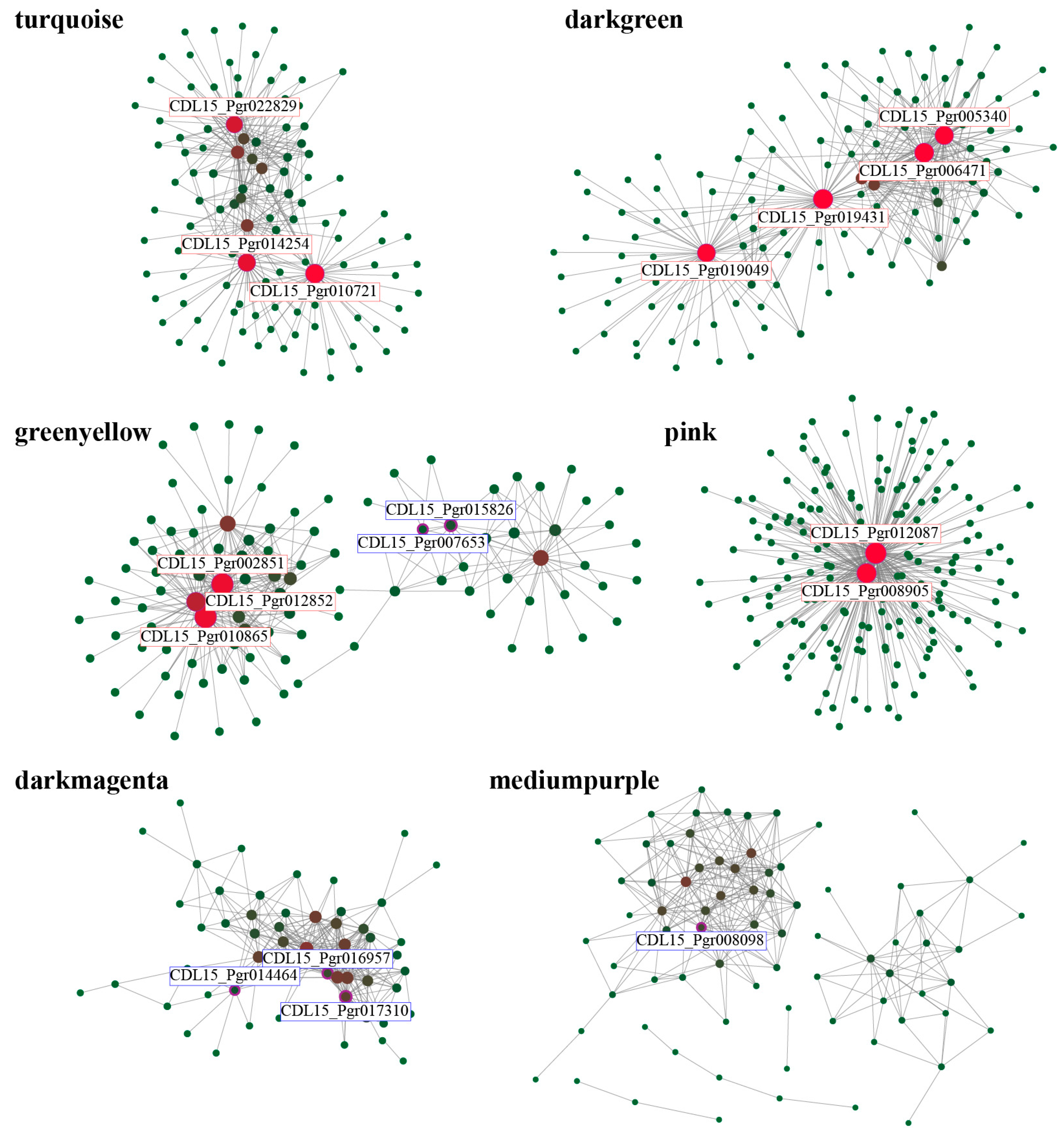
3.3.1. Identification and Visual Analysis of the Specific Modules
3.3.2. GO and KEGG Analysis of the Specific Modules
3.3.3. Candidate Gene Identification Associated with Salt Stress
3.4. Validation of the Candidate Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaleem, F.; Shabir, G.; Aslam, K.; Rasul, S.; Manzoor, H.; Shah, S.M.; Khan, A.R. An overview of the genetics of plant response to salt stress: Present status and the way forward. Appl. Biochem. Biotechnol. 2018, 186, 306–334. [Google Scholar] [CrossRef] [PubMed]
- Benny, J.; Marchese, A.; Giovino, A.; Marra, F.P.; Perrone, A.; Caruso, T.; Martinelli, F. Gaining insight into exclusive and common transcriptomic features linked to drought and salinity responses across fruit tree crops. Plants 2020, 9, 1059. [Google Scholar] [CrossRef]
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Wakamatsu, A.; Mori, I.C.; Matsuura, T.; Taniwaki, Y.; Ishii, R.; Yoshida, R. Possible roles for phytohormones in controlling the stomatal behavior of Mesembryanthemum crystallinum during the salt-induced transition from C3 to crassulacean acid metabolism. J. Plant Physiol. 2021, 262, 153448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Luo, F.; Zou, R.; Liu, J.X.; Yan, Y.M. Integrated physiological and chloroplast proteome analysis of wheat seedling leaves under salt and osmotic stresses. J. Proteom. 2021, 234, 104097. [Google Scholar] [CrossRef]
- Koselski, M.; Trebacz, K.; Dziubinska, H. The role of vacuolar ion channels in salt stress tolerance in the liverwort Conocephalum conicum. Acta Physiol. Plant. 2019, 41, 110. [Google Scholar] [CrossRef]
- Shen, W.Q.; Liu, D.; Zhang, H.; Zhu, W.; He, H.Z.; Li, G.H.; Liu, J.W. Overexpression of β-cyanoalanine synthase of Prunus persica increases salt tolerance by modulating ROS metabolism and ion homeostasis. Environ. Exp. Bot. 2021, 186, 104431. [Google Scholar] [CrossRef]
- Rahimi, E.; Nazari, F.; Javadi, T.; Samadi, S.; Teixeira da Silva, J.A. Potassium-enriched clinoptilolite zeolite mitigates the adverse impacts of salinity stress in perennial ryegrass (Lolium perenne L.) by increasing silicon absorption and improving the K/Na ratio. J. Environ. Manag. 2021, 285, 112142. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, M.; Tehranifar, A.; Davarynejad, G.H.; Sayyari-Zahan, M.H. Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-e-Saveh’ and ‘Shishe-Kab’ pomegranates in response to salinity stress. Photosynthetica 2014, 52, 301–312. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; He, S.P.; Gong, W.F.; Xu, F.F.; Pan, Z.E.; Jia, Y.H.; Geng, X.L.; Du, X.M. Integration of proteomic and transcriptomic profiles reveals multiple levels of genetic regulation of salt tolerance in cotton. BMC Plant Biol. 2018, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.K.; Chen, H.; Zhang, Z.H.; Chen, C.; Yu, F.Y.; Guy, R.D. Effects of fruit shading on gene and protein expression during starch and oil accumulation in developing Styrax tonkinensis kernels. Front. Plant Sci. 2022, 13, 905633. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, C.Y.; Wang, M.K.; Fu, F.F.; El-Kassaby, Y.A.; Wang, T.L.; Wang, G.B. Metabolome and transcriptome analyses reveal flavonoids biosynthesis differences in Ginkgo biloba associated with environmental conditions. Ind. Crops Prod. 2020, 158, 112963. [Google Scholar] [CrossRef]
- Shi, J.L.; Wang, S.; Yao, J.A.; Cui, M.Y.; Hu, B.Q.; Wang, J.; Li, F.; Wang, S.; Tong, R.R.; Li, M.; et al. Ultrasound treatment alleviates external pericarp browning and improves fruit quality of pomegranate during storage. J. Sci. Food Agric. 2024, 104, 391–399. [Google Scholar] [CrossRef]
- Zhang, P.; Duo, T.Q.; Wang, F.D.; Zhang, X.Z.; Yang, Z.Z.; Hu, G.F. De novo transcriptome in roots of switchgrass (Panicum virgatum L.) reveals gene expression dynamic and act network under alkaline salt stress. BMC Genom. 2021, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Ren, Y.J.; Zhang, D.Y.; Chen, X.W.; Huang, J.Z.; Xu, Y.; Aucapiña, C.B.; Zhang, Y.; Miao, Y. Transcriptome-based WGCNA analysis reveals regulated metabolite fluxes between floral color and scent in Narcissus tazetta flower. Int. J. Mol. Sci. 2021, 22, 8249. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Han, C.; Liu, Z.M.; Yu, F.Y.; Wu, Q.K. 24-Epibrassinolide and methyl jasmonate promoted seed development of Styrax tonkinensis and affected seed chemical compositions, especially seed lipid metabolism. J. Plant Growth Regul. 2023, 42, 2162–2175. [Google Scholar] [CrossRef]
- Wu, Q.K.; Cao, Y.Y.; Zhao, X.; Zhang, Z.H.; Yu, F.Y.; Guy, R.D. A comparative study of seed reserve accumulation in five Styrax species with potential for biofuel production. Trees Struct. Funct. 2020, 34, 891–902. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, S.P.; Zhu, Z.Q.; Xu, Z.D.; Qi, S.; Xing, S.T.; Yu, Y.Y.; Wu, Q.K. Transcriptome and chemical analyses revealed the mechanism of flower color formation in Rosa rugosa. Front. Plant Sci. 2022, 13, 1021521. [Google Scholar] [CrossRef]
- Pei, G.; Chen, L.; Zhang, W. WGCNA application to proteomic and metabolomic data analysis. Methods Enzymol. 2017, 585, 135–158. [Google Scholar] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhao, Y.J.; Zhao, X.Q.; Dong, J.M.; Yuan, Z.H. Genome-wide identification and expression analysis of the CLC gene family in pomegranate (Punica granatum) reveals its roles in salt resistance. BMC Plant Biol. 2020, 20, 560. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Dong, J.M.; Liu, C.Y.; Wang, Y.Y.; Zhao, Y.J.; Ge, D.P.; Yuan, Z.H. Genome-wide identification of the NHX gene family in Punica granatum L. and their expressional patterns under salt stress. Agronomy 2021, 11, 264. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C.F. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Bishi, S.K.; Goswami, N.; Singh, A.L.; Zala, P.V. Differential fine-regulation of enzyme driven ROS detoxification network imparts salt tolerance in contrasting peanut genotypes. Environ. Exp. Bot. 2016, 128, 79–90. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.D.; Li, J.; Zhang, J.; Zhang, X.D.; Hu, L.X.; Ding, D.X.; Bakpa, E.P.; Xie, J.M. Trehalose alleviated salt stress in tomato by regulating ROS metabolism, photosynthesis, osmolyte synthesis, and trehalose metabolic pathways. Front. Plant Sci. 2022, 13, 772948. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Nafees, M.; Moosa, A.; Ferrante, A.; Darras, A. Melatonin induces proline, secondary metabolites, sugars and antioxidants activity to regulate oxidative stress and ROS scavenging in salt stressed sword lily. Heliyon 2024, 10, E32569. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.K.; Zhao, X.; Chen, C.; Zhang, Z.H.; Yu, F.Y. Metabolite profiling and classification of developing Styrax tonkinensis kernels. Metabolites 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhou, H.P. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, K.L.; Chang, X.K.; Ouyang, Z.P.; Meng, G.; Han, Y.A.; Shen, S.S.; Yao, Q.J.; Piao, F.Z.; Wang, Y. Comparative physiological and transcriptomic analyses of two contrasting pepper genotypes under salt stress reveal complex salt tolerance mechanisms in seedlings. Int. J. Mol. Sci. 2022, 23, 9701. [Google Scholar] [CrossRef]
- Zheng, X.; Su, Y.L.; Chen, Y.G.; Huang, H.N.; Shen, Q.T. Global transcriptional responses of denitrifying bacteria to functionalized single-walled carbon nanotubes revealed by weighted gene-coexpression network analysis. Sci. Total Environ. 2018, 613, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, R.M.; Shobbar, Z.S.; Jelodar, N.B.; Ghaffari, M.; Mohammadi, S.M.; Daryani, P. Salt tolerance involved candidate genes in rice: An integrative meta-analysis approach. BMC Plant Biol. 2020, 20, 452. [Google Scholar]
- Qaisar, U.; Irfan, M.; Meqbool, A.; Zahoor, M.; Khan, M.Y.; Rashid, B.; Riazuddin, S.; Husnain, T. Identification, sequencing and characterization of a stress induced homologue of fructose bisphosphate aldolase from cotton. Can. J. Plant Sci. 2010, 90, 41–48. [Google Scholar] [CrossRef][Green Version]
- Niu, J.P.; Chen, Z.; Guo, Z.P.; Xu, N.; Sui, X.; Roy, M.; Kareem, H.A.; Ul Hassan, M.; Cui, J.; Wang, Q.Z. Exogenous melatonin promotes the growth of alfalfa (Medicago sativa L.) under NaCl stress through multiple pathways. Ecotoxicol. Environ. Saf. 2022, 242, 113938. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Song, Z.Q.; Ti, Y.J.; Liu, Y.X.; Li, Q.W. Physiological response and transcriptome analysis of Prunus mume to early salt stress. J. Plant Biochem. Biotechnol. 2022, 31, 330–342. [Google Scholar] [CrossRef]
- Hou, R.M.; Yang, L.Z.; Wuyun, T.; Chen, S.Y.; Zhang, L. Genes related to osmoregulation and antioxidation play important roles in the response of Trollius chinensis seedlings to saline-alkali stress. Front. Plant Sci. 2023, 14, 1080504. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.H.; Chen, R.; Wei, X.; Liu, Y.H.; Zhao, S.J.; Yin, X.P.; Xie, T. Genome-wide identification of R2R3-MYB family in wheat and functional characteristics of the abiotic stress responsive gene TaMYB344. BMC Genom. 2020, 21, 792. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.A.; Wu, Y.X.; He, L.Y. A wheat WRKY transcription factor TaWRKY17 enhances tolerance to salt stress in transgenic Arabidopsis and wheat plant. Plant Mol. Biol. 2023, 113, 171–191. [Google Scholar] [CrossRef]
- Li, W.Y.; Wang, C.; Shi, H.H.; Wang, B.; Wang, J.X.; Liu, Y.S.; Ma, J.Y.; Tian, S.Y.; Zhang, Y.W. Genome-wide analysis of ethylene-response factor family in adzuki bean and functional determination of VaERF3 under saline-alkaline stress. Plant Physiol. Biochem. 2020, 147, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Guo, X.; Zhang, M.H.; Wang, X.; Zhao, Y.; Yin, Z.G.; Zhang, Z.Y.; Wang, Y.M.; Xiong, H.Y.; Zhang, H.L.; et al. OsERF71 confers drought tolerance via modulating ABA signaling and proline biosynthesis. Plant Sci. 2018, 270, 131–139. [Google Scholar] [CrossRef]
- Chen, H.H.; Lai, L.Y.; Li, L.X.; Liu, L.P.; Jakada, B.H.; Huang, Y.M.; He, Q.; Chai, M.N.; Niu, X.P.; Qin, Y. AcoMYB4, an Ananas comosus L. MYB transcription factor, functions in osmotic stress through negative regulation of ABA signaling. Int. J. Mol. Sci. 2020, 21, 5727. [Google Scholar] [CrossRef]
- Chen, K.Q.; Song, M.R.; Guo, Y.N.; Liu, L.F.; Xue, H.; Dai, H.Y.; Zhang, Z.H. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Q.; Wang, C.; Chen, X.B.; Lu, W.J.; Li, H.; Wang, X.L.; Hao, L.L.; Guo, X.Q. The cotton WRKY gene GhWRKY41 positively regulates salt and drought stress tolerance in transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0143022. [Google Scholar] [CrossRef] [PubMed]
- Han, G.L.; Lu, C.X.; Guo, J.R.; Qiao, Z.Q.; Sui, N.; Qiu, N.W.; Wang, B.H. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | Raw Reads | Clean Reads | Q30/% | GC Content/% | Mapping Rate/% |
|---|---|---|---|---|---|
| CK | 70,565,333 | 67,423,747 | 96.86 | 50.83 | 96.48 |
| HH24 | 66,980,421 | 64,152,333 | 96.86 | 50.56 | 95.81 |
| HH48 | 67,902,700 | 65,028,698 | 96.81 | 50.58 | 96.29 |
| LH24 | 67,390,393 | 64,555,540 | 96.74 | 50.65 | 95.92 |
| LH48 | 65,291,753 | 62,395,161 | 96.86 | 50.71 | 96.19 |
| Mean | 67,626,120 | 64,711,096 | 96.83 | 50.67 | 96.14 |
| SD | 5,818,599 | 5,485,938 | 0.08 | 0.12 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Wang, C.; Mei, J.; Feng, L.; Wu, Q.; Yin, Y. Transcriptome Analysis Revealed the Response Mechanism of Pomegranate to Salt Stress. Agronomy 2024, 14, 2261. https://doi.org/10.3390/agronomy14102261
Tang H, Wang C, Mei J, Feng L, Wu Q, Yin Y. Transcriptome Analysis Revealed the Response Mechanism of Pomegranate to Salt Stress. Agronomy. 2024; 14(10):2261. https://doi.org/10.3390/agronomy14102261
Chicago/Turabian StyleTang, Haixia, Chuanzeng Wang, Jian Mei, Lijuan Feng, Qikui Wu, and Yanlei Yin. 2024. "Transcriptome Analysis Revealed the Response Mechanism of Pomegranate to Salt Stress" Agronomy 14, no. 10: 2261. https://doi.org/10.3390/agronomy14102261
APA StyleTang, H., Wang, C., Mei, J., Feng, L., Wu, Q., & Yin, Y. (2024). Transcriptome Analysis Revealed the Response Mechanism of Pomegranate to Salt Stress. Agronomy, 14(10), 2261. https://doi.org/10.3390/agronomy14102261






