Abstract
Various rapid propagation strategies have been discovered, which has facilitated large-scale plant reproduction and cultivar development. These methods, in many plant species, are used to rapidly generate large quantities (900 mini-tubers/m2) of high-quality propagule (free from contamination) at a relatively low cost in a small space. They are also used for plant preservation. This review article aims to provide potential applications for regeneration and clonal propagation. Plant propagation using advanced agrotechnology, such as aeroponics, is becoming increasingly popular among academics and industrialists. The advancement of asexual aeroponic propagation has been achieved through advancements in monitoring and control systems using IoT and smart sensor technology. New sensor technology systems have gained substantial interest in agriculture in recent years. It is used in agriculture to precisely arrange various operations and objectives while harnessing limited resources with minimal human intervention. Modern intelligent technologies and control systems simplify sensor data collection, making it more efficient than manual data collection, which can be slow and prone to errors. Specific ambient variables like temperature, humidity, light intensity, stock solution concentrations (nutrient water), EC (electrical conductivity), pH values, CO2 content, and atomization parameters (frequency and interval) are collected more effectively through these systems. The use of intelligent technologies provides complete control over the system. When combined with IoT, it aids in boosting crop quality and yield while also lowering production costs and providing data directly to tablets and smartphones in aeroponic propagation systems. It can potentially increase the system’s productivity and usefulness compared to the older manual monitoring and operating methods.
1. Introduction
Plant propagation is a technique of producing new plants from a stock plant. There are many methods to propagate plants, including taking cuttings from leaves, stems, or roots to generate new plants from these tissues. Other methods include grafting and layering [1]. Propagation is either sexual or asexual. Sexual propagation produces new plants from seeds at a modest rate of reproduction involving genetic recombination. Plants typically require more time to establish and mature due to the developmental stages involved [2]. Because of genetic variability, sexual propagation cannot produce true-to-type plants from seeds, especially where maintaining specific traits, like hybrids or cultivars, is crucial [3]. However, doubled haploid plants and inbred lines are exceptions. Due to their uniform genetic makeup and reduced variability, they are typically true-to-type. Asexual propagation has integrated the conveyance of stock plant’s genetic characteristics to progeny; it enables replicating plants with limited or no seed production, along with flexible production scheduling, because it is no longer dependent on the bearing (fruiting) seasons [4]. This asexual propagation method preserves traits and accelerates plant growth; such grafted bushes begin to blossom in less than 5 years, as opposed to 8–23 years for seed propagation [5]. Sexual reproduction of hybrid seeds and interspecific crossbreeds cannot yield true-to-type plants [6]. At the same time, asexual propagation can be obtained genetically similar to that of an individual parent. This propagation method can also rapidly mass-produce many cloned plants [7]. Table 1 provides a list of plants regenerated using various rapid propagation approaches. This review presents a novel use of intelligent aeroponic technology for rapid asexual propagation. In contrast to earlier research, this study focuses on the fusion of sensor-based and IoT technologies, enabling real-time control and optimization, a significant improvement over the conventional propagation approach. The objective of this review is to explore the potential of intelligent aeroponic systems for rapid asexual propagation, evaluate their impact on asexual smart plant production, and assess their viability in conserving species and improving asexual methods of propagation rapidly.

Table 1.
List of crops that can be regenerated using rapid propagation techniques.
The review approach considered for this article addressed multiple methods for rapid asexual propagation, focusing on intelligent aeroponic technology. Initially, the scope included other asexual propagation approaches, such as synthetic seed techniques. However, it was decided to reduce secondary material and focus primarily on aeroponics. The review utilized Scopus and Google Scholar as primary databases, with search queries including terms like ‘aeroponics’, ‘asexual propagation’, and ‘intelligent sensor technology’. Approximately 300 research articles, books, and reports were initially identified. Following a thorough evaluation, 176 materials were selected for inclusion. These selected references comprise original research, review articles, and book chapters from major academic publication groups, including Elsevier, Springer, Taylor & Francis, and MDPI.
1.1. Propagation Organs
Vegetative propagation refers to any asexual multiplication in plant species wherein a new plant develops from a part of the parent plant or a specific reproductive organ such as a stolon, rhizomes, tubers, and bulb. Stolons are stems that grow underground form incidental roots at nodes, and generate new vegetation from shoots [34]. A rhizome is a modified plant stem that grows underground and develops roots and shoots along its length from nodes. They are sometimes called rootstocks or creeping rootstalk [35]. A bulb is a short stem with soft, thick, undeveloped leaves around it. The bulb leaf bases, also known as scales, do generally not support leaves. However, they contain nutritional reserves, which help the plant survive under harsh conditions, and membrane exterior scaling, which shields the continual lamina of fleshy scales [36,37]. Tubers are swollen structures utilized as nutritional storing organ systems in some plants for asexual reproduction [38]. The word tuber, which refers to a stem structure made of stems comes from the Latin tuber, which means “swelling, bump, lump” [39]. The above-discussed parts of the plant that are used for asexual propagation in tissue culture technology and its primary stages are shown in Figure 1.
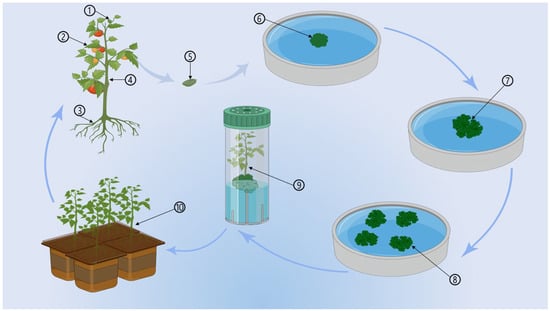
Figure 1.
Plant tissue culture cycle: 1. Bud; 2. Leaf; 3. Root system; 4. Stem; 5. Plant tissue sample; 6. Tissue sample in culture; 7. Forming of callus; 8. Separation and multiplication; 9. Regenerated plantlet; 10. Plantlet hardening.
1.2. Plant Growth Regulators
Growth regulators (PGRs) are required for the asexual propagation of plants. Plants thrive using hormones that are crucial for their development. Their species and proportions control cell dedifferentiation, growth and proliferation morphogenesis, and cell differentiation [40]. Even though many plants in the nursery trade do not require PGRs for propagation, many of them still necessitate their involvement for the formation of adventitious shoots [41]. Application of PGRs may be needed to initiate the cellular and tissue development necessary for successful propagation. Plant hormones and PGRs are substances that regulate plant development [42]. Plant developmental processes involve six widely used types of phytohormones, which are illustrated in Table 2.

Table 2.
Commonly used growth hormones and their involvement in the development process [43,44].
The chemical structures of each hormone class are different and members of the same class typically have identical physiological roles, though some variations may occur. Initially, plant hormone research identified five main classes: auxins, cytokinins, ethylene, gibberellins, and abscisic acid [45]. This classification has since been updated to include jasmonates, brassinosteroids, and salicylic acid as important plant growth hormones. PGRs have been used to manipulate plant growth, rapid flowering, and yield patterns in a variety of horticultural and other crops, including fruits [46], herbs [47,48,49], vegetables [50], pulses [51], and ornamental plants [52]. These chemicals can promote plant yield, quality, and growth [53]. Regulators such as auxin and gibberellin have been identified to regulate plant development when applied at low concentrations, usually by stimulating a component of the endogenous growth regulatory system [54]. PGRs, also known as growth stimulants and bioregulators, were developed by scientists to modulate plant growth and development processes. These compounds are often used to enhance desired traits in plants, regardless of their reproductive method. Most of them are available in liquid, granular, and powder form.
2. Micropropagation Technique
Micropropagation is a rapid vegetative propagation technique similar to tissue culture [55]. It bypasses the juvenile period (which may last days to years), allowing a rapid reproduction of desired plant cultivars. It is the in vitro cultivation of tissues, organs, cells, and their components under controlled chemical and physical conditions for growth and propagation [56]. In a sterile and controlled setting in the microenvironment of the culture vessel, the plants exhibit growth patterns that are comparable to those observed in natural environments. The structure of the culture environment, media, and gene expression all play a role in the success of micropropagation [57]. The essential idea of micropropagation is the ability of cells or tissues to create all cell types and renew a plant [58].
Tissue Culture
Plant tissue culture is a valuable tool for fundamental and applied research and commercial applications. It is a series of processes for cultivating or developing plant tissues, cells, or organs in a sterile environment utilizing a nutrient-stabilized medium [59]. Various tissue culture methods have advantages over traditional propagation methods, and their shortcomings were addressed by turning to tissue culture while enabling rapid propagation [60]. This technique is incredibly extensible and allows rapid growth in a shorter span [61]. It originated from the concept of Gottlieb Haberlandt, a German botanist in the early twentieth century. Early research led to the development of embryo cultures, root cultures, and the first tissue cultures [62]. The initial test of root culture was utilized for virus research and eventually became an essential tool for physiological studies [63]. The application of in vitro techniques to many plant species has steadily increased over time. These propagation techniques have been used on various plants, including vegetable crops, grasses, cereals, other tuber or root crops, legumes, forest trees, tropical fruits, oilseeds, plantation crops, and ornamental plants [62]. Healthy tissue development and morphology can vary according to the nutritive requirement of the plant.
Furthermore, tissues from different regions of plants may have distinct growth requirements. These requirements are met by combining several components known as plant media or basal media; a list of commonly employed basal media is shown in Table 3. Gautheret first created tissue culture media from nutrient solutions to grow entire plants. Over time, scientists developed many media types to meet the demands of plant tissue culture operations. However, this article will discuss the most widely used basal media. Tissue culture delivers all water, nutrients, and energy necessary for plant or explant development via the basal medium. It also regulates incubation conditions to offer ideal light and temperature settings for development.

Table 3.
Commonly employed basal media for propagation techniques.
3. The Fundamental Elements of Propagation Technique
Plant tissue culture should generally contain the following components: primary nutrients, secondary nutrients, amino acids, vitamins or nitrogen substances, undefined organic additives, carbon source (s), solidifying agents, and some growth regulators. However, the descriptive composition of these elements is shown in Table 4. It should be noted that the optimal quantity of each element for reaching maximal growth rates differs between organisms.
3.1. Primary Elements
For proper development and morphogenesis, nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) are required [73]. The culture medium should include at least 25–60 mM of inorganic nitrogen for optimum plant cell development. Most plant species require K for cell development. Optimal amounts of P, Mg, S, and Ca are 1–3 mM if other criteria for cell development are met [74].

Table 4.
The nutritional concentrations of widely used basal media [44,75].
Table 4.
The nutritional concentrations of widely used basal media [44,75].
| All Basal Media Components | MS | W | B5 | NN | BDS | WPM | DKW | BABI | MMS |
|---|---|---|---|---|---|---|---|---|---|
| Components of primary elements (mg L−1) | |||||||||
| KNO3 | 1900.0 | 80.0 | 2500.0 | 950.0 | 2500.0 | - | - | 2500.0 | 950.0 |
| K2SO4 | - | - | - | - | - | 990.0 | 1559.0 | - | - |
| NH4NO3 | 1650.0 | - | - | 720.0 | 320.0 | 400.0 | 1416.0 | 320.0 | 825.0 |
| CA(NO3)2·4H2O | - | 300.0 | - | - | - | 556.0 | 1948.0 | - | - |
| NH4H2PO4 | - | - | - | - | 230.0 | - | - | 230.0 | - |
| NaH2PO4·H2O | - | 16.5.0 | 150.0 | - | 150.0 | - | - | 150.0 | - |
| (NH4)2SO4 | - | - | 134.0 | - | 134.0 | - | - | 134.0 | - |
| MgSO4·7H2O | 370.0 | 720.0 | 250.0 | 185.0 | 250.0 | 370.0 | 740.0 | 250.0 | 185.0 |
| KH2PO4 | 170.0 | - | - | 68.0 | - | 170.0 | 265.0 | - | 85.0 |
| CaCl2·2H2O | 440.0 | - | 150.0 | 166.0 | 150.0 | 96.0 | 149.0 | 440.0 | 220.0 |
| Na2SO4 | - | 200.0 | - | - | - | - | - | - | - |
| KCI | - | 65.0 | - | - | - | - | - | - | - |
| Components of secondary elements (mg L−1) | |||||||||
| H3BO3 | 6.2 | 1.5 | 3.0 | 10.0 | 3.0 | 6.2 | 4.8 | 3.0 | 6.2 |
| KI | 0.83 | 0.75 | 0.75 | - | 0.75 | - | - | 0.75 | 0.83 |
| MnSO4.4H2O | 22.30 | 7.0 | - | 25.0 | - | - | - | - | 22.30 |
| MnSO4·H2O | 16.9 | - | 10.0 | - | 10.0 | 22.3 | 33.5 | 10.0 | 16.9 |
| ZnSO4·7H2O | 10.6 | 2.6 | 2.0 | 10.0 | 2.0 | 8.6 | - | 2.0 | 10.6 |
| Zn (NO3)2·6H2O | - | - | - | - | - | - | 17 | - | - |
| CuSO4·5H2O | 0.025 | - | 0.039 | 0.025 | 0.039 | 0.25 | 0.25 | 0.039 | 0.025 |
| Na2MoO4·2H2O | 0.25 | - | 0.25 | 0.25 | 0.25 | 0.25 | 0.39 | 0.25 | 0.25 |
| CoCl2·6H2O | 0.025 | - | 0.025 | - | 0.025 | - | - | 0.025 | 0.025 |
| NiSO4·6H2O | - | - | - | - | - | - | 0.005 | - | - |
| FeSO4·7H2O | 27.8 | - | 27.8 | 27.8 | 27.8 | 27.8 | 33.8 | 27.8 | 27.8 |
| Na2EDTA | 37.3 | - | 37.3 | 37.3 | 37.3 | 37.3 | 45.4 | 37.3 | 37.3 |
| Organic compounds and vitamins (mg L−1) | |||||||||
| Myo-inositol | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Nicotinic acid | 0.5 | 0.5 | 1.0 | 0.5 | 1 | 0.5 | 1.0 | 1.0 | 1.0 |
| Pyridoxine HCl | 0.5 | 0.1 | 1.0 | 0.5 | 1 | - | 0.5 | 1.0 | 1.0 |
| Thiamine HCl | 0.1 | 0.1 | 10 | 1.0 | 10 | 1.6 | 2.0 | 10.0 | 10.0 |
| Cysteine HCl | - | 1.0 | - | - | - | - | - | - | - |
| Ca-pantothenate | - | 1.0 | - | - | - | - | - | - | - |
| Biotin | - | - | - | 0.05 | - | - | - | - | - |
| Folic acid | - | - | - | 0.5 | - | - | - | - | - |
| Glycine | 2.0 | 3.0 | - | 2.0 | - | - | 20.0 | - | - |
| L-Glutamine | - | - | - | - | - | 250.0 | - | - | |
| Sucrose (g/L) | 30.0 | 20.0 | 20.0 | 20.0 | 30.0 | 20.0 | 30.0 | 30.0 | 30.0 |
| pH | 5.8 | - | 5.5 | - | 5.8 | 5.6 | 5.5 | 5.8 | 5.8 |
Note: 1. Murashige and Skoog (MS); 2. White Medium (W); 3. Gamborg (B5); 4. Nitsch and Nitsch media (NN); 5. Modified Gamborg (BDS); 6. Woody Plant Medium (WPM); 7. Driver and Kuniyuki Woody Plant (DKW); 8. Modified BDS (BABI); 9. Modified Murashige and Skoog (MMS).
3.2. Secondary Elements
Iron (Fe), manganese (Mn), zinc (Zn), boron (B), copper (Cu), and molybdenum (Mo) are essential sources of nutrients (microelements) for plant cell and tissue development. However, their requirements are generally low [73]. Iron is usually the most important key element and is employed in culture media as citrate or tartarated salts. However, these compounds have specific issues due to their difficulties in staying in solution following media preparation. There have been experiments utilizing EDTA-iron chelate (FeEDTA) to overcome this issue. A method for preparing non-precipitating iron-chelating solvents was also devised. Cobalt (Co) and iodine (I) can be used in some media, although their requirements for cell development remain less explored. Sodium (Na) and chlorine (Cl) are also utilized in some media, although it is suggested that these are for development. Copper and cobalt are supplied at 0.1 μM levels to the culture medium, iron, and molybdenum at 1 μM, iodine at 5 μM, zinc at 5–30 μM, manganese at 20–90 μM, and boron at 25–100 μM [74].
3.3. Source of Carbon
A carbohydrate source is vital for the basal media because, though in vitro organisms have some ability to photosynthesize, their capacity is often insufficient to support organ development, stimulation, and differentiated growth [76,77]. Plant cell culture media contains carbohydrates in addition to sucrose, which is often used as a carbon source in concentrations ranging from 2% to 5%. This source is used because galactose, starch, and lactose are less effective than sucrose or glucose, as glucose is first absorbed by the cells, followed by fructose, which is proportionally more effective than fructose. It is generally accepted that autoclaved sucrose is superior to filter-sterilized sucrose for development. Autoclaving hydrolyses the sucrose into more conveniently absorbed carbohydrates like fructose [78]. Sucrose has been shown to function as a morphogenetic activator in the production of adventitious root branching and auxiliary shoot production. The addition of cane sugar syrup (molasses), coconut water, and bananas could be an excellent way to reduce the cost of the medium [78]. In addition to carbohydrates, these surfactants provide vitamins and inorganic ions essential for development.
3.4. Vitamins
Many plants can synthesize the vitamins they need for their development. Some vitamins are necessary for optimal plant growth; they act as catalysts in various metabolic mechanisms. In vitro tissue culture may limit cell proliferation and differentiation. Pyridoxine, thiamin (B1), and nicotinic acid are the most often utilized vitamins in cell and tissue culture media (B6). Thiamin is used in amounts ranging from 0.1 to 10 mg L−1 and is required for the growth of all cells [79]. Although several species do not necessitate pyridoxine or nicotinic acid for the formation of the cell, they are often placed in culture media. Nicotinic acid and pyridoxine are used in quantities of 0.1 to 5 mg L−1 and 0.1 to 10 mg L−1. Other vitamins added to the cell culture medium include folic acid, vitamin E (tocopherol), biotin, ascorbic acid, pantothenic acid, vitamin b10 (para-aminobenzoic acid), and riboflavin. It is recommended to add vitamins to the culture media only when the thiamin concentration is low or when the cells are cultured in an environment with a low population density. Myo-inositol, a type of carbohydrate rather than a vitamin, is used moderately to drive cell development in most plant genera [80]. The composition of these vitamins is shown in Table 4.
3.5. Amino Acids
Amino acids required for cell viability and proliferation are present in basal media: L-glutamine, an amino acid required for nucleic acid synthesis and protein and energy generation. However, at normal pH in aqueous solutions, L-glutamine is unstable and rapidly converted into pyroglutamate and ammonium, which may interfere with cell proliferation [81]. Most plants synthesize the amino acids essential for optimum development; however, the incorporation of specific amino acids is especially crucial for creating cultures of protoplasts and cells. The added amino acids are digested by tissues and cells faster than inorganic compounds of nitrogen, and they are also used to promote cell proliferation in the medium.
3.6. Unspecified Organic Additives
Naturally existing organic additives such as coconut milk, protein hydrolysates, yeast extract, pulverized banana, malt extract, and vegetable and fruit juices are just a few examples that can be added to boost plant development [82]. Although their chemical origin is unknown, these substances are also complex organics. This means that they include elements whose impact on plant growth and advancement has not been determined yet [83]. Activated charcoal in culture media has been demonstrated to increase growth and differentiation in carrots and onions [84,85], orchids [86], and tomatoes [87] but hinder cell development in soybeans. Activated charcoal increases the hydrolysis of sucrose during autoclaving, causing the medium to become acidic. However, in some cases, the stated tissue culture medium does not produce the desired outcomes. Consequently, it is advantageous to experiment with various organic substances in such settings to acquire better outcomes. Once the various quantities of complex organisms in the medium have been adequately studied, a protocol can be established to favor the in vitro growth of plant tissue.
3.7. Solidifying Gel Agents
Gelling agents, or solidifying agents, are used to thicken the basal media mixture to a gel-like consistency (Table 4). The gel-like consistency provides the structure for tissues and organisms to grow efficiently and exchange gases without restriction while also offering an external environment suited for plant tissues to regenerate under controlled (in vitro) conditions. While many reagents, including agar, gelrite, and gellan gum, are used to support materials in plant tissue culture [88], agar is most common in (semi-solid) culture media [89,90,91]. Agar is an organic natural substance derived from aquatic algae (seaweed); however, other gelling agents such as gelatin, agarose, alginate, and gelrite are sometimes used. Other than gelling agents, the composition of the basal medium plays an essential role in forming plant tissues. The concentrations of important elements in commonly used basal media are shown in Table 4 below.
Even though no customized method exists to explain these media’s unique uses, the studies detailed in Table 4 can offer an understanding of them. W may be limited to Apiaceae and Musaceae family plants, B5 is best for different legumes, NN is suitable for another culture in plant propagation, BDS was modified for Allium spp., so it may be suitable for bulbous flowering plants, BABI or MS media may be recommended for many herbaceous plant tissue culture implementations, and for woody plants, other basal media such as MMS, WPM, or DKW may be advised.
4. Asexual Propagation Using Intelligent Technology, Sensor and IoT
The term “soilless culture” applies to any method of growing plants without the need for soil as a rooting surface [92,93]; a soilless medium made up of mostly organic matter is used instead; it provides anchoring for effective plant development [93], while the plant thrives on the provided mixture of nutrients and water [94]. When equated to conventional agriculture, these plant-growing techniques are the most intense, employing all available resources effectively and maximizing crop production [95]. Soilless cultivation is considered a modern method, but there have been many attempts throughout history to grow crops in above-ground containers. The wall art at the Temple of Deir el Bahari appears to be the first known container-grown plant [96]. Since native soils were not suited for the specific plant, workers transferred matured trees across their native places and grew them in soilless culture.
Nonetheless, this technique has been used for centuries, the Aztecs and Egyptians (logographic) used water culture to nurture specific species, and the hanging garden of Babylon is a prime example [97]. Almost any plant can be cultivated in a soilless culture. This method has mainly been used to cultivate garden and vegetable plants or when an agricultural territory is limited and irrigation water is scarce [98]. Another significant application of this method is plant propagation and cloning, many commercial plants can be propagated using this method. Hydroponics is one of the most widely used soilless growth technologies, with less use for the regeneration of different species, while aeroponics systems are the most effective in propagating various plant varieties. Table 5 depicts a variety of plants regenerated with various soilless culture techniques.

Table 5.
List of crops that can be regenerated using rapid soilless propagation techniques.
Agriculture requires further precision for significant returns in today’s modern time-controlled world. Humans require decision making and automatic advanced computer control and monitoring mechanisms to decrease manual input and improve system performance. Adopting computer intelligence strategies can improve the accuracy of propagation operations in a controlled environment. The advancement of asexual aeroponic propagation lies in the application of monitoring and control systems using IoT and smart sensor technology. New sensor technology has gained substantial interest in agriculture in recent years. It is used in agriculture to arrange various operations precisely. The application of intelligent technology for fault diagnosis in the aeroponics system can be accomplished through computer programming techniques. The information for fault detection in the system is provided by operational intelligent sensors that are used for environmental, chemical, and mechanical parameters [112], and this intelligent technology is also capable of detecting biological faults that develop in the system [113]. Furthermore, the advantages of adopting intelligent technology for asexual aeroponic propagation are wireless communication and cloud data monitoring. In IoT technologies, Wi-Fi, Zigbee, Lora, GSM, and other technologies are used to transmit the data to a smartphone or laptop [114].
4.1. Aeroponic Propagation
Aeroponics is a farming technology that produces plants in a closed tank with a soilless environment where roots are suspended in the air and receive nutrients through misting nozzles [115]. The term “aeroponic” is derived from the Greek words “aero” (air) and “pónos” (worker) [116]. Although scientists first created aeroponics to investigate root systems and related issues, its unparalleled performance in commercial crops and large-scale vegetative multiplication has made aeroponics a viable concept for the future. This technology has outperformed traditional propagation techniques in terms of water efficiency, reduced space, and plant cycle and its time requirements, seasonal freedom, propagation of disease-free clones, and vast plant growth. As a result, it is widely used to grow in the fields of forestry [117], vegetables [118], leafy vegetables [119], tubers [103,120], and horticulture [121]. However, this growth is mainly accomplished through sexual propagation, which is slower than asexual reproduction. Intelligent rapid asexual propagation is a new technology that provides precision in management and massive systematic data collection to create a mechanism in which components can be operated with automation, used to collect data precisely, and connected to the Internet via sensors. However, data gathering of rapid asexual propagation in a traditional aeroponic system is reportedly slow because such data are typically collected manually.
Furthermore, the reliability of manually collected propagule performance data is low because an experienced person is required to take the data by hand and the quantity of data is greater. Additionally, manual data collection is time-consuming and prone to human error. In contrast, intelligent technology can make decisions based on available data and can make changes with the help of sensors. Figure 2 shows a schematic representation of an aeroponic system.
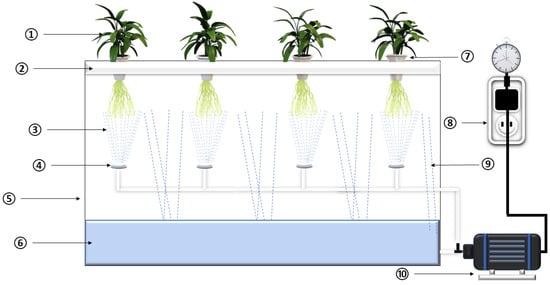
Figure 2.
Schematic view of aeroponic system: 1. Plant; 2. Plant supporting raft; 3. Nutrient mist; 4. Misting nozzle; 5. Growth chamber; 6. Nutrient solution; 7. Plant holder; 8. Timer; 9. Nutrient dropdown; 10. Pump.
4.2. History of Aeroponic Propagation
Plant propagation via aeroponic technology is not a new concept; aeroponic propagation of plants has been around since the 1960s [122]; following the success of aeroponic propagation, it became well-known and was employed as a commercial technique for plant propagation, attracting many individuals. B. A Briggs, a pioneer in the soilless propagation field, was among them. He excelled in stimulating roots on hardwood cuttings by air-rooting that were stronger and more rigid than those generated in traditional methods [123]. In 1981, an Israeli scientist named Nir Isaac presented the results of five different ornamental plant varieties, successfully propagated via cuttings in aeroponic chambers with varied plant densities. He discovered that the density did not affect the development of cuttings [124]. Richard J. Stoner was the first businessman to establish a corporation dedicated to manufacturing aeroponic machines, and he is regarded as the “Father of Modern Aeroponics”. He introduced the “Genesis Machine”, which was the earliest aeroponic cloning equipment [125]. Different variations with progress through time are still employed today for the purpose of propagation. Plants that were previously assumed to be inconvenient or impossible to propagate, such as softwoods and hardwoods, were now achievable in aeroponics propagation by stem cuttings as a result of the highly aerated microenvironment around the root zone, which promoted root hair formation [126]. Because air-rooted plants were less likely to be affected by diseases, farmers could clone and transplant them straight into field soil without suffering transplant shock [127]. Ref. [128] propagated chrysanthemum cuttings in an aeroponic chamber, recording varied spray intervals in the winter and summer. They advised a 120 s (summer) and 30 s (winter) spray duration with intervals of 30 min during the day and 60 min at night.
4.3. Workability of Aeroponic Propagation
Plant organs or cuttings are placed in the system, where they contact a nutrient mist delivered by an evenly distributed nozzle network. These nozzles are fixed in a piping system designed to dispense nutrients for propagating plants in aeroponics. The fundamental challenge with the aeroponics model is the size of the nutrient droplet. Bigger droplets render deficient oxygen concentration in the root zone, while the small droplets create an excessive amount of root hair without creating a lateral root system, which is not good for sustainable plant development [129]. The pipes are connected to the water pump, which uses strong suction to push the nutritious solution. A programmable time controller is linked to the pump to control the nutrition spraying at predetermined intervals [130]. The pressure of mist and its nozzle spacing, the density of propagules, the pumping capability of the motor, the timing of nutrient misting, and the time interval between sprays may vary depending on the scale of the aeroponics structure arrangement and the farmed plants [131].
4.4. Rapid Propagation Using Aeroponic Technique
Aeroponics asexual propagation is the fastest method of plant regeneration. Potato plants may produce more than 900 mini-tubers per m2 to the traditional potato propagation method [132], which produces roughly 8 offspring tubers only throughout 12 months. In the greenhouse, after only 3 to 5 months of in vitro propagation using a nutrient culture of potato nodes, each potato shrub produced some tubers. [133]. Aeroponics provides exact plant nutritional requirements for the crops, reducing fertigation requirements and the potential of high fertilizer residues entering the groundwater [134]. Aeroponics should also be considered an effective method of yam propagation, which showed that vines cut from five-month-old plants were successfully rooted (95%) within 14 days [111]. The five yam genotypes propagated in aeroponics averaged an 83 percent rooting, ranging from 68 percent to 98 percent. However, this system must be carefully monitored because it is susceptible to high temperatures. Aeroponic cloning improves root elongation, rapid maturation, growth rate, and survival [135]. The previous study had shown that the average yield of tuberous species grown in aeroponics is greater than the same plant grown in traditional techniques [132,136]. Aeroponics has also been adopted in the regeneration and culture of medicinal plants in recent years. Plantation of medicinal rhizomes Zingiber officinale [137] and high-valued root crops such as Withania somnifera [138], Arctium lappa [139], and Anemopsis Californica [140], as well as clonal propagation of three critically endangered medicinal plants, Tylophora indica, Caralluma edulis, and Leptadenia reticulata by [110] and discovered that aeroponically produced stem cuttings had a greater rooting rate than soil-grown stem cuttings. In another study [141], researchers studied the impact of accessible root zone volume on the production and nutritional quality of propagated Ocimum basilicum in an aeroponic setup. It has also been tested for antifungal, antibacterial, and cytotoxic effects in aeroponically grown medicinal herbs [142].
4.5. Precision in Rapid Propagation
With the progression of time, every branch of study is advancing toward more exact procedures that employ scientific and technical approaches to obtain the highest production while employing the fewest and most accurate resources. Such advancements not only improve the method’s performance but also give rise to fresh unique approaches in agriculture that have never been employed before. While some developments will be greater, certain propagation technologies have already reached almost all their potential. There is no doubt that tissue culture is most extensively used for the commercial production of plants; however, tissue culture technology has fully matured in the last decades, and all key discoveries in plant tissue culture have already been made [143], limiting its future progress. The same is evident for synthetic seeds, which, while a marvel of science, have limitations. For example, only a few seeds mature into a fully developed plant, and storage is a leading difficulty because it can only be preserved in a controlled atmosphere [144]. These approaches may develop through time to yield better results in rapid asexual propagation. Still, rapid asexual aeroponic propagation has already achieved significant advances, making it an essential tool for plant regeneration due to its flexibility in adaptation to different plant species. This method is helpful for the growth of many plant species, including rare and threatened plants, with the source of explants for mass regeneration. Aeroponic plant regeneration is a highly beneficial approach for inducing an adventitious root system, thus developing healthy plants [109]; compared to other techniques, it also has the potential to be profitable from an economic standpoint [145]. It is also a potential method for rapidly cloning plants with ideal phenotypic characteristics for commercial sectors, and domestic and conservation purposes [146]. Moving on to propagation precision, several characteristics, such as chemical and physical environments, mechanical parameters, and agronomical parameters, such as plant density, significantly influence the system’s overall performance. Physical characteristics include the potential hydrogen (pH) and electrical conductivity (EC) of the stock media, humidity inside and outside the chamber, temperature of the stock solution and growth environment, and adequate oxygen supply [147].
The inside temperature of the chamber and ambient temperature are recorded, as the plant, depending on its topographical location, has different requirements depending on the biome of the plant; temperature also affects the pH value and EC of the nutrient solution. The pH value is always maintained so that the system does not fail; if the pH value is not balanced, the plants will struggle to absorb nutrients and die due to nutrient depletion; the average pH in an aeroponic system is kept at 5.7 and 6.3. The supply of oxygen is provided in the form of dilution in the nutrient solution, which is designated as DO (dissolved oxygen); the circulation of air together with the nutrient mixture is delivered to the plant roots via a pressure pump coupled mist. Another crucial characteristic is electrical conductivity; maintaining the right amount of EC is critical for a successful system; if the EC is too high, the plant will go into stress and exhibit indications of salt toxicity, and if it is too low, the plant will show evidence of nutrient deprivation [148]. Chemical parameters, such as macro- and micronutrient concentrations in the solution, and mechanical properties, including the type of misting nozzle and the type of light, play a crucial role in influencing plant growth and development in rapid aeroponic propagation, as shown in Table 6.

Table 6.
Influence of various parameters on the rapid aeroponic propagation technique.
The data reviewed in the table demonstrate that improvements in specific parameters can lead to positive outcomes. However, collecting and tracking this information takes time. Manual data collection can sometimes result in significant mistakes or data loss. New trends like the Internet of Things (IoT), intelligent control, and sensor technology have made the field of agriculture easier and more precise, particularly in the area of plant rapid propagation, where a single error could lead to the failure of the entire process.
4.6. Potential Approaches for Monitoring and Smart Control of Rapid Aeroponic Propagation
Currently, rapid defect detection and diagnosis utilizing an intelligent agriculture monitoring system has been regarded as the most significant instrument for monitoring plants without the need for sophisticated operations and laboratory examination, which need domain expertise and significant time. The development of these valuable traits has also sparked a lot of interest in agriculture [155]. The use of intelligent technology for agricultural prediction is not new; however, there has been limited work conducted with rapid aeroponic propagation to develop an intelligent rapid propagation machine using aeroponic technology, despite the fact that the approaches used for aeroponic cultivation method have many achievements in the field of artificial intelligence, smart control and monitoring, data logging using a sensor with a controller, and IoT, which can be used for aeroponic propagation. Additionally, this approach could forecast various parameters in aeroponic propagation based on entirely automated control systems in which the survival rate, adventitious root development, and other parameter rates could be optimized by controlling the variables. Ref. [156] used artificial intelligence technologies in an aeroponic model to predict the yield of six distinct types of crops. They emphasize that data fusion is possible and even improves yield forecasting in aeroponics only by providing sensor data for AI models. Ref. [157] employed intelligent control to sustain the LED lights; three types of lights were used, each regulated by a defined value of lumen flux; this usage of intelligent control influenced and enhanced plant height and leaf number. Ref. [158] designed a reprogrammable electrical circuit that employs software MATLAB-Simulink R2019b (The MathWorks, Inc., Natick, MA, USA) to generate multiple sprinkler cycles to achieve enough plant development; this technique could be employed for better water scheme propagation if programmed according to the type of cutting or explant. Ref. [159] conducted an experiment on a Lattepanda windows-based smart microcontroller unit (MCU) that was fitted with a light, humidity, and temperature sensor. It also controlled the intervals of the pressure pump and the photoperiod of the LED light with a relay connected to the MCU, which kept the aeroponic chamber atmosphere stable. Ref. [160] stated that an aeroponic model equipped with an IoT device that is integrated with a mobile app, service platform, and control module allows the user to monitor and manage the aeroponics system remotely, a graphical representation of an IoT-based intelligent controlled rapid aeroponic propagation machine is shown in Figure 3.
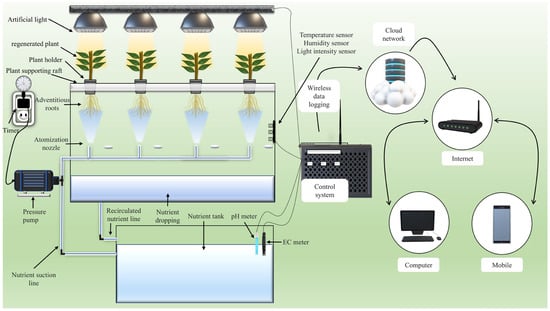
Figure 3.
Rapid aeroponic propagation machine control system process.
While these precision aeroponic technologies will make research more manageable, they will also allow many plant species to be swiftly propagated on a broader commercial scale. Intelligent rapid propagation will bring several benefits to the user, including lower beginning costs, precise material utilization, maintenance and upgrading conducted in the backend by machine, lower human labor costs, and easy and rapid production of high-quality clonal plants. The implementation of IoT with a rapid aeroponic propagation machine will allow the administrator to stay accessible to the system via portable gadgets such as a smartphone, tablet, and laptop from any location through the Internet, which is not bound by any restriction caused by location or time.
5. Smart Control and Monitoring System
The implications of an automated aeroponic propagation machine far outweigh the limitations of traditional propagation methods. While traditional aeroponic models may perform better for sexual propagation, asexual propagation is a complex method in which each parameter must be precisely controlled, which is critical to the success of the propagation cycle. This precision can only be accomplished by utilizing sensors and control systems that enable advanced computer control and monitoring mechanisms to reduce manual input and improve system performance. Data collection from sensors has become much easier with modern intelligence technology and control systems. These sensors are powerful tools that provide precise data in seconds. In addition to sensors, an actuator equipped with a relay module is used. Relay modules are precise devices that provide efficient operation. These can be used to atomize spray, turn on and off fans, and control spectrum lights at specific times. The sensor data collection includes ambient variables (temperature and humidity), light intensity, stock solution (nutrient-rich water), EC and pH values, CO2 content, atomization frequency, and atomization interval length. Temperature is regarded as one of the most critical factors influencing plant propagation in an aeroponic system, particularly the root system. The architectural structure of an intelligent control and monitoring system is composed of five divisions. Figure 4 shows the components of the smart control and monitoring system, as well as their use.
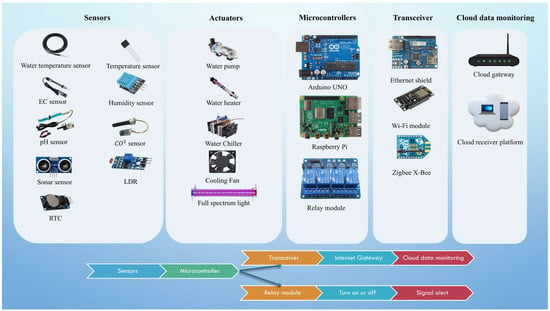
Figure 4.
Smart control and monitoring asexual aeroponic propagation system components and purpose of application.
5.1. Sensors
Much research has been conducted on smart sensor use in aeroponics. The sensors minimize high expenses, since they offer essential information in real time [155]. By wirelessly providing the operator with real-time information gathered in the system, the sensor network approach helps to increase the efficiency and functionality of the devices already placed in the greenhouse. In order to conserve energy and lower consumption, sensors are more precise, rapid, and energy efficient.
5.2. Temperature and Humidity Sensors
The plant root’s capacity to absorb the nutrient solution is influenced by temperature. According to [161], plants exposed to hot or higher temperatures typically exhibit symptoms of nutrient insufficiency. In addition to temperature, humidity is another consideration. Because regular misting increases the humidity, if the moisture level in the tank is too high or too low, it might cause several issues, including plant mortality. Temperatures must be within 15 °C and 25 °C and should not drop below 4 °C or rise above 30 °C [155]. Most plant species are maintained at humidity levels between 60 and 70% [162]. The most popular sensors used for aeroponic asexual propagation are the DS18B20, LM35, DHT11, and DHT22, as shown in Figure 5. Modern temperature sensors are simple to use and require a single installation. Some of them can record humidity as well as temperature (DHT11 and DHT22) from air [163]. Others just measure temperature (LM35), while one can record data from water or even chemical solutions (DS18B20).

Figure 5.
Temperature and humidity sensors used in rapid asexual aeroponics propagation technique.
5.3. pH and EC Probe Sensors
The pH of the stock solution is an essential feature of a soilless propagation technique; it is the measure of acidity or alkalinity of a stock solution; fluctuations in this value result in a situation in which a plant cannot obtain basic nourishment [164]. As a result, monitoring pH levels is crucial for enhanced management and optimum propagule development. While pH levels indicate the equilibrium of accessible nutrients in a stock solution, EC indicates the amount of accessible nutrients. A higher EC level will cause toxicity [165], resulting in resistance to nutrient uptake. If the level is low, propagules will face severe nutritional deficits. Battery-operated meters are used to assess the levels of EC and Ph; however, it takes time if the data have to be gathered manually each day. Therefore, smart sensors could be used to determine pH and EC levels. These analog sensors equipped with sensor nodes are linked to microcontrollers and cloud devices, allowing for easy remote monitoring and control of pH and EC readings.
5.4. Carbon Dioxide (CO2) Sensor
When using a rapid aeroponic propagation approach, the shortage of carbon dioxide (CO2) is a more critical issue than the shortage of mineral nourishment nutrient components. Ref. [166] found that increased CO2 levels during plant propagation boosted cacao and yam growth and eliminated the requirement for supplementary carbohydrates. According to [167], The plant develops 94% of its mass (dry matter) from CO2 and water, while the remaining 6% comes from mineral fertilizers. Supervising and controlling CO2 levels for the growth and emergence of propagule roots in the rapid aeroponic propagation system is vital to optimize production while reducing operational time and costs with the least amount of human engagement. Therefore, the CO2 sensor should be utilized to monitor and detect fluctuations of CO2 content within the aeroponics growth chamber for rapid and precise monitoring and detection. CO2 sensors are classified into two types (shown in Figure 6): non-dispersive infrared (NDIR), sometimes known as an infrared sensor, and chemical CO2 (CDS). Infrared sensors are spectroscopy sensors that detect CO2 in a volatile condition by measuring its absorption. When comparing the CDS sensor to the NDIR sensor, the CDS sensor has a shorter overall life, and NDIR is significantly more accurate than CDS.
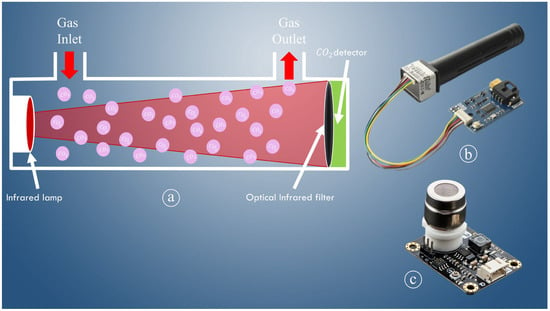
Figure 6.
Working mechanism of NDIR sensor (a), NDIR CO2 sensor (b), CDS carbon dioxide sensor (c).
5.5. Water Level Sensor
The nutrient solution tank must be maintained throughout the propagation phase to ensure the clone has enough nutrients. In a traditional aeroponic system, the operator has to adjust the nutritional solution level in the tank manually. Smart wireless sensors, on the other hand, can detect the liquid level in reservoirs and make it easier for operators to acquire water nutrient level data in real time by implementing intelligent precision agriculture techniques. Some of these sensors will also notify the operator of any possible leakage or spills, as well as when a container is close to being empty. There are many kinds of sensors available to be used in rapid asexual aeroponics propagation systems that can wirelessly transmit data to growers and can also adjust the nutrient solution level. Sonar sensors, infrared sensors, radar sensors, and conductivity sensors are examples of regularly used sensors. Ref. [162] mentioned using a sonar (ultrasonic) sensor. The sonar sensor is made up of a control circuit, a receiver, and a transmitter. It uses the principle of ultrasonic waves to measure the amount of water nutrient solution in tanks. The sensor calculates the reflected waves from the liquid surface and the time it takes to reach the surface to give the level of available water in the tank. Radar sensors work in the same way that sonar sensors do. These sensors, however, broadcast microwaves through an antenna on the radar sensor and reflect these microwaves back to the receiver antenna. Hence, the time between signal transmission and receiving is proportional to the level of water. Infrared sensors function by turning light beams into electronic pulses that measure a physical luminance and then transform it into a calculation [168]. Conductivity sensors, also called resistive sensors, are used to detect the level of water by using a probe to read conductivity. The probe is made up of a pair of electrodes that are connected by an alternating current. These sensors (shown in Figure 7) are helpful for measuring tank levels, highly corrosive liquids, and reagent tracking.

Figure 7.
Types of sensors for water level used in rapid aeroponic propagation technique.
5.6. Synthetic Light and Sensors
In order to provide a precise environment for better overall propagule growth, asexual propagation using the aeroponic technique is typically carried out indoors. However, there is no natural light present in indoor systems; artificial LED light has been used as a substitute for at least 8 to 10 h per day. However, no temporal automation has been incorporated into synthetic lightning. The real-time clock or RTC module (shown in Figure 4) provides the time pulses required for automation. With the aid of a microcontroller and Arduino, the LED light of the indoor aeroponic system can be automatically switched on and off. Light intensity also has an important impact on propagule growth. According to the findings of [169], the density of photosynthetically active photons had a more significant impact on average plant weight than the fertilizer density. A sensor known as an LDR (shown in Figure 4) or light-dependent resistor, is a light sensor that monitors light intensity via resistance and the passing of volts (via electron movement). This sensor is linked to a microcontroller for cloud monitoring and control.
5.7. Control and Relay Module
The control and relay modules are the two primary circuits of an intelligent aeroponics system that control and monitor the whole operation (shown in Figure 4). A microcontroller or control module is an integrated circuit (IC) component that controls other elements of an electronic system, often through memory, interfaces, and a microprocessor unit [170]. The two most prominent control modules in smart aeroponic propagation systems are the Arduino Uno and Raspberry Pi. The control modules are programmed using the official software “Arduino IDEA (version 1.8.19)”. Once programmed, they can be instructed to do specific tasks [171]. Relay modules are electrical circuits that are equipped with components that cannot be automated on their own and need a mechanism or an automated relay module. These electrical devices are also known as actuators. A relay essentially functions as a switch, in contrast to an actuator, which is a tool that aids in bringing about necessary mechanical movements. Consequently, a sensor sends data directly to the control module, which then signals the relay module to act. Although they are fundamentally opposing technologies, sensors and actuators usually function together. A sensor tracks the environment and alerts users when something changes. An actuator responds to a signal (given by a relay module) by taking action, frequently by moving a mechanical device.
5.8. Intelligent Control and Internet of Things (IoT) Systems in the Asexual Aeroponics Propagation
The Internet of Things (IoT) in intelligent aeroponic machines has become an important tool for improving asexual propagation systems. IoT is a modern technology that makes use of an Internet connection. It refers to a set of equipment that interacts independently over the Internet and does comprehensive data analysis to create a system in which products can be linked to the online network via integrated circuits and sensors [172]. As a result, traditionally stagnant and less accurate work becomes rapid and more precise. The IoT system relies on a wireless sensor network (WSN) consisting of a data server, nodes for wireless convergence, wireless routers, and wireless sensor nodes. These wireless sensor nodes collect sensor data and transmit them to the intelligent agriculture monitoring system, which is further managed and monitored [173]. Ref. [174] developed a ZigBee-based WSN system in which the WSN nodes were coupled with an actuator to regulate and monitor the parameters of an aeroponic system. Ref. [175] developed a WSN system to monitor an aeroponic system’s physical characteristics; the sensors’ data were transferred to a computer using the Global System for Mobile (GSM) module. IoT devices link to the Internet via an ethernet shield module or WiFi module (ESP8266) with the assistance of a microcontroller, which transmits sensor data to an IoT device or transceiver (shown in Figure 4). There are microcontrollers such as the ESP8266 Node MCU, which is a control module with a built-in Wi-Fi module coupled on the same platform. However, this data transport is not just dependent on a wireless fidelity network; it can also be achieved using Zigbee, GSM, Lora, and other platforms.
5.9. Cloud Monitoring and Storage Platform
When large amounts of data are gathered, they require storage space. A microcontroller can save data for a limited duration, but a platform is required when large amounts of data are acquired. A cloud server platform gathers, analyses, and stores data [114]. A cloud-based server, such as thingSpeak, is a free platform. This platform collects and displays data received by WSN-based IoT devices. Another cloud-based server is the Blynk mobile application, which is an open source that utilizes cloud storage and presents data on the smartphone screen in graphical and digital forms.
6. Advantages of Intelligent Rapid Asexual Propagation
Aeroponics is one of the new intelligent and rapid technologies in agriculture. It is a modern asexual propagation method in which the plant cutting or organ is propagated in a suspended air atmosphere without soil support. The technology saves labor by 98%, uses 60% fewer nutrients, near-to-no pesticides and herbicides, and increases plant output by 45% to 75% over a hydroponics or conventional system [127,176]. Ref. [103] stated that, in aeroponic rapid propagation, the average survival rate of casava plants was 90.5%, compared to 51.5% in the conventional method. These results indicate that the aeroponic method of asexual propagation is more efficient and productive. However, in the absence of a natural growth medium (soil), commonly, a non-soil organic media is provided to the plants in the aeroponic system to support them instead of soil, which is an alternative artificial environment that requires a range of variables to be regulated for optimum plant development. However, this difficulty has been remedied by applying sophisticated monitoring and control systems. When the clone is subjected to unexpected stress in the traditional method of aeroponic propagation and no one is around, the cloned plant would perish.
Along with that, a highly experienced professional is also required to monitor the environmental, chemical, and mechanical parameter levels using various instruments and checking the readings while manually gathering data, which is a time-consuming and labor-intensive process. An aeroponic machine equipped with an actuator and IoT-enabled sensors can be designed to monitor fundamental parameters, addressing the challenges mentioned above while reducing labor costs and requiring less technical experience. The use of intelligent technology with the wireless sensor and actuator network has several advantages, including the ability to respond faster to unfavorable environmental conditions and better propagation quality control with the best output with precision. A mobile application designed to operate IoT applications on smartphones and laptops can be used for data monitoring. It obtains information from the server platform. Wireless sensors can be used by growers to automatically monitor data of plant development during cloning and propagation from remote areas and display them on the screen [114].
Disadvantages of Intelligent Rapid Asexual Propagation
Future aeroponic propagation technologies will rely increasingly on monitor and control system-based IoT technologies with large-scale sensors, but these will be costly for large-scale productions.
Rapid asexual propagation requires a certain degree of expertise to run the aeroponic system; currently, less effort is being made in this regard, and maximizing the full potential of this technology will take time.
Since plant cloning and propagation in the system differs from plant cultivating culture in an aeroponic system, the operator must be familiar with the correct quantity and kind of nutrient or growth hormone. As a result, it is vital to offer the appropriate nutritional content. If the concentration is too high, the clone will die; if it is too low, the adventitious root and propagule development will suffer.
The cost of system design is significantly influenced by the need for modern equipment. While cheaper alternatives exist, it is the superior equipment that provides the precise readings necessary for effective aeroponic cultivation, albeit at a higher cost.
7. What We Know About Asexual Rapid Propagation Techniques and What Remains
Currently, fields associated with propagation require more productivity, necessitating the use of more complex technologies. The preliminary experiments present the following facts regarding the various rapid techniques: These propagation techniques (tissue culture/micropropagation) produce at a rapid rate, including aeroponic and synthetic seed production, which are new in the propagation domain with high potential and output, and thus these approaches increase yields and quality across diverse plant species such as fruits, vegetables, medicinal herbs, and certain endangered plant species. However, because tissue culture is the first stage in most propagation processes, improved formulations for basal medium synthesis can increase efficacy and pace of production, hence boosting all other connected techniques. Economic evaluations should be undertaken to establish the system’s viability. In order to produce cost-effective procedures, the initial cost of the facility and equipment must be considered. At the same time, renewable energy (solar/wind) should be employed to reduce energy prices while ensuring the commercial usage of conventional power supplies. These rapid techniques should also be investigated in order to produce plants that are resistant to a range of stresses, as well as for the creation of key plant-based phytochemicals. Aeroponics necessitates the investigation of nutrient solutions for each variety since various systems may have different optimal nutrient solutions. The initial step toward the commercialization of this approach will be the large-scale manufacture of asexual aeroponic propagation machines with excellent regeneration potential at a reasonable cost.
Moreover, extended research is required in order to optimize the operation of rapid propagation techniques through automation of explant selection to culture growth, shoot multiplication to rooting the plant, and plantlet selection to hardening and growing in the field. Other approaches of AI and IoT should also be considered for better performance; furthermore, aeroponics coupled with automation will boost the efficacy of production. Finally, other related disciplines, such as aquaponics, should be studied for rapid plant propagation while coupling them with automation. While propagating tuberous plants, additional economically valuable plants should be grafted and tested to increase the productivity of the propagation process, such as in aeroponics systems.
Prospects
According to earlier research, the rapid propagation approach is a commercially viable way for growing a high number of clonal plants. Several particular protocols for the mass production of a varied range of plants have been developed; the chosen propagation method depends on the plant species and type, as well as the production goals. Micropropagation is best suited for the rapid multiplication of plantlets produced from tissue culture, while artificial seeds could preserve propagules for a more extended period and are a better technique to transfer them overseas. Overall aeroponics is a good way of growing diverse tuberous plants, plant cuttings, and other explants with the possibility of producing faster and of higher quality than the conventional approach. Aeroponics has an advancement compared to other asexual propagation methods as it is equipped with a smart intelligent control system. A smart aeroponic propagation machine with mechanized atomization and a controlled environment with the aid of computers having cloud control feature is the current need, as will be the case with artificial intelligence, intelligent control systems, and high-end sensor optimization, as well as the refinement of current procedures for the production of numerous plant species, which are discussed above.
8. Conclusions
This review article addresses the use of intelligent aeroponic technology for rapid asexual propagation. The rapid propagation technique, as explained in this article, is vital for sizeable vegetative regeneration because it reduces the process cost per plant. Using rapid asexual propagation via intelligent controlled and monitored aeroponic systems enables plant production without sacrificing quality. This new technique provides plant scientists with a variety of information needed to have a better grasp of the rapid multiplication of asexually. However, limited work has been conducted in this direction, requiring the attention of researchers as it has the potential to surpass other asexual propagation techniques when fully developed to its full potential. The use of this approach in the preservation of endemic and endangered plant species would aid in the conservation of natural assets as well as the prevention of natural disasters caused by deforestation and degradation of natural habitats that could result in the extinction of the species, resulting in a decrease in biodiversity and a negative impact on the ecosystem. Furthermore, asexual propagation employing smart aeroponic systems is the best potential future technology in plant propagation, allowing plant scientists and researchers to remotely monitor and control many factors without wasting time on human labor.
Author Contributions
Conceptualization, A.-u.-A.S.; validation, M.H.T., A.R.O., and M.N.H.A.A.; formal analysis, L.T., A.R.J., and L.H.; investigation, A.R.O., M.N.H.A.A., S.A.B., S.A.O., and J.A.C.; writing—original draft preparation, A.-u.-A.S., L.T., and M.H.T.; writing—review and editing, A.R.J.; funding acquisition, L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China, grant number 2023YFD1900704-02, the National Natural Science Foundation of China, grant number 52109105, and the Jiangsu Association for Science and Technology Young Science and Technology Talent Support Project, grant number JSTJ-2023-XH029.
Data Availability Statement
Data is contained within the article.
Acknowledgments
All the authors are thankful to figdraw (www.figdraw.com) for their assistance in drawing Figure 1 (accessed on 21 June 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- ICRAF. World Agroforestry Centre (ICRAF): Annual Report 2011–2012. Available online: https://cgspace.cgiar.org/handle/10947/4607 (accessed on 13 January 2022).
- Weismann, A. The significance of sexual reproduction in the theory of natural selection. Essays Hered. Kindred Biol. Probl. 1889, 1, 255–332. [Google Scholar]
- Weismann, A. Das Keimplasma Eine Theorie der Vererbung. Nature 1893, 47, 265–266. [Google Scholar] [CrossRef]
- Assogbadjo, A.E.; Sinsin, B.; De Caluwé, E.; Van Damme, P. Développement et Domestication du Baobab au Bénin. 2009. Available online: http://hdl.handle.net/1854/LU-868477 (accessed on 13 January 2022).
- Sidibe, M.; Williams, J.T. Baobab (Adasonia digitata L.) Propagation Manual; RPM Reprographics: Chichester, UK, 2002; pp. 1–102. ISBN 0854327649. [Google Scholar]
- Sharma, K.; Thapa, T. The Various Methods used for the Clonal Propagation in Horticultural Crops: A Review. Agric. Rev. 2021, 43, 229–233. [Google Scholar] [CrossRef]
- BD Editors. Asexual Reproduction—The Definitive Guide|Biology Dictionary. 2020. Available online: https://biologydictionary.net/asexual-reproduction/ (accessed on 13 January 2022).
- Chhalgri, M.A.; Khan, M.T.; Nizamani, G.S.; Yasmeen, S.; Khan, I.A.; Aslam, M.M.; Rajpar, A.A.; Tayyaba; Nizamani, F.; Nizamani, M.R.; et al. Effect of plant growth hormones on shoot and root regeneration in rose under in vitro conditions. Adv. Life Sci. 2020, 8, 93–97. [Google Scholar]
- Soni, M.; Kaur, R. Rapid in vitro propagation, conservation and analysis of genetic stability of Viola pilosa. Physiol. Mol. Biol. Plants 2014, 20, 95–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, A.S.; Lin, M.G.; Liu, F.X. Rapid propagation of cut flower varieties of Oncidium by tissue culture. Biology 2009, 40, 801–806. [Google Scholar]
- Reinhardt, D.H.R.C.; Bartholomew, D.P.; Souza, F.V.D.; de Carvalho, A.C.P.P.; de Pádua, T.R.P.; Junghans, D.T.; de Matos, A.P. Advances in pineapple plant propagation. Rev. Bras. Frutic. 2018, 40, 1–22. [Google Scholar] [CrossRef]
- Tumuhimbise, R.; Talengera, D. Improved propagation techniques to enhance the productivity of Banana (Musa spp.). Open Agric. 2018, 3, 138–145. [Google Scholar] [CrossRef]
- Beshir, W.; Alemayehu, M.; Dessalegn, Y. Effect of grafting time and technique on the success rate of grafted Mango (Mangifera indica L.) in Kalu District of Amhara Region, North Eastern Ethiopia. Cogent Food Agric. 2019, 5, 01–19. [Google Scholar] [CrossRef]
- Shan, F.; Seaton, K. Semi-sterilized tissue culture for rapid propagation of grapevines (Vitis vinifera L.) using immature cuttings. HortScience 2014, 49, 949–954. [Google Scholar] [CrossRef]
- Bowman, K.D.; Albrecht, U. Efficient propagation of citrus rootstocks by stem cuttings. Sci. Hortic. 2017, 225, 681–688. [Google Scholar] [CrossRef]
- Patil, V.M.; Dhande, G.A.; Thigale, D.M.; Rajput, J.C. Micropropagation of pomegranate (Punica granatum L.) “Bhagava” cultivar from nodal explant. Afr. J. Biotechnol. 2011, 10, 18130–18136. [Google Scholar] [CrossRef]
- MeiFang, J.; Zhi, C.; JunJie, C.; MaoZi, L. Tissue culture and rapid propagation technique of red-flowered strawberry. Guangxi Zhiwu/Guihaia 2017, 37, 1395–1405. [Google Scholar]
- Nordine, A.; Bousta, D.; El Khanchoufi, A.; El Meskaoui, A. An Efficient and Rapid in Vitro Propagation System of Thymus Hyemalis Lange, a Wild Medicinal and Aromatic Plant of Mediterranean Region. Int. J. Pharma Biosci. Technol. 2013, 1, 118–129. [Google Scholar]
- Nongdam, P.; Chongtham, N. In vitro rapid propagation of Cymbidium aloifolium (L.) Sw.: A medicinally important orchid via seed culture. J. Biol. Sci. 2011, 11, 254–260. [Google Scholar] [CrossRef]
- Poornima, D.V.; Jayanna, S.G.; Kumar, V.; Gajula, H.; Rajashekar, J.; Sannabommaji, T.; Basappa, G.; Anuradha, C.M. Rapid in vitro propagation of Lucas aspera Spreng. A potential multipurpose Indian medicinal herb. Ind. Crops Prod. 2017, 107, 281–287. [Google Scholar] [CrossRef]
- Chavan, J.J.; Kshirsagar, P.R.; Gaikwad, N.B. Rapid in vitro propagation of Clematis heynei M. A. Rau: An important medicinal plant. Emir. J. Food Agric. 2012, 24, 79–84. [Google Scholar] [CrossRef]
- Doğan, M. In Vitro rapid propagation of an aquatic plant Pogostemon erectus (Dalzell) Kuntze. Anatol. J. Bot. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Kam, M.Y.Y.; Chai, L.C.; Chin, C.F. The biology and in vitro propagation of the ornamental aquatic plant, Aponogeton ulvaceus. Springerplus 2016, 5, 01–11. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.M.; Ghosh, B. A submerged culture system for rapid micropropagation of the commercially important aquarium plant, ‘Amazon sword’ (Echinodorus ‘Indian Red’). Vitr. Cell. Dev. Biol.—Plant 2019, 55, 81–87. [Google Scholar] [CrossRef]
- Ramgareeb, S.; Snyman, S.J.; van Antwerpen, T.; Rutherford, R.S. Elimination of virus and rapid propagation of disease-free sugarcane (Saccharum spp. cultivar NCo376) using apical meristem culture. Plant Cell. Tissue Organ Cult. 2010, 100, 175–181. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, W.; Saha, M.C.; Udvardi, M.K.; Kang, Y. Improved node culture methods for rapid vegetative propagation of switchgrass (Panicum virgatum L.). BMC Plant Biol. 2021, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Shetty, N.P.; Giridhar, P.; Ravishankar, G.A. Rapid in vitro regeneration method for Moringa oleifera and performance evaluation of field grown nutritionally enriched tissue cultured plants. 3 Biotech 2012, 2, 187–192. [Google Scholar] [CrossRef]
- Balaraju, K.; Agastian, P.; Ignacimuthu, S.; Park, K. A rapid in vitro propagation of red sanders (Pterocarpus santalinus L.) using shoot tip explants. Acta Physiol. Plant 2011, 33, 2501–2510. [Google Scholar] [CrossRef]
- Trueman, S.J.; Hung, C.D.; Wendling, I. Tissue culture of Corymbia and Eucalyptus. Forests 2018, 9, 84. [Google Scholar] [CrossRef]
- Fan, B.; He, R.; Shang, Y.; Xu, L.; Wang, N.; Gao, H.; Liu, X.; Wang, Z. System construction of virus-free and rapid-propagation technology of Baodi garlic (Allium sativum L.). Sci. Hortic. 2017, 225, 498–504. [Google Scholar] [CrossRef]
- Armas, I.; Pogrebnyak, N.; Raskin, I. A rapid and efficient in vitro regeneration system for lettuce (Lactuca sativa L.). Plant Methods 2017, 13, 58. [Google Scholar] [CrossRef]
- Sariçam, Ş.; Kantoğlu, K.Y.; Ellialtioğlu, Ş.Ş. Tissue Culture Applications in Lettuce (Lactuca sativa L.). Eurasian J. Agric. Res. 2014, 1, 88–95. [Google Scholar]
- Pandino, G.; Lombardo, S.; Monaco, A.L.; Ruta, C.; Mauromicale, G. Mycorrhizal Inoculation Improves Plant Growth and Yield of Micropropagated Early Globe Artichoke under Field Conditions. Agriculture 2022, 12, 114. [Google Scholar] [CrossRef]
- Hickey, M.; King, C. The Cambridge Illustrated Glossary of Botanical Terms; Cambridge University Press: Cambridge, UK, 2001; Volume 208. [Google Scholar]
- Jang, C.S.; Kamps, T.L.; Skinner, D.N.; Schulze, S.R.; Vencill, W.K.; Paterson, A.H. Functional classification, genomic organization, putatively cis-acting regulatory elements, and relationship to quantitative trait loci, of sorghum genes with rhizome-enriched expression. Plant Physiol. 2006, 142, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. Plant form: An Illustrated Guide to Flowering Plant Morphology; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Mishra, S. Plant Reproduction; Discovery Publishing House: Delhi, India, 2005; pp. 120–125. ISBN 978-81-7141-955-5. [Google Scholar]
- Longman, K.A.; Wilson, R.H.F. Tropical Trees: Propagation and Planting Manuals, Rooting Cuttings of Tropical Trees, 1st ed.; Commonwealth Science Council: London, UK, 2002; p. 138. ISBN 978-0-85092-394-0. [Google Scholar]
- Mauseth, J.D. Botany: An Introduction to Plant Biology, 5th ed.; Sudbury, M.A., Ed.; Jones and Bartlett Learning: Burlington, MA, USA, 2012; p. 672. ISBN 978-1-4496-6580-7. [Google Scholar]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissue cultures in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–131. [Google Scholar] [PubMed]
- Hasegawa. In vitro propagation of rose (Tissue culture). HortScience 1979, 14, 610–612. [Google Scholar] [CrossRef]
- VanDerZanden, A.M. How Hormones and Growth Regulators Affect your Plants|OSU Extension Service. 2012. Available online: https://extension.oregonstate.edu/gardening/techniques/how-hormones-growth-regulators-affect-your-plants (accessed on 13 January 2022).
- Curaba, J.; Singh, M.B.; Bhalla, P.L. miRNAs in the crosstalk between phytohormone signalling pathways. J. Exp. Bot. 2014, 65, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol.—Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Rost, T.L.; Barbour, M.G.; Thornton, R.M.; Weier, T.E.; Stocking, C.R. Botany: A Brief Introduction to Plant Biology; Wiley: Hoboken, NJ, USA, 1984; ISBN 9780471874546. [Google Scholar]
- Bisht, T.S.; Rawat, L.; Chakraborty, B.; Yadav, V. A Recent Advances in Use of Plant Growth Regulators (PGRs) in Fruit Crops—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1307–1336. [Google Scholar] [CrossRef]
- Batool, Z.; Arash, F.; Zahra, T. Salicylic Acid In Reducing Effect of Salinity on Some Growth Parameters of Black Cumin (Nigella sativa). J. Plant Process Funct. 2019, 8, 287–298. [Google Scholar]
- El-Keltawi, N.; Barham, I.H.; El-Naggar, A.I.; Rekaby, A.F. Investigation on the responses of cumin plants to certain horticultural agro-chemicals. 2.-influences of gibberellic acid, GA3 on foliage growth, fruit yield, essential oil and chemical composition. Egypt. J. Hortic. 2000, 27, 459–478. [Google Scholar]
- Reddy, P.P.; Hore, J.K. Role of Growth Regulators on Fenugreek (Trigonella foenum-graecum L.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 25–32. [Google Scholar] [CrossRef]
- Khatoon, R.; Moniruzzaman, M.; Moniruzzaman, M. Effect of foliar spray of GA3 and NAA on sex expression and yield of bitter gourd. Bangladesh J. Agric. Res. 2019, 44, 281–290. [Google Scholar] [CrossRef]
- Reja, M.S.; Sikder, S.; Hasan, M.A.; Pramanik, S.K. Effect of Gibberellic Acid (GA3) on Morpho-physiological Traits and Yield Performance of Chickpea (Cicer arietinum L.). IOSR J. Agric. Vet. Sci. 2020, 13, 20–28. Available online: https://www.researchgate.net/publication/344042940_Effect_of_Gibberellic_Acid_GA3_on_Morpho-physiological_Traits_and_Yield_Performance_of_Chickpea_Cicer_arietinum_L#fullTextFileContent (accessed on 28 September 2024).
- Ashwini, A.; Pm, M.; Kulkarni, B.S.; Kumar, R.; Taj, A.; Kumar, M.S. Effect of plant growth regulators on vegetative and flowering parameters of gladiolus (Gladiolus hybridus L.) cv. adigo yellow. Int. J. Chem. Stud. 2019, 7, 1553–1556. [Google Scholar]
- Geetha, K.; Sadewarte, K.T.; Mahorkar, V.K.; Joshi, P.S.; Deo, D.D. A note on the effect of foliar application of plant growth regulators on seed yield in China aster. Orissa J. Hortic. 2000, 28, 113–114. [Google Scholar]
- Dalai, S.; Singh, M.K.; Singh, K.; Kumar, M.; Malik, S.; Kumar, V. Effect of Foliar Application of GA 3 and NAA on Growth, Flower-Ing Yield and Yield Attributes of Cucumber [Cucumis Sativus L.]). Ann. Hortic. 2015, 8, 181. [Google Scholar] [CrossRef]
- Aryal, S. Micropropagation of plants. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Springer: Heidelberg, Germany, 2019; pp. 329–346. [Google Scholar]
- Yeole, M.P.; Ghosle, Y.N.; Gurunani, S.G.; Dhole, S.M. Plant Tissue Culture Techniques: A Review for Future View. Crit. Rev. Pharm. Sci. 2016, 5, 16–24. [Google Scholar]
- Gaikwad, A.V.; Singh, S.K.; Gilhotra, R. Plant Tissue Culture—A Review. SGVU J. Pharm. Res. Educ. 2017, 2, 217–220. [Google Scholar]
- Horn, W.; Schlegel, G.; John, K. Micropropagation of Roses (ROSA HYBR.). Acta Hortic. 1988, 226, 623–630. [Google Scholar] [CrossRef]
- Bhojwani, S.S. Plant Tissue Culture: Applications and Limitations; Elsevier: Amsterdam, Netherlands, 1990; Volume 461. [Google Scholar]
- Struik, P.C.; Wiersema, S.G. Seed Potato Technology; Wageningen Acdemic Publishers: Wageningen, The Netherlands, 1999. [Google Scholar]
- Naik, P.; Chakrabarti, S.; Sarkar, D.; Birhman, R. Potato Biotechnology: Indian Perspective; Indian Potato Association: Shimla, India, 2000; pp. 194–211. [Google Scholar]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Street, H.E. Growth in Organised and Unorganised Systems, 5th ed.; Steward, F.C., Ed.; Plant physiology; Press NYA: New York, NY, USA, 1969; pp. 3–224. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- White, P.R. Nutrient deficiency studies and improved inorganic nutrients for cultivation of excised tomato roots. Growth 1963, 7, 53–65. [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension culture of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.I.; Short, K.C. Improved growth of tissue cultures of the onion Allium cepa. Physiol. Plant 1977, 41, 70–77. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Comb. Proc. Int. Plant Prop. Soc. 1980, 30, 421–427. [Google Scholar]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstocks. HortScience 1984, 18, 506–509. [Google Scholar] [CrossRef]
- Greenway, M.B.; Phillips, I.C.; Lloyd, M.N.; Hubstenberger, J.F.; Phillips, G.C. A nutrient medium for diverse applications and tissue growth of plant species in vitro. In Vitro Cell Dev. Biol.–Plant 2012, 48, 403–410. [Google Scholar] [CrossRef]
- Herman, E.B. Recent advances in plant tissue culture XXI. In Media and Techniques for Growth, Regeneration and Storage; Agritech Consultants Inc.: Shrub Oak, NY, USA, 2015; p. 148. [Google Scholar]
- Singh, P.R.; Singh, L.J. In vitro propagation for improvement of medicinal plants: A review. J. Pharmacogn. Phytochem. 2021, 10, 1484–1489. [Google Scholar]
- Torres, K.C. Tissue Culture Techniques for Horticultural Crops; Springer: Boston, MA, USA, 1988; p. 224. ISBN 978-1-4615-9758-2. [Google Scholar]
- Abobkar, I.M.S.; Elshahed, A.M. Plant Tissue Culture Media. Recent Adv. Plant Vitr. Cult. 2012, 2, 29–40. [Google Scholar] [CrossRef]
- Debergh, P.C.; Read, P.E. Micropropagation; Springer: Dordrecht, The Netherlands, 1991; pp. 1–13. [Google Scholar]
- George, E.F. Plant Propagation by Tissue Culture: The Technology, 2nd ed.; Exegetics: Worcester, UK, 1993. [Google Scholar]
- Nguyen, S.K.; Sophonputtanaphoca, S.; Kim, E.; Penner, M.H. Hydrolytic Methods for the Quantification of Fructose Equivalents in Herbaceous Biomass. Appl. Biochem. Biotechnol. 2009, 158, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Ohira, K.; Makoto, I.; Ojima, K. Thiamine requirements of various plant cells in suspension culture. Plant Cell Physiol. 1976, 17, 583–590. [Google Scholar]
- Vasil, I.K.; Thorpe, T.A. Plant Cell and Tissue Culture, 1st ed.; Kluwer Academic: Norwell, MA, USA, 1994. [Google Scholar]
- Alm, J.J.; Qian, H.; Le Blanc, K. Clinical Grade Production of Mesenchymal Stromal Cells. In Tissue Engineering, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 427–469. [Google Scholar] [CrossRef]
- Bhatia, N. 7 Organic Supplements Used in Plant Tissue Culture Media. Lab Assocciates. 2021. Available online: https://labassociates.com/8-organic-supplements-used-in-plant-tissue-culture-media (accessed on 8 March 2022).
- Thejaswini, R.; Narasimhan, S. Undefined Organic Additives Stimulates in Vitro Seed Germination of Dendrobium Ovatum (Willd.) Kraenzl, a Medicinal Orchid. Int. J. Pharma Med. Biol. Sci. 2017, 6, 29–31. [Google Scholar] [CrossRef]
- Fridborg, G.; Eriksson, T. Effects of activated charcoal on growth and morphogenesis in cell cultures. Physiol. Plant 1975, 34, 306–308. [Google Scholar] [CrossRef]
- Fridborg, G.; Pederson, M.; Landstrom, L.; Eriksson, T. The effect of activated charcoal on tissue cultures: Adsorption of metabolites inhibiting morphogenesis. Physiol. Plant 1978, 43, 104–106. [Google Scholar] [CrossRef]
- Wang, W.C.; Yung, Y.L.; Lacis, T.M.; Hansen, J.E. Greenhouse effects due to man-made perturbation of trace gases. Science 1976, 194, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Anagnostakis, S.L. Haploid plants from anthers of tobacco enhancement with charcoal. Planta 1974, 115, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.; Taha, R.M.; Noor, N.N.M.; Alimon, H. Potential of alternative gelling agents in media for the in vitro micro-propagation of Celosia sp. Int. J. Bot. 2011, 7, 183–188. [Google Scholar] [CrossRef][Green Version]
- Cohen, D. The culture medium. Acta Hort. 1995, 393, 15–24. [Google Scholar] [CrossRef]
- Kumar, A.; Palni, L.M.S.; Nandi, S.K. The effect of light source and gelling agent on micropropagation of Rosa damascenes Mill. and Rhynchostylis retusa L.) Bl. J. Hort. Sci. Biotech 2003, 78, 786–792. [Google Scholar] [CrossRef]
- MacRae, S.; Van Staden, J. In vitro culture of Eucalyptus grandis: Effect of gelling agents on propagation. J. Plant Physiol. 1990, 137, 249–251. [Google Scholar] [CrossRef]
- Savvas, D. Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. J. Food Agric. Environ. 2003, 1, 80–86. [Google Scholar]
- Gruda, N. Do Soilless Culture Systems Have an Influence on Product Quality of Vegetables. J. Appl. Bot. Food Qual. 2009, 82, 141–147. Available online: https://www.researchgate.net/publication/237821108_Does_soilless_culture_have_an_influence_on_product_quality_of_vegetables (accessed on 28 September 2024).
- Olubanjo, O.O.; Alade, A.E. Growth and Yield Response of Tomato Plants Grown under Different Substrates Culture. J. Sustain. Technol. 2018, 9, 110–123. [Google Scholar]
- Syed, A.-U.-A.; Khan, Z.A.; Chattha, S.H.; Shaikh, I.A.; Ali, M.N.H.A.; Bughio, Z.-U.-R.; Dahri, S.H.; Buriro, G.B. Comparative Assessment of Hydroponic and Geoponic Cultivation Systems for Sustainable Spinach Cultivation. Pak. J. Agric. Res. 2021, 34, 678–688. [Google Scholar] [CrossRef]
- Naville, E.H. The Temple of Deir El-Bahari (Parts I–III); Memoirs of the Egypt Exploration Fund: London, UK, 1913; Volume 16, pp. 12–17. [Google Scholar]
- Tagle, S.; Benoza, H.; Pena, R.; Oblea, A. Development of an Indoor Hydroponic Tower for Urban Farming. In Proceedings of the DLSU Innovation and Technology Fair, Manila, Philippines, 22–23 November 2018; pp. 1–13. [Google Scholar]
- Okasha, A.M.; Eldib, E.M.; Elmetwalli, A.H.; Farooque, A.A.; Yaseen, Z.M.; Elsayed, S. Maximization of Water Productivity and Yield of Two Iceberg Lettuce Cultivars in Hydroponic Farming System Using Magnetically Treated Saline Water. Agriculture 2022, 12, 101. [Google Scholar] [CrossRef]
- Benzle, K.; Cornish, K. Improved axenic hydroponic whole plant propagation for rapid production of roots as transformation target tissue. Plant Methods 2017, 13, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.K.; Sabir, A. Effects of Different Storage Conditions on Rooting and Shooting Performance of Grapevine (Vitis vinifera L.) Cuttings in Hydroponic Culture System. Int. J. Sustain. Agric. Res. 2018, 5, 46–53. [Google Scholar] [CrossRef]
- Ritter, E.; Angulo, B.; Riga, P.; Herrán, C.; Relloso, J.; San Jose, M. Comparison of hydroponic and aeroponic cultivation systems for the production of potato minitubers. Potato Res. 2001, 44, 127–135. [Google Scholar] [CrossRef]
- Farran, I.; Mingo-Castel, A.M. Potato minituber production using aeroponics: Effect of plant density and harvesting intervals. Am. J. Potato Res. 2006, 83, 47–53. [Google Scholar] [CrossRef]
- Tokunaga, H.; Anh, N.H.; Van Dong, N.; Ham, L.H.; Hanh, N.T.; Hung, N.; Ishitani, M.; Tuan, L.N.; Utsumi, Y.; Vu, N.A.; et al. An efficient method of propagating cassava plants using aeroponic culture. J. Crop Improv. 2020, 34, 64–83. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Development of an in vitro hydroponic culture system for wasabi nursery plant production—Effects of nutrient concentration and supporting material on plantlet growth. Sci. Hortic. 2019, 245, 237–243. [Google Scholar] [CrossRef]
- Angel, R.D.V.-E.; Kantar, M.B.; Branca, J.; Moore, S.; Frederiksen, M.K.; Hagen, L.; Hussain, T.; Baumler, D.J. Aeroponic cloning of Capsicum spp. Horticulturae 2019, 5, 30. [Google Scholar] [CrossRef]
- Srikanth, S.; Choong, T.W.; Yan, A.; He, J.; Chen, Z. An efficient method for adventitious root induction from stem segments of brassica species. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oakes, A.D.; Kazcmar, N.A.; Maynard, C.A.; Powell, V.A. Vegetative Propagation of American Elm (Ulmus Americana) Varieties from Softwood Cuttings. J. Environ. Hortic. 2012, 30, 73–76. [Google Scholar] [CrossRef]
- Peterson, B.J.; Burnett, S.E.; Sanchez, O. Submist is effective for propagation of korean lilac and inkberry by stem cuttings. Horttechnology 2018, 28, 378–381. [Google Scholar] [CrossRef]
- Choudhary, P.; Kataria, V. In vitro culture in combination with aeroponics is an efficient means of mass propagation of Sarcostemma acidum: A rare medicinal plant of Indian arid zone. In Vitro Cell. Dev. Biol.—Plant 2022, 58, 372–381. [Google Scholar] [CrossRef]
- Mehandru, P.; Shekhawat, N.S.; Rai, M.K.; Kataria, V.; Gehlot, H.S. Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica—Three threatened medicinal Asclepiads. Physiol. Mol. Biol. Plants 2014, 20, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Maroya, N.; Balogun, M.; Asiedu, R.; Aighewi, B.; Kumar, P.L.; Augusto, J. Yam Propagation Using ‘Aeroponics’ Technology. Annu. Res. Rev. Biol. 2014, 4, 3894–3903. [Google Scholar] [CrossRef]
- Pala, M.; Mizenko, L.; Mach, M.; Reed, T. Aeroponic Greenhouse as an Autonomous System Using Intelligent Space for Agriculture Robotics. In Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2014; Volume 274, pp. 83–93. ISBN 9783319055817. [Google Scholar] [CrossRef]
- Ferentinos, K.P.; Albright, L.D.; Selman, B. Neural network-based detection of mechanical, sensor and biological faults in deep-trough hydroponics. Comput. Electron. Agric. 2003, 40, 65–85. [Google Scholar] [CrossRef]
- Francis, F.; Vishnu, P.L.; Jha, M.; Rajaram, B. IOT-based automated aeroponics system. Lect. Notes Electr. Eng. 2018, 492, 337–345. [Google Scholar] [CrossRef]
- Gopinath, P.; Vethamoni, P.I.; Gomathi, M. Aeroponics Soilless Cultivation System for Vegetable Crops. Chem. Sci. Rev. Lett. 2017, 6, 838–849. [Google Scholar]
- Barker, B.T.P. Studies on root development. Long Asht. Res. Stn. Ann. Rep. 1921, 9, 9–57. [Google Scholar]
- Eshel, A.; Grünzweig, J.M. Root-shoot allometry of tropical forest trees determined in a large-scale aeroponic system. Ann. Bot. 2012, 112, 291–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bohme, M.; Pinker, I. Asian leafy vegetables and herbs cultivated in substrate culture and aeroponics in greenhouse. Acta Hortic. 2014, 1034, 155–162. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Lakhiar, I.A.; Solangi, K.A.; Qureshi, W.A.; Shaikh, S.A.; Chen, J. Influence of Atomization Nozzles and Spraying Intervals on Growth, Biomass Yield, and Nutrient Uptake of Butter-Head Lettuce under Aeroponics System. Agronomy 2021, 11, 97. [Google Scholar] [CrossRef]
- Rykaczewska, K. The potato minituber production from microtubers in aeroponic culture. Plant Soil Environ. 2016, 62, 210–214. [Google Scholar] [CrossRef]
- Scoggins, H.L.; Mills, H.A. Poinsettia growth, tissue nutrient concentration, and nutrient uptake as influenced by nitrogen form and stage of growth. J. Plant Nutr. 2008, 21, 191–198. [Google Scholar] [CrossRef]
- Casillas, A. Propagation of Cannabis sativa for commercial production. Acta Hortic. 2017, 1174, 157–158. [Google Scholar] [CrossRef]
- Briggs, B.A. An experiment in air-rooting. Int. Plant Propagator’s Soc. 1966, 16, 139–140. [Google Scholar]
- Nir, I. Growing plants in aeroponics growth system. Acta Hortic. 1982, 126, 435–448. [Google Scholar] [CrossRef]
- Stoner, R.J. Rooting in Air. Greenh. Grow. 1983, 11, 1–7. [Google Scholar]
- Soffer, H.; Burger, D.W. Effects of Dissolved Oxygen Concentrations in Aero-hydroponics on the Format ion and Growth of Adventitious Roots. J. Am. Soc. Hortic. Sci. 1988, 113, 218–221. [Google Scholar] [CrossRef]
- Stoner, R.J. Aeroponics Versus Bed and Hydroponic Propagation. Florists’ Rev. 1983, 173, 4477. [Google Scholar]
- Molitor, H.; Fischer, M. Effect of several parameters on the growth of chrysanthemum stock plants in aeroponics. Acta Hortic. 1999, 481, 179–186. [Google Scholar] [CrossRef]
- Stoner, R.; Clawson, J. A High Performance, Gravity Insensitive, Enclosed Aeroponic System for Food Production in Space; NASA Small Business Innovation Research (SBIR) Program, Report Number NAS10-98030, 1998. Available online: https://legacy.www.sbir.gov/content/high-performance-gravity-insensitive-enclosed-aeroponic-system-food-production-space-0 (accessed on 28 September 2024).
- Hussain Tunio, M.; Gao, J.; Ahmed Qureshi, W.; Ali Sheikh, S.; Chen, J.; Ali Chandio, F.; Ali Lakhiar, I.; Ali Solangi, K. Effects of droplet size and spray interval on root-to-shoot ratio, photosynthesis efficiency, and nutritional quality of aeroponically grown butterhead lettuce. Int. J. Agric. Biol. Eng. 2022, 15, 79–88. [Google Scholar] [CrossRef]
- Sharma, U.; Barupal, M.; Shekhawat, N.; Kataria, V. Aeroponics for propagation of horticultural plants: An approach for vertical farming. Hortic. Int. J. 2018, 2, 443–444. [Google Scholar] [CrossRef]
- Rodriguez Julian, F.M.; de Haan, S.; Piedra, J.L.A.; Maldonado, L.; Hareau, G.; Barker, I. Technical and Economic Analysis of Aeroponics and other Systems for Potato Mini-Tuber Production in Latin America. Am. J. Potato Res. 2013, 90, 357–368. [Google Scholar] [CrossRef]
- Hussey, G.; Stacey, N.J. In Vitro Propagation of Potato (Solanum tuberosum L.). Ann. Bot. 1981, 48, 787–796. [Google Scholar] [CrossRef]
- Nichols, M.A. Aeroponics and Potatoes. Acta Hortic. 2005, 670, 201–206. [Google Scholar] [CrossRef]
- Stoner, R.; Schorr, S. Aeroponics versus bed and hydroponic propagation “The process of propagating and growing plants in air”. Florists’ Rev. 1985, 173, 49–51. [Google Scholar]
- Tsoka, O.; Demo, P.; Nyende, A.B.; Kamau, N. Seed Production of Selected Potato (Solanum tuberosum L.) Clones under Aeroponic Conditions; Jomo Kenyetta University of Agriculture and Technology: Nairobi, Kenya, 2008. [Google Scholar]
- Hayden, A.L.; Brigham, L.A.; Giacomelli, G.A. Aeroponic cultivation of ginger (Zingiber officinale) rhizomes. Acta Hortic. 2004, 659, 397–402. [Google Scholar] [CrossRef]
- Xu, Y.; Marron, M.T.; Seddon, E.; McLaughlin, S.P.; Ray, D.T.; Whitesell, L.; Leslie Gunatilaka, A.A. 2,3-Dihydrowithaferin A-3beta-O-sulfate, a new potential prodrug of withaferin A from aeroponically grown with Ania somnifera. Bioorg. Med. Chem. 2009, 17, 2210–2214. [Google Scholar] [CrossRef]
- Pagliarulo, C.L.; Hayden, A. Potential for Greenhouse Aeroponic Cultivation of Medicinal Root Crops. 2010. Available online: https://www.semanticscholar.org/paper/POTENTIAL-FOR-GREENHOUSE-AEROPONIC-CULTIVATION-OF-Pagliarulo-Hayden/0e07ef58d004cf47765b27e765f9bec5d6e3ad0c (accessed on 2 February 2022).
- Hayden, A.L. Aeroponic and hydroponic systems for medicinal herb, rhizome, and root crops. Hortscience 2006, 41, 536–538. [Google Scholar] [CrossRef]
- Salachas, G.; Savvas, D.; Argyropoulou, K.; Tarantillis, P.A.; Kapotis, G. Yield and nutritional quality of aeroponically cultivated basil as affected by the available root-zone volume. Emirates J. Food Agric. 2015, 27, 911–918. [Google Scholar] [CrossRef]
- Kumari, A.; Baskaran, P.; Chukwujekwu, J.C.; de Kock, C.A.; Smith, P.J.; Van Staden, J. The changes in morphogenesis and bioactivity of Tetradenia riparia, Mondia whitei and Cyanoptis speciosa by an aeroponic system. Ind. Crops Prod. 2016, 84, 199–204. [Google Scholar] [CrossRef]
- Haque, M.I.; Singh, P.K.; Ghuge, S.; Kumar, A.; Chandra Rai, A.; Kumar, A.; Modi, A. A general introduction to and background of plant tissue culture: Past, current, and future aspects. In Advances in Plant Tissue Culture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–30. ISBN 9780323907958. [Google Scholar]
- Rihan, H.; Kareem, F.; El-Mahrouk, M.; Fuller, M. Artificial Seeds (Principle, Aspects and Applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef]
- Peterson, B.J.; Sanchez, O.; Burnett, S.E.; Hayes, D.J. Comparison of four systems for propagation of coleus by stem cuttings. Horttechnology 2018, 28, 143–148. [Google Scholar] [CrossRef]
- Roberto, S.R.; Colombo, R.C. Innovation in Propagation of Fruit, Vegetable and Ornamental Plants. Horticulturae 2020, 6, 23. [Google Scholar] [CrossRef]
- Kakuhenzire, R.; Otazu, V.; Tibanyendera, D.; Kashaija, I.N.; Lemaga, B.; Kimoone, G.; Kesiime, V.E.; Ortiz, O.; Barker, I. Improving minituber production from tissue-cultured potato plantlets with aeroponic technology. Int. J. Agric. Environ. Res. 2017, 234–241. [Google Scholar]
- Joe, M. Signs That Your Plants May Be Struggling with Incorrect EC. Blog by Bluelab. 2021. Available online: https://blog.bluelab.com/signs-of-incorrect-ec-plants#:~:text=EC is really important%2C as,will have a nutrient deficiency (accessed on 27 July 2022).
- Sumarni, E.; Farid, N.; Darjanto, D.; Ardiansyah, A.; Soesanto, L. Effect of electrical conductivity (EC) in the nutrition solution on aeroponic potato seed production with root zone cooling application in tropical Lowland, Indonesia. Agric. Eng. Int. CIGR J. 2019, 21, 70–77. [Google Scholar]
- da Silva Filho, J.B.; Fontes, P.C.R.; Cecon, P.R.; McGiffen, M.E. Evaluation of “UFV Aeroponic System” to Produce Basic Potato Seed Minitubers. Am. J. Potato Res. 2018, 95, 443–450. [Google Scholar] [CrossRef]
- Abdullateef, S.; Böhme, M.H.; Pinker, I. Potato minituber production at different plant densities using an aeroponic system. Acta Hortic. 2012, 927, 429–436. [Google Scholar] [CrossRef]
- Regas, T.; Han, J.-H.; Pauli, C.S.; Park, S.-H. Employing Aeroponic Systems for the Clonal Propagation of Cannabis. J. Vis. Exp. 2021, 178, 102–117. [Google Scholar] [CrossRef]
- Rahman, M.H.; Islam, M.J.; Azad, M.O.K.; Rana, M.S.; Ryu, B.R.; Lim, Y.-S. LED Light Pre-Treatment Improves Pre-Basic Seed Potato (Solanum tuberosum L. cv. Golden King) Production in the Aeroponic System. Agronomy 2021, 11, 1627. [Google Scholar] [CrossRef]
- Calori, A.H.; Factor, T.L.; Feltran, J.C.; de Araujo, H.S.; Purquerio, L.F.V. Can nitrogen reduction be used to increase seed potato minituber production in an aeroponic system? J. Plant Nutr. 2022, 45, 1572–1581. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Jianmin, G.; Syed, T.N.; Chandio, F.A.; Buttar, N.A.; Qureshi, W.A. Monitoring and Control Systems in Agriculture Using Intelligent Sensor Techniques: A Review of the Aeroponic System. J. Sens. 2018, 2018, 8672769. [Google Scholar] [CrossRef]
- Torres-Tello, J.; Ko, S.-B. Interpretability of artificial intelligence models that use data fusion to predict yield in aeroponics. J. Ambient Intell. Humaniz. Comput. 2021, 14, 3331–3342. [Google Scholar] [CrossRef]
- Caya, M.V.C.; Cruz, J.C.D.; Estrella, A.M.C.; Mendoza, J.C.A. Fuzzy Controlled LED Lighting Compensation for Aeroponics System. In Proceedings of the 2021 IEEE 13th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Manila, Philippines, 28–30 November 2021; pp. 1–6. [Google Scholar]
- Salazar, J.D.R.; Becerra, C.J.E.; Velasco, F.E.H. Growing arugula plants using aeroponic culture with an automated irrigation system. Int. J. Agric. Biol. Eng. 2020, 13, 52–56. [Google Scholar] [CrossRef]
- Rahmad, F.I.; Tanti, L.; Puspasari, R.; Ekadiansyah, E.; Agung Fragastia, V. Automatic Monitoring and Control System in Aeroponic Plant Agriculture. In Proceedings of the 2020 8th International Conference on Cyber and IT Service Management (CITSM), Pangkal Pinang, Indonesia, 23–24 October 2020; pp. 1–5. [Google Scholar]
- Kerns, S.C.; Lee, J.-L. Automated Aeroponics System Using IoT for Smart Farming. Eur. Sci. J. 2017, 13, 10–15. [Google Scholar] [CrossRef]
- Jie, H.; Kong, L.S. Growth and photosynthetic responses of three aeroponically grown lettuce cultivars (Lactuca sativa L.) to different rootzone temperatures and growth irradiances under tropical aerial conditions. J. Hortic. Sci. Biotechnol. 1998, 73, 173–180. [Google Scholar] [CrossRef]
- Rahman, F.; Ritun, I.J.; Ahmed Biplob, M.R.; Farhin, N.; Uddin, J. Automated Aeroponics System for Indoor Farming using Arduino. In Proceedings of the 2018 Joint 7th International Conference on Informatics, Electronics & Vision (ICIEV) and 2018 2nd International Conference on Imaging, Vision & Pattern Recognition (icIVPR), Kitakyushu, Japan, 25–29 June 2018; pp. 137–141. [Google Scholar]
- Srivastava, D.; Kesarwani, A. Shivani Dubey Measurement of Temperature and Humidity by using Arduino Tool and DHT11. Int. Res. J. Eng. Technol. 2018, 876, 876–878. [Google Scholar]
- Resh, H.M. A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439878699. [Google Scholar]
- Teixeira, J.; Pereira, S. High salinity and drought act on an organ-dependent manner on potato glutamine synthetase expression and accumulation. Environ. Exp. Bot. 2007, 60, 121–126. [Google Scholar] [CrossRef]
- Trauger, M.; Hile, A.; Sreenivas, K.; Shouse, E.M.; Bhatt, J.; Lai, T.; Mohandass, R.; Tripathi, L.; Ogden, A.J.; Curtis, W.R. CO2 supplementation eliminates sugar-rich media requirement for plant propagation using a simple inexpensive temporary immersion photobioreactor. Plant Cell Tissue Organ Cult. 2022, 150, 57–71. [Google Scholar] [CrossRef]
- He, J.; Austin, P.T.; Lee, S.K. Effects of elevated root zone CO2 and air temperature on photosynthetic gas exchange, nitrate uptake, and total reduced nitrogen content in aeroponically grown lettuce plants. J. Exp. Bot. 2010, 61, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Lee, J.J.; Shin, J.; Kim, J.H.; Hong, S.; Kim, S. Resistive Water Level Sensors Based on AgNWs/PEDOT:PSS-g-PEGME Hybrid Film for Agricultural Monitoring Systems. ACS Omega 2022, 7, 15459–15466. [Google Scholar] [CrossRef]
- Sutulienė, R.; Laužikė, K.; Pukas, T.; Samuolienė, G. Effect of Light Intensity on the Growth and Antioxidant Activity of Sweet Basil and Lettuce. Plants 2022, 11, 1709. [Google Scholar] [CrossRef]
- Keim, R. What Is a Microcontroller? The Defining Characteristics and Architecture of a Common Component—Technical Articles. 2019. Available online: https://www.allaboutcircuits.com/technical-articles/what-is-a-microcontroller-introduction-component-characteristics-component/ (accessed on 9 December 2022).
- Khaing, K.K.; Srujan Raju, K.; Sinha, G.R.; Swe, W.Y. Automatic Temperature Control System Using Arduino; Springer: Singapore, 2020; pp. 219–226. [Google Scholar] [CrossRef]
- Chan, H.C.Y. Internet of Things Business Models. J. Serv. Sci. Manag. 2015, 8, 552–568. [Google Scholar] [CrossRef]
- Park, D.-H.; Kang, B.-J.; Cho, K.-R.; Shin, C.-S.; Cho, S.-E.; Park, J.-W.; Yang, W.-M. A Study on Greenhouse Automatic Control System Based on Wireless Sensor Network. Wirel. Pers. Commun. 2011, 56, 117–130. [Google Scholar] [CrossRef]
- Laksono, P.; Idris, I.; Sani, M.I.; Putra, D.N. Lab Prototype of Wireless Monitoring and Control for Seed Potatoes Growing Chamber. Proc. Asia-Pac. Adv. Netw. 2014, 37, 20. [Google Scholar] [CrossRef][Green Version]
- Anitha, P.; Periasamy, P.S. Energy Efficient Green House Monitoring in the Aeroponics System using Zigbee Networks. Asian J. Res. Soc. Sci. Humanit. 2016, 6, 2243. [Google Scholar] [CrossRef]
- NASA. NASA Spinoff; Progressive plant growing has business blooming. In Environmental and Agricultural Resources; NASA Spinoff: New York, NY, USA, 2006. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).