γ-Aminobutyric Acid Alleviates Salinity-Induced Impairments in Rice Plants by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice (Oryza sativa L.) Cultivation and Experimental Design
2.2. RNA Extraction, RNA-Seq, and Transcriptome Analysis
2.3. Chlorophyll Content, SPAD Value, Relative Growth Rate, and Leaf Photosynthesis
2.4. Stomatal Morphology
2.5. The Surface Area of Mesophyll Cells Exposed to Intercellular Airspace per Leaf Area (Sm) and the Surface Area of Chloroplasts Exposed to Intercellular Airspace per Leaf Area (Sc)
2.6. The Contents of GABA, ABA, Malondialdehyde, Proline, and Fructose Content
2.7. The Activities of Superoxide Dismutase, Peroxidase, and Catalase
2.8. The Contents of Na+ and K+
2.9. Statistical Analysis
3. Results
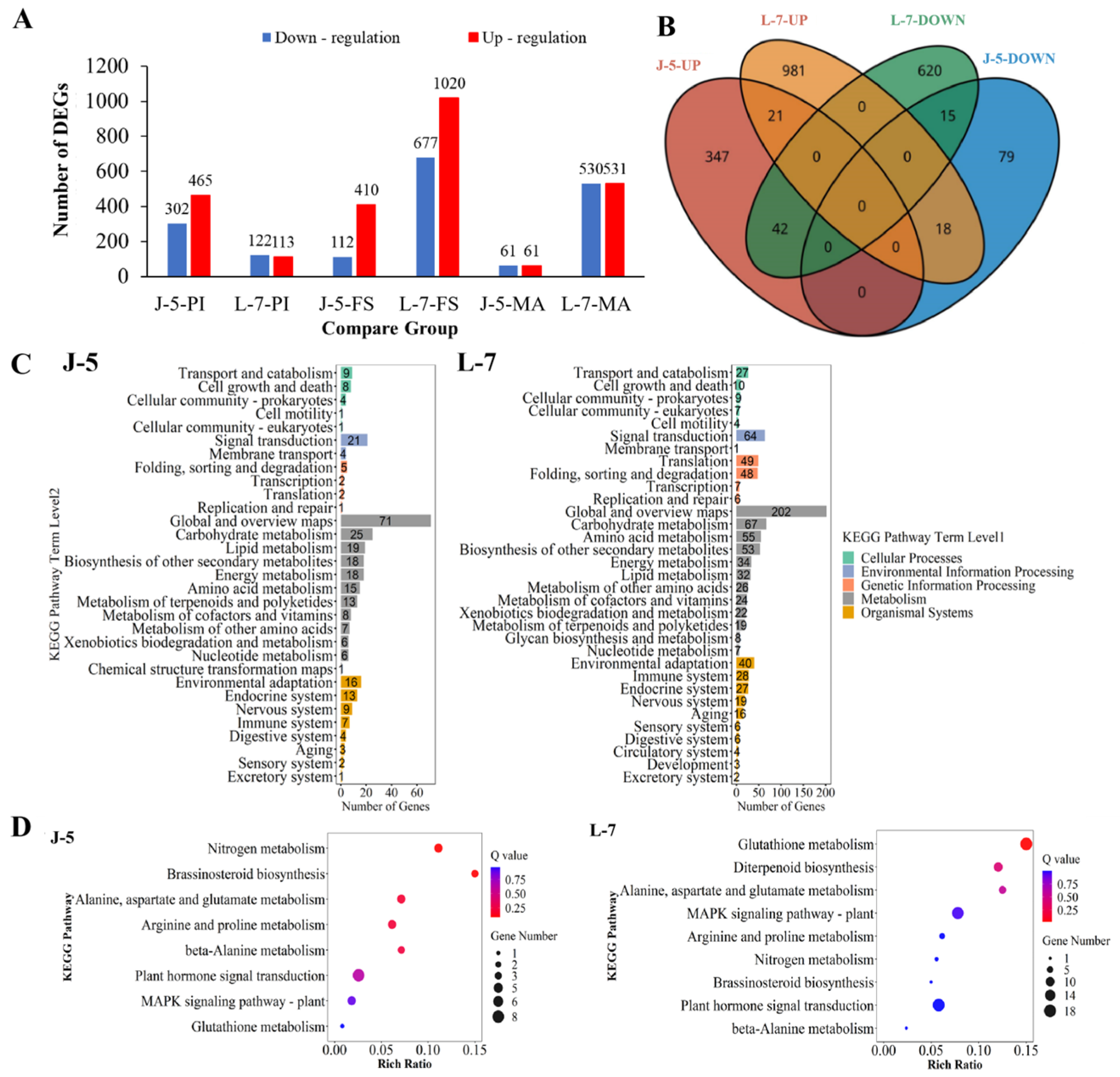
3.1. Transcriptomic Profiling of Rice Cultivars with Varying Salt Tolerance
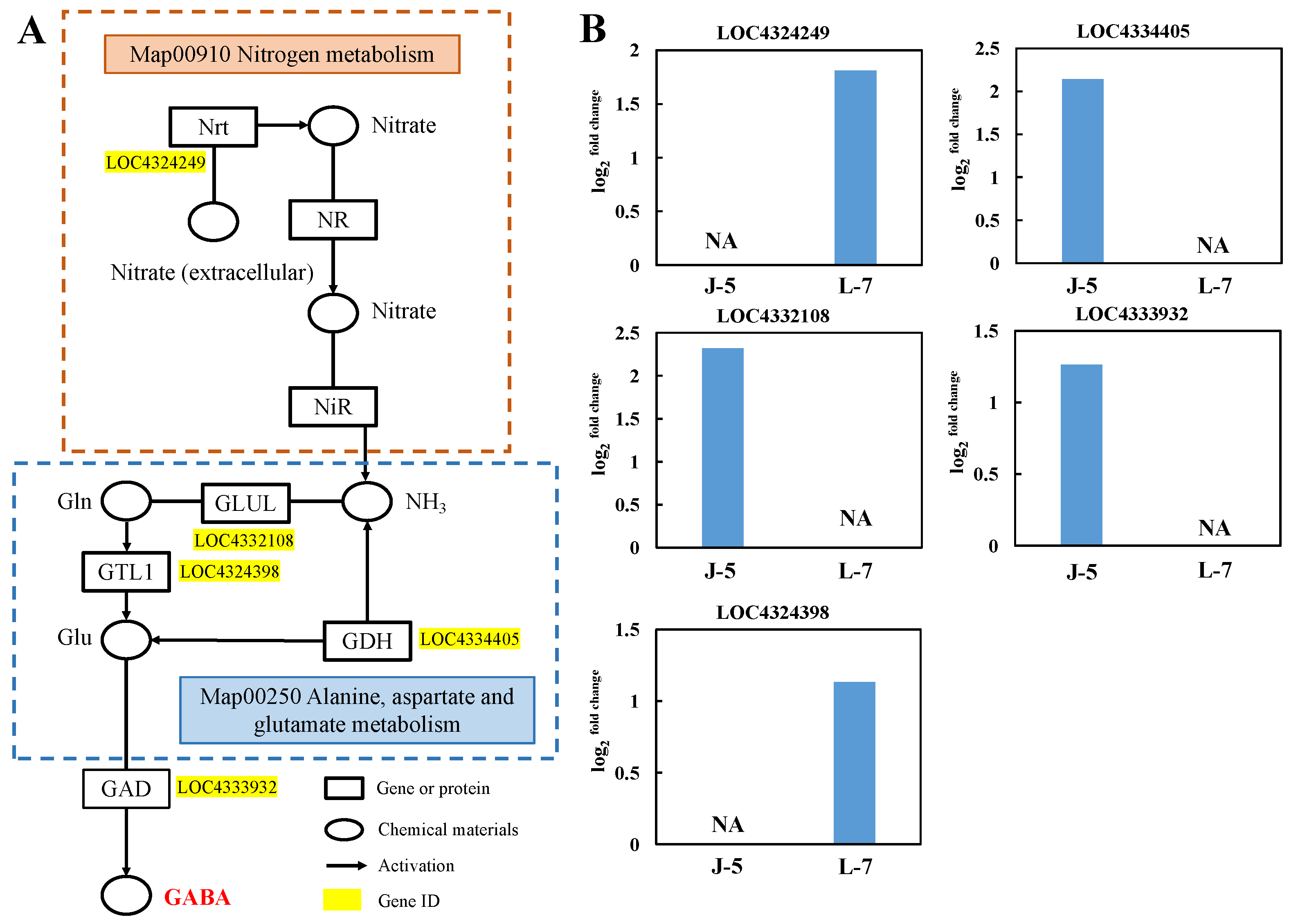
3.2. Analysis of Gene Expression of Metabolism of GABA
3.3. Grain Yield
3.4. Dry Matter Accumulation and Plant Growth Rate
3.5. Transpiration Rate and Photosynthesis
3.6. Chlorophyll Content and SPAD Value
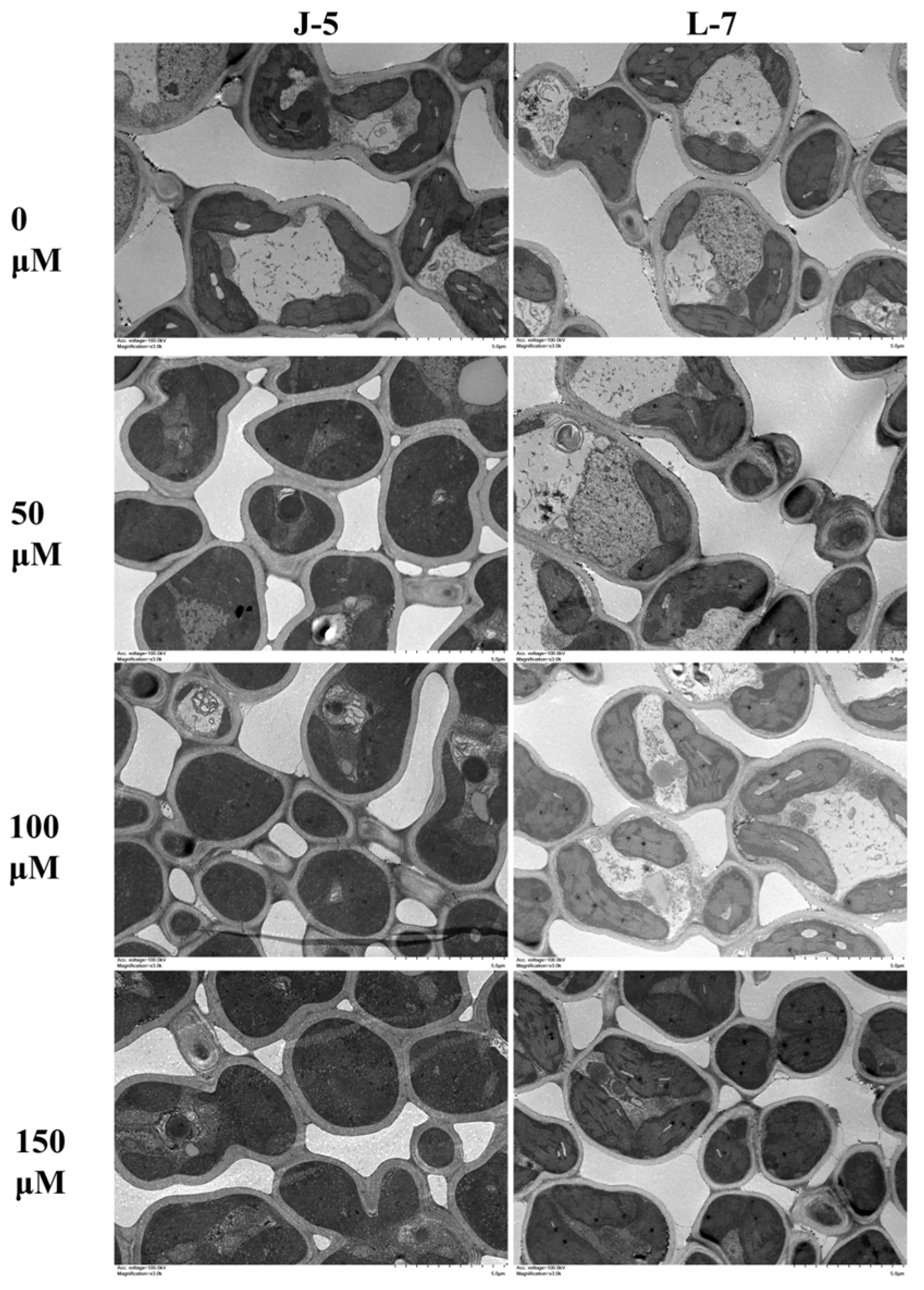
3.7. Stomatal Density and Size, and Leaf Anatomical Properties
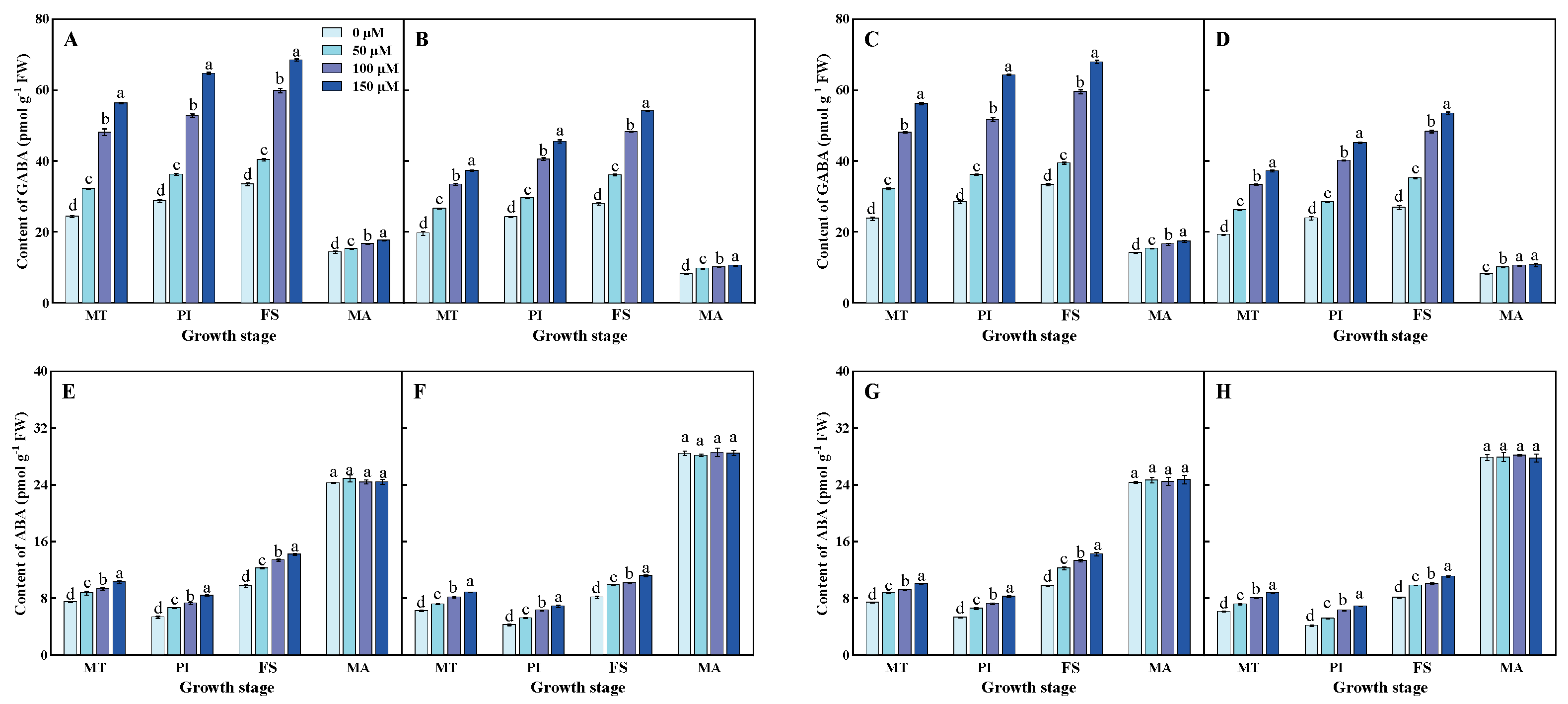
3.8. Contents of GABA and ABA in Flag Leaf
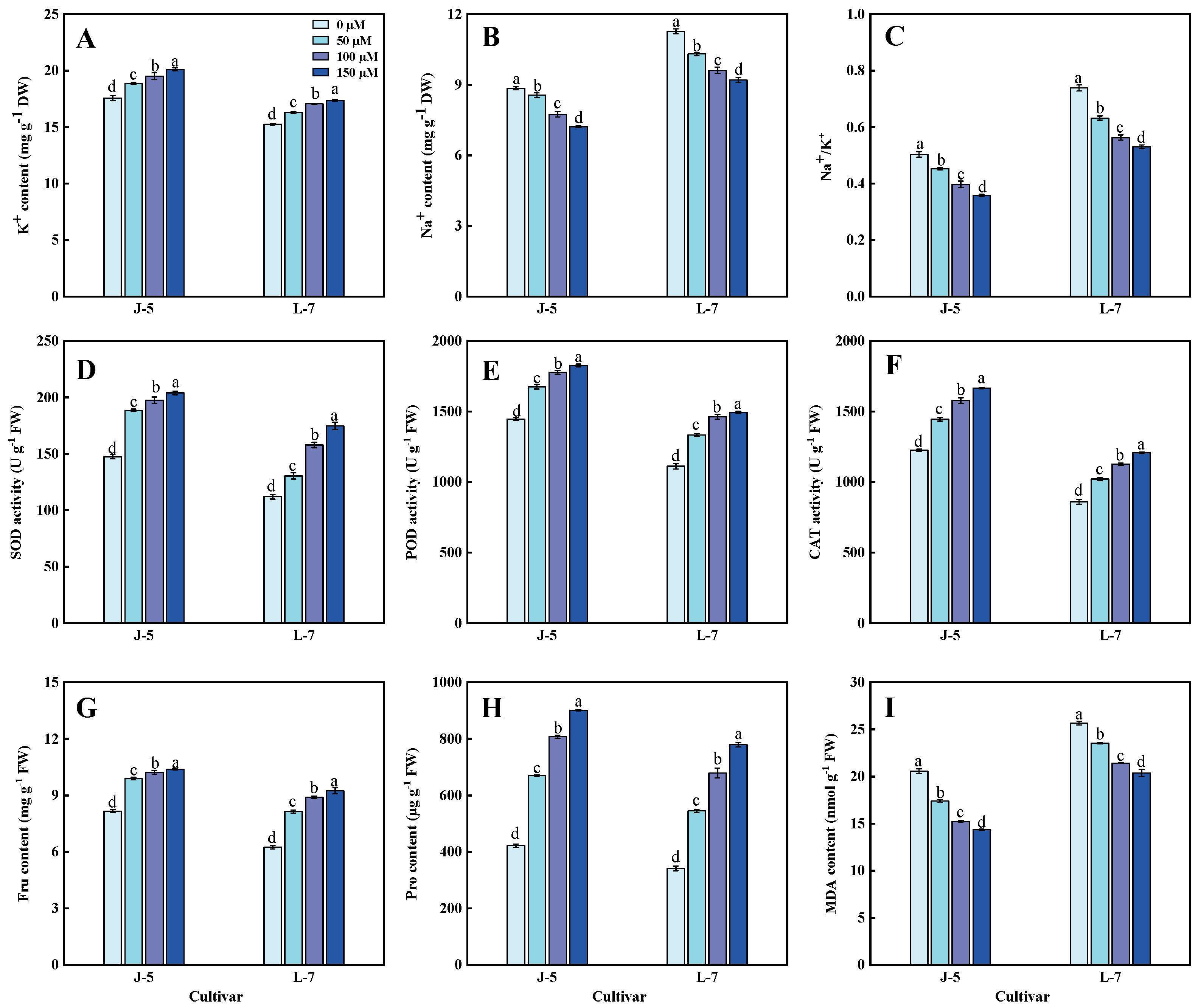
3.9. Contents of K+, Na+, Malondialdehyde, and Osmotic Adjustment Substances and Activities of Antioxidant Enzymes
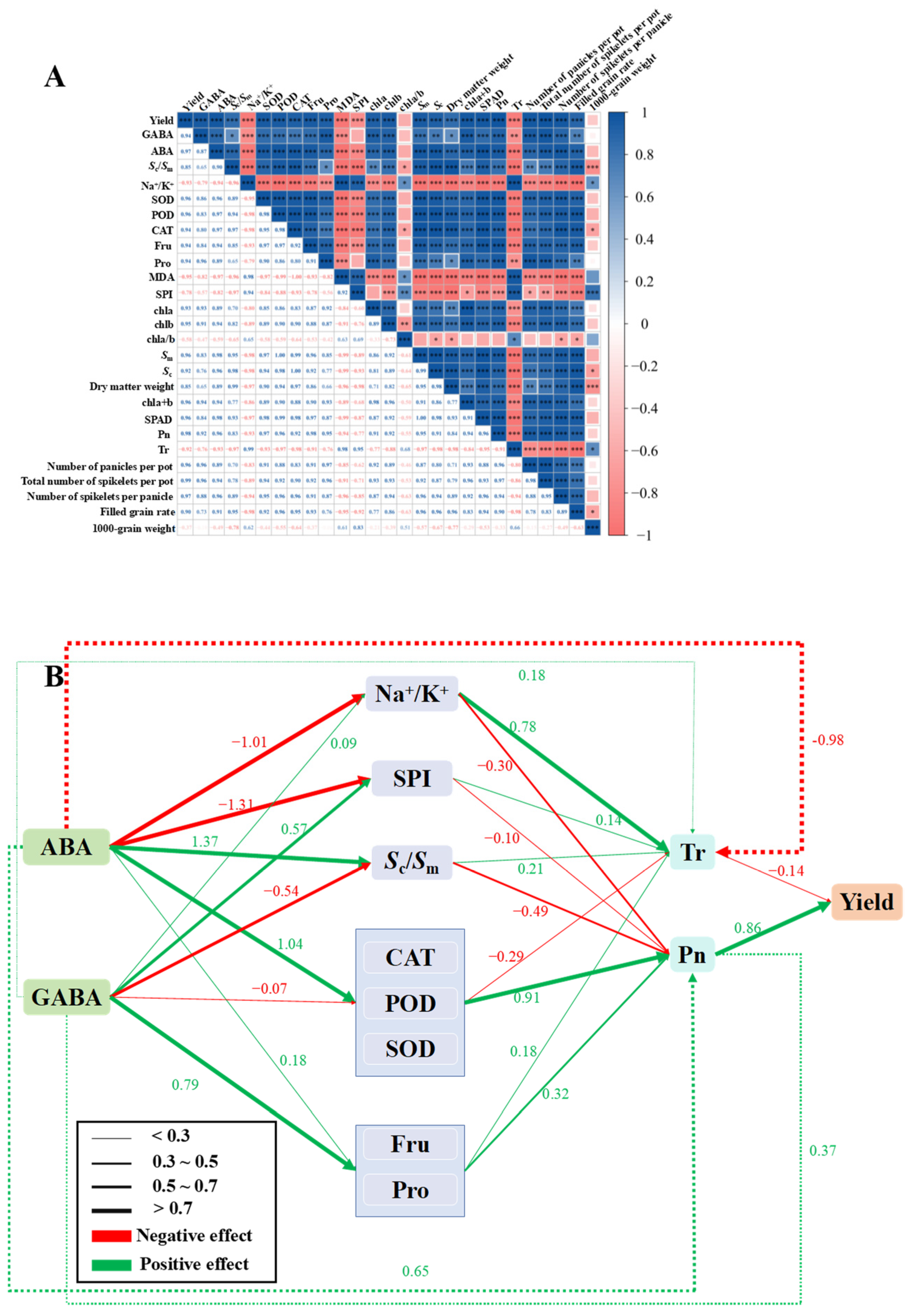
3.10. Correlation Analysis and Analysis of Structural Equation Model
4. Discussion
4.1. Effects of Genes Related to GABA Synthesis
4.2. Effects of Exogenous GABA Application on Rice Yield and Growth and Development
4.3. Exogenous GABA Regulates the Physiological and Biochemical Properties of Rice Under Salt Stress to Improve Salt Tolerance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.P.; Sun, J.; Huang, G. Optimizing water resources allocation and soil salinity control for supporting agricultural and environmental sustainable development in Central Asia. Sci. Total Environ. 2020, 704, 135281. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, T.; Lv, Z.; Yue, Y.; Liu, A.; Long, X.; Rengel, Z. The mechanisms of improving coastal saline soils by planting rice. Sci. Total Environ. 2020, 703, 135529. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, T.; Zhu, K.; Wang, W.; Zhang, W.; Zhang, H.; Yang, J. Effects of salt stress on grain yield and quality parameters in rice cultivars with differing salt tolerance. Plants 2023, 12, 3243. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Liu, L.; Tan, Y.; Sheng, X.; Yu, D.; Sun, Z.; Sun, X.; Chen, J.; Yuan, C.; et al. Effect of salinity stress on rice yield and grain quality: A meta-analysis. Eur. J. Agron. 2023, 144, 126765. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.K.; Yoon, I.S.; Kim, K.H.; Kwon, T.R. Salt tolerance in rice: Focus on mechanisms and approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, J.; Li, W.; Ding, Y.; Zhong, Q.; Xu, X.; Li, G. Exogenous melatonin alleviates salt stress by improving leaf photosynthesis in rice seedlings. Plant Physiol. Biochem. 2021, 163, 367–375. [Google Scholar] [CrossRef]
- Hussain, S.; Zhong, C.; Bai, Z.; Cao, X.; Zhu, L.; Hussain, A.; Zhu, C.; Fahad, S.; James, A.B.; Zhang, J.; et al. Effects of 1-methylcyclopropene on rice growth characteristics and superior and inferior spikelet development under salt stress. J. Plant Growth Regul. 2018, 37, 1368–1384. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.; Wang, Y.; Tian, Y.; Zheng, H.; Zhou, Z.; Liu, X. The protein phosphatase PC1 dephosphorylates and deactivates CatC to negatively regulate H2O2 homeostasis and salt tolerance in rice. Plant Cell 2023, 35, 3604–3625. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, W.; Lai, J.; Liu, Q.; Zhang, W.; Chen, Z.; Gao, J.; Song, S.; Liu, J.; Xiao, Y. OsGLYI3, a glyoxalase gene expressed in rice seed, contributes to seed longevity and salt stress tolerance. Plant Physiol. Biochem. 2022, 183, 85–95. [Google Scholar] [CrossRef]
- Song, T.; Shi, Y.; Shen, L.; Cao, C.; Shen, Y.; Jing, W.; Tian, Q.; Lin, F.; Li, W.; Zhang, W. An endoplasmic reticulum-localized cytochrome b5 regulates high-affinity K+ transport in response to salt stress in rice. Proc. Nat. Acad. Sci. USA 2021, 118, e2114347118. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Ponce, K.S.; Guo, L.; Leng, Y.; Meng, L.; Ye, G. Advances in sensing, response and regulation mechanism of salt tolerance in rice. Int. J. Mol. Sci. 2021, 22, 2254. [Google Scholar] [CrossRef]
- Ganie, S.A.; Molla, K.A.; Henry, R.; Bhat, K.V.; Mondal, T.K. Advances in understanding salt tolerance in rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef]
- Steward, F.C.; Thompson, J.F.; Dent, C.E. γ-Aminobutyric acid: A constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.D.; Abd El-Gawad, H.G.; El-Yazied, A.A.; Ibrahim, M.F.; Jahan, M.S.; et al. GABA: A key player in drought stress resistance in plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Gahlowt, P.; Singh, S.; Dubey, N.K.; Singh, S.P.; Tripathi, D.K.; Singh, V.P. GABA: A key player of abiotic stress regulation. Plant Signal. Behav. 2023, 18, 2163343. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, V.; Tanou, G.; Belghazi, M.; Diamantidis, G.; Molassiotis, A. Characterization of β-amino-and γ-amino butyric acid-induced citrus seeds germination under salinity using nanoLC–MS/MS analysis. Plant Cell Rep. 2017, 36, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, H.; Wang, S.; Guo, J.; Dou, H.; Qiao, J.; Yang, Q.; Shao, R.; Wang, H. Exogenous γ-aminobutyric acid (GABA) improves salt-inhibited nitrogen metabolism and the anaplerotic reaction of the tricarboxylic acid cycle by regulating GABA-shunt metabolism in maize seedlings. Ecotoxicol. Environ. Safe 2023, 254, 114756. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Ali, I.; Noor, J.; Zeng, F.; Bawazeer, S.; Eldin, S.M.; Asghar, M.A.; Javed, H.H.; Saleem, K.; Ullah, S.; et al. Exogenous γ-aminobutyric acid (GABA) mitigated salinity-induced impairments in mungbean plants by regulating their nitrogen metabolism and antioxidant potential. Front. Plant Sci. 2023, 13, 1081188. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Hewedy, O.A.; Battaglia, M.L.; Jalal, R.S.; Alhammad, B.A.; Al-Doss, A. Field crop responses and management strategies to mitigate soil salinity in modern agriculture: A review. Agronomy 2021, 11, 2299. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Ramezanpour, S.S.; Soltanloo, H.; Seifati, S.E. RNA-seq analysis and reconstruction of gene networks involved in response to salinity stress in quinoa (cv. Titicaca). Sci. Rep. 2023, 13, 7308. [Google Scholar] [CrossRef]
- Guo, X.; Meng, Z.; Zhu, M.; Long, J.; Li, J.; Zhou, B.; Ai, Z.; Deng, H. Comparative transcriptomic analysis of the super hybrid rice Chaoyouqianhao under salt stress. BMC Plant Biol. 2022, 22, 233. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Jin, X.; Liu, T.; Xu, J.; Gao, Z.; Hu, X. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Jerbi, A.; Brereton, N.J.B.; Sas, E.; Amiot, S.; Lachapelle-T, X.; Comeau, Y.; Pitre, F.; Labrecque, M. High biomass yield increases in a primary effluent wastewater phytofiltration are associated to altered leaf morphology and stomatal size in Salix miyabeana. Sci. Total Environ. 2020, 738, 139728. [Google Scholar] [CrossRef] [PubMed]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.H.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.; Fiorani, F.; Ottosen, C.O. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef]
- Xiong, D.; Huang, J.; Peng, S.; Li, Y. A few enlarged chloroplasts are less efficient in photosynthesis than a large population of small chloroplasts in Arabidopsis thaliana. Sci. Rep. 2017, 7, 5782. [Google Scholar] [CrossRef]
- Xie, W.; Li, Y.; Li, Y.; Ma, L.; Ashraf, U.; Tang, X.; Pan, S.; Tian, H.; Mo, Z. Application of γ-aminobutyric acid under low light conditions: Effects on yield, aroma, element status, and physiological attributes of fragrant rice. Ecotox. Environ. Safe 2021, 213, 111941. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Zhen, W.; Sun, T.; Hu, X. Abscisic acid alleviates harmful effect of saline–alkaline stress on tomato seedlings. Plant Physiol. Biochem. 2022, 175, 58–67. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, Y.; Zhang, Z.; Yue, H.; Wang, L.; Li, J.; Jiao, Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth. Regul. 2017, 36, 436–449. [Google Scholar] [CrossRef]
- Nayyar, H.; Kaur, R.; Kaur, S.; Singh, R. γ-Aminobutyric acid (GABA) imparts partial protection from heat stress injury to rice seedlings by improving leaf turgor and upregulating osmoprotectants and antioxidants. J. Plant Growth Regul. 2014, 33, 408–419. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Q.; Cheng, F. Sugar starvation enhances leaf senescence and genes involved in sugar signaling pathways regulate early leaf senescence in mutant rice. Rice Sci. 2020, 27, 201–214. [Google Scholar]
- Gu, Y.; Liang, C. Responses of antioxidative enzymes and gene expression in Oryza sativa L and Cucumis sativus L seedlings to microcystins stress. Ecotoxicol. Environ. Safe 2020, 193, 110351. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-aminobutyric acid (GABA): Biosynthesis, role, commercial production, and application. In Study in Natural Products Chemistry, 1st ed.; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 413–452. [Google Scholar]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against abiotic stress tolerance in legumes: A review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; O’Leary, B.; Tabares-da Rosa, S.; Borsani, O.; Monza, J. GABA and Proline Metabolism in Response to Stress. In Hormones and Plant Response. Plant in Challenging Environments; Gupta, D.K., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2021; pp. 291–314. [Google Scholar]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. (Eds.) World Agriculture towards 2030/2050: The 2012 Revision; ESA Working Papers 12-03; ESA: Paris, France, 2012. [Google Scholar]
- Mustafa, G.; Akhtar, M.S.; Abdullah, R. Global concern for salinity on various agro-ecosystems. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Springer: Singapore, 2019; pp. 1–19. [Google Scholar]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Ashraf, U.; Li, G.; Li, Y.; Lu, W.; Hu, J. Exogenous γ-aminobutyric acid (GABA) application improved early growth, net photosynthesis, and associated physio-biochemical events in maize. Front. Plant Sci. 2016, 7, 919. [Google Scholar] [CrossRef]
- Parveen, A.; Ahmar, S.; Kamran, M.; Malik, Z.; Ali, A.; Riaz, M.; Abbasi, G.H.; Khan, M.; Bin Sohail, A.; Rizwan, M.; et al. Abscisic acid signaling reduced transpiration flow, regulated Na+ ion homeostasis and antioxidant enzyme activities to induce salinity tolerance in wheat (Triticum aestivum L.) seedlings. Environ. Technol. Inno. 2021, 24, 101808. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef]
- Badr, A.; Basuoni, M.M.; Ibrahim, M.; Salama, Y.E.; Abd-Ellatif, S.; Abdel Razek, E.S.; Amer, K.; Ibrahim, A.A.; Zayed, E.M. Ameliorative impacts of gamma-aminobutyric acid (GABA) on seedling growth, physiological biomarkers, and gene expression in eight wheat (Triticum aestivum L.) cultivars under salt stress. BMC Plant Biol. 2024, 24, 605. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Z.; Wu, M.; Li, H.; Gu, J.; Yang, J.; Zhang, H.; Zhang, Z. Optimized leaf anatomy improves photosynthetic producing capacity of mid-season indica rice in the Yangtze River Basin during the genetic improvement. Eur. J. Agron. 2024, 158, 127196. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Jalil, S.U.; Ansari, M.I. Physiological role of gamma-aminobutyric acid in salt stress tolerance. In Salt and Drought Stress Tolerance in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 337–350. [Google Scholar]
- Wang, G.; Zhang, L.; Zhang, S.; Li, B.; Li, J.; Wang, X.; Zhang, J.; Guan, C.; Ji, J. The combined use of a plant growth promoting Bacillus sp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system, enhancing photosynthesis and improving soil enzyme activities. Microbiol. Res. 2023, 266, 127225. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kundu, A. Sugars and sugar polyols in overcoming environmental stresses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Aryadeep Roychoudhury, A., Tripathi, D.K., Eds.; John Wiley Sons, Ltd.: West Sussex, UK, 2020; pp. 71–101. [Google Scholar]
- Pandey, G.K.; Mahiwal, S. Potassium in Abiotic Stress. In Role of Potassium in Plants; Springer: Cham, Switzerland, 2020; pp. 45–49. [Google Scholar]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Abbasi, H.; Jamil, M.; Haq, A.; Ali, S.; Ahmad, R.; Malik, Z.; Parveen, Z. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: A review. Zemdirbyste 2016, 103, 229–238. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Ali, B.; Ren, X.; Chen, X.; Li, Q.; Saqib, M.; Ahmad, N. Recent progress in understanding salinity tolerance in plants: Story of Na+/K+ balance and beyond. Plant Physiol. Biochem. 2021, 160, 239–256. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef]


| Year | Growth Stage | Treatment | Soil Salinity (g kg−1) | Soil Electrical Conductivity (μS cm−1) |
|---|---|---|---|---|
| 2020 | MT | CK | 0 | 225.26 ± 3.14 b |
| 0.2% | 0.2 | 4832.37 ± 36.24 a | ||
| PI | CK | 0 | 224.56 ± 3.05 b | |
| 0.2% | 0.2 | 4831.23 ± 26.31 a | ||
| MA | CK | 0 | 224.31 ± 3.66 b | |
| 0.2% | 0.2 | 4828.11 ± 17.34 a | ||
| 2021 | MT | CK | 0 | 223.13 ± 4.72 b |
| 0.2% | 0.2 | 4815.51 ± 21.96 a | ||
| PI | CK | 0 | 222.96 ± 5.12 b | |
| 0.2% | 0.2 | 4811.30 ± 36.32 a | ||
| MA | CK | 0 | 222.94 ± 4.62 b | |
| 0.2% | 0.2 | 4812.37 ± 22.56 a |
| Year | Cultivar | Treatment | Number of Panicles per Pot | Total Number of Spikelets per Pot (103) | Number of Spikelets per Panicle | Filled Grain Rate (%) | 1000-Grain Weight (g) | Yield (g pot−1) | Yield Increase Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2020 | J-5 | 0 μM | 16.33 ± 0.58 c | 1.90 ± 0.01 c | 116.33 ± 3.79 c | 65.30 ± 2.54 b | 22.84 ± 0.08 c | 28.31 ± 1.06 d | |
| 50 μM | 19.00 ± 1.00 b | 2.27 ± 0.12 b | 119.67 ± 0.58 c | 69.60 ± 2.96 a | 23.07 ± 0.03 b | 36.47 ± 1.30 c | 29.04 ± 3.12 c | ||
| 100 μM | 22.67 ± 0.58 a | 2.84 ± 0.10 a | 125.33 ± 1.53 b | 72.50 ± 2.21 a | 23.14 ± 0.02 b | 47.65 ± 2.02 b | 68.48 ± 4.25 b | ||
| 150 μM | 23.00 ± 1.00 a | 2.98 ± 0.10 a | 129.67 ± 1.53 a | 73.80 ± 0.70 a | 23.27 ± 0.03 a | 51.19 ± 1.20 a | 81.07 ± 5.65 a | ||
| L-7 | 0 μM | 14.00 ± 1.00 d | 1.46 ± 0.08 d | 104.33 ± 1.53 b | 45.27 ± 2.76c | 24.30 ± 0.02 d | 16.07 ± 1.65 d | ||
| 50 μM | 16.33 ± 0.58 c | 1.75 ± 0.10 c | 107.33 ± 2.31 b | 55.53 ± 0.32 b | 24.40 ± 0.02 c | 23.77 ± 1.52 c | 49.15 ± 3.02 c | ||
| 100 μM | 18.33 ± 0.58 b | 2.08 ± 0.07 b | 113.33 ± 3.21 a | 59.27 ± 0.42 a | 24.92 ± 0.03 b | 30.68 ± 0.98 b | 91.99 ± 1.25 b | ||
| 150 μM | 20.33 ± 1.15 a | 2.33 ± 0.16 a | 114.67 ± 2.08 a | 60.87 ± 0.60 a | 24.97 ± 0.02 a | 35.44 ± 2.14 a | 121.37 ± 4.01 a | ||
| 2021 | J-5 | 0 μM | 15.67 ± 1.15 c | 1.77 ± 0.14 d | 112.67 ± 2.52 d | 64.70 ± 0.85 d | 22.81 ± 0.04 d | 26.04 ± 1.72 d | |
| 50 μM | 18.33 ± 0.58 b | 2.19 ± 0.06 c | 119.67 ± 1.15 c | 69.13 ± 0.64 c | 23.05 ± 0.01 c | 34.95 ± 1.08 c | 34.40 ± 3.21 c | ||
| 100 μM | 21.67 ± 0.58 a | 2.71 ± 0.09 b | 125.00 ± 1.00 b | 72.20 ± 1.13 b | 23.14 ± 0.01 b | 45.27 ± 1.88 b | 74.17 ± 4.36 b | ||
| 150 μM | 22.33 ± 0.58 a | 2.94 ± 0.05 a | 131.637 ± 2.08 a | 74.43 ± 0.80 a | 23.28 ± 0.01 a | 50.94 ± 1.49 a | 96.24 ± 2.24 a | ||
| L-7 | 0 μM | 13.67 ± 0.58 d | 1.41 ± 0.04 d | 103.33 ± 1.53 d | 41.00 ± 0.62 d | 24.35 ± 0.02 d | 14.10 ± 0.54 d | ||
| 50 μM | 16.33 ± 0.58 c | 1.74 ± 0.10 c | 106.67 ± 2.08 c | 55.00 ± 1.44 c | 24.44 ± 0.03 c | 23.41 ± 0.74 c | 66.34 ± 4.55 c | ||
| 100 μM | 18.67 ± 0.58 b | 2.12 ± 0.04 b | 113.33 ± 1.53 b | 58.63 ± 0.96 b | 24.91 ± 0.02 b | 30.89 ± 0.47 b | 119.42 ± 5.12 b | ||
| 150 μM | 20.33 ± 0.58 a | 2.37 ± 0.09 a | 116.67 ± 1.53 a | 60.50 ± 0.20 a | 24.98 ± 0.04 a | 35.86 ± 1.40 a | 154.81 ± 4.18 a |
| Year | Cultivar | Treatment | Dry Matter Weight (g Pot−1) | Plant Growth Rate (g d−1 Pot−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| MT | PI | FS | MA | MT-PI | PI-FS | FS-MA | |||
| 2020 | J-5 | 0 μM | 11.36 ± 0.09 d | 45.30 ± 0.34 d | 66.26 ± 1.02 d | 112.76 ± 1.51 d | 1.31 ± 0.01 d | 1.40 ± 0.05 ab | 1.01 ± 0.02 d |
| 50 μM | 13.36 ± 0.06 c | 49.60 ± 0.47 c | 71.63 ± 0.85 c | 136.86 ± 3.01 c | 1.39 ± 0.02 c | 1.47 ± 0.06 a | 1.42 ± 0.05 c | ||
| 100 μM | 14.76 ± 0.12 b | 53.38 ± 0.13 b | 74.50 ± 0.43 b | 157.61 ± 0.93 b | 1.49 ± 0.01 b | 1.41 ± 0.02 a | 1.81 ± 0.03 b | ||
| 150 μM | 16.25 ± 0.15 a | 56.25 ± 0.36 a | 76.08 ± 0.09 a | 166.47 ± 1.01 a | 1.54 ± 0.02 a | 1.32 ± 0.02 b | 1.96 ± 0.02 a | ||
| L-7 | 0 μM | 6.53 ± 0.02 d | 30.48 ± 0.12 d | 43.36 ± 0.17 d | 66.22 ± 0.98 d | 0.86 ± 0.00 d | 0.76 ± 0.02 c | 0.48 ± 0.02 d | |
| 50 μM | 8.77 ± 0.07 c | 34.66 ± 0.31 c | 49.64 ± 0.94 c | 77.97 ± 0.68 c | 0.92 ± 0.01 c | 0.88 ± 0.07 b | 0.59 ± 0.01 c | ||
| 100 μM | 9.77 ± 0.21 b | 36.42 ± 0.37 b | 53.75 ± 0.48 b | 85.43 ± 0.25 b | 0.95 ± 0.02 b | 1.02 ± 0.03 a | 0.66 ± 0.01 b | ||
| 150 μM | 10.29 ± 0.12 a | 39.55 ± 0.36 a | 57.58 ± 0.42 a | 90.52 ± 0.23 a | 1.05 ± 0.01 a | 1.06 ± 0.03 a | 0.69 ± 0.01 a | ||
| 2021 | J-5 | 0 μM | 11.22 ± 0.22 d | 44.83 ± 0.49 d | 65.89 ± 1.07 d | 111.25 ± 2.59 d | 1.29 ± 0.01 d | 1.40 ± 0.06 a | 0.99 ± 0.03 d |
| 50 μM | 13.45 ± 0.09 c | 50.17 ± 0.05 c | 71.68 ± 0.39 c | 136.22 ± 1.94 c | 1.42 ± 0.00 c | 1.43 ± 0.03 a | 1.40 ± 0.05 c | ||
| 100 μM | 14.56 ± 0.26 b | 53.19 ± 0.09 b | 74.28 ± 0.11 b | 156.60 ± 1.83 b | 1.49 ± 0.01 b | 1.41 ± 0.01 a | 1.79 ± 0.04 b | ||
| 150 μM | 16.19 ± 0.16 a | 55.59 ± 0.49 a | 76.18 ± 0.12 a | 165.27 ± 0.96 a | 1.52 ± 0.02 a | 1.37 ± 0.04 a | 1.94 ± 0.02 a | ||
| L-7 | 0 μM | 6.48 ± 0.03 d | 29.34 ± 0.20 d | 42.93 ± 2.13 d | 64.89 ± 0.62 d | 0.82 ± 0.01 d | 0.80 ± 0.13 c | 0.46 ± 0.04 c | |
| 50 μM | 8.71 ± 0.13 c | 34.23 ± 0.25 c | 49.77 ± 0.57 c | 77.72 ± 0.65 c | 0.91 ± 0.01 c | 0.91 ± 0.05 bc | 0.58 ± 0.02 b | ||
| 100 μM | 9.83 ± 0.18 b | 36.14 ± 0.08 b | 53.46 ± 0.45 b | 84.75 ± 0.52 b | 0.94 ± 0.01 b | 1.02 ± 0.02 ab | 0.65 ± 0.01 a | ||
| 150 μM | 10.23 ± 0.11 a | 39.26 ± 0.17 a | 57.25 ± 0.08 a | 90.11 ± 0.88 a | 1.04 ± 0.01 a | 1.06 ± 0.01 a | 0.68 ± 0.02 a | ||
| Year | Cultivar | Treatment | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Chl a + b (mg g−1 FW) | Chl a/b | SPAD |
|---|---|---|---|---|---|---|---|
| 2020 | J-5 | 0 μM | 1.32 ± 0.04 b | 0.62 ± 0.02 b | 1.94 ± 0.04 c | 2.13 ± 0.07 a | 34.23 ± 0.04 d |
| 50 μM | 1.35 ± 0.02 b | 0.65 ± 0.03 b | 2.00 ± 0.04 c | 2.08 ± 0.08 a | 37.34 ± 0.20 c | ||
| 100 μM | 1.41 ± 0.03 a | 0.69 ± 0.01 a | 2.10 ± 0.03 b | 2.04 ± 0.06 a | 38.24 ± 0.07 b | ||
| 150 μM | 1.45 ± 0.03 a | 0.72 ± 0.02 a | 2.17 ± 0.03 a | 2.02 ± 0.09 a | 39.06 ± 0.14 a | ||
| L-7 | 0 μM | 1.28 ± 0.04 b | 0.58 ± 0.01 c | 1.86 ± 0.04 c | 2.21 ± 0.06 a | 31.10 ± 0.09 c | |
| 50 μM | 1.31 ± 0.04 ab | 0.60 ± 0.03 bc | 1.91 ± 0.04 bc | 2.19 ± 0.13 a | 33.36 ± 0.26 b | ||
| 100 μM | 1.35 ± 0.05 ab | 0.63 ± 0.02 ab | 1.98 ± 0.07 ab | 2.14 ± 0.02 a | 34.53 ± 0.51 a | ||
| 150 μM | 1.36 ± 0.03 a | 0.64 ± 0.01 a | 2.00 ± 0.02 a | 2.13 ± 0.07 a | 34.78 ± 0.19 a | ||
| 2021 | J-5 | 0 μM | 1.28 ± 0.03 c | 0.62 ± 0.01 b | 1.91 ± 0.03 d | 2.06 ± 0.02 a | 34.12 ± 0.03 d |
| 50 μM | 1.34 ± 0.01 b | 0.65 ± 0.01 b | 1.99 ± 0.01 c | 2.08 ± 0.03 a | 37.28 ± 0.11 c | ||
| 100 μM | 1.42 ± 0.01 a | 0.69 ± 0.03 a | 2.11 ± 0.04 b | 2.06 ± 0.08 a | 38.25 ± 0.09 b | ||
| 150 μM | 1.45 ± 0.03 a | 0.71 ± 0.02 a | 2.16 ± 0.02 a | 2.03 ± 0.08 a | 39.06 ± 0.05 a | ||
| L-7 | 0 μM | 1.25 ± 0.03 b | 0.58 ± 0.02 c | 1.84 ± 0.05 c | 2.15 ± 0.04 a | 31.09 ± 0.10 d | |
| 50 μM | 1.32 ± 0.01 a | 0.61 ± 0.01 b | 1.93 ± 0.01 b | 2.15 ± 0.03 a | 33.26 ± 0.26 c | ||
| 100 μM | 1.35 ± 0.03 a | 0.63 ± 0.01 ab | 1.98 ± 0.03 ab | 2.13 ± 0.05 a | 34.68 ± 0.14 b | ||
| 150 μM | 1.36 ± 0.02 a | 0.65 ± 0.01 a | 2.01 ± 0.01 a | 2.09 ± 0.05 a | 35.09 ± 0.09 a |
| Cultivar | Treatment | SL (μm) | SW (μm) | SA (μm2) | SD (mm−2) | SPI (10−2 μm2 μm−2) | Sm (μm2 μm−2) | Sc (μm2 μm−2) | Sc/Sm (%) |
|---|---|---|---|---|---|---|---|---|---|
| J-5 | 0 μM | 16.45 ± 0.19 d | 9.31 ± 0.07 d | 120.20 ± 1.91 d | 444.44 ± 10.18 a | 5.34 ± 0.12 a | 18.25 ± 0.07 d | 14.31 ± 0.06 d | 78.41 ± 0.20 c |
| 50 μM | 17.80 ± 0.42 c | 10.33 ± 0.01 c | 144.34 ± 3.50 c | 348.89 ± 10.18 b | 5.03 ± 0.13 b | 20.30 ± 0.11 c | 16.76 ± 0.19 c | 82.58 ± 0.81 b | |
| 100 μM | 18.36 ± 0.18 b | 11.23 ± 0.08 b | 161.83 ± 1.91 b | 293.33 ± 6.67 c | 4.75 ± 0.16 c | 21.37 ± 0.16 b | 17.94 ± 0.21 b | 83.95 ± 0.87 ab | |
| 150 μM | 18.98 ± 0.13 a | 11.37 ± 0.09 a | 169.48 ± 0.81 a | 264.44 ± 10.18 d | 4.48 ± 0.19 d | 21.89 ± 0.05 a | 18.47 ± 0.23 a | 84.40 ± 0.89 a | |
| L-7 | 0 μM | 26.66 ± 0.45 c | 16.27 ± 0.06 d | 340.55 ± 6.20 d | 271.11 ± 10.18 a | 9.24 ± 0.49 a | 15.25 ± 0.09 d | 10.32 ± 0.06 d | 67.64 ± 0.05 c |
| 50 μM | 27.51 ± 0.16 b | 16.71 ± 0.06 c | 360.81 ± 1.05 c | 242.22 ± 3.85 cb | 8.74 ± 0.13 b | 17.25 ± 0.10 c | 12.24 ± 0.11 c | 70.98 ± 0.72 b | |
| 100 μM | 27.91 ± 0.10 ab | 17.25 ± 0.12 b | 377.95 ± 1.94 b | 211.11 ± 3.85 c | 7.98 ± 0.17 c | 18.14 ± 0.18 b | 13.23 ± 0.12 b | 72.90 ± 0.23 a | |
| 150 μM | 28.35 ± 0.11 a | 17.83 ± 0.18 a | 396.89 ± 3.25 a | 184.44 ± 3.85 d | 7.32 ± 0.20 d | 18.54 ± 0.08 a | 13.68 ± 0.15 a | 73.80 ± 0.74 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Zhou, T.; Gu, Y.; Shu, C.; Zhu, K.; Zhang, W.; Zhang, H.; Liu, L.; Wang, Z.; Gu, J.; et al. γ-Aminobutyric Acid Alleviates Salinity-Induced Impairments in Rice Plants by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants. Agronomy 2024, 14, 2524. https://doi.org/10.3390/agronomy14112524
Feng J, Zhou T, Gu Y, Shu C, Zhu K, Zhang W, Zhang H, Liu L, Wang Z, Gu J, et al. γ-Aminobutyric Acid Alleviates Salinity-Induced Impairments in Rice Plants by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants. Agronomy. 2024; 14(11):2524. https://doi.org/10.3390/agronomy14112524
Chicago/Turabian StyleFeng, Jiaxin, Tianyang Zhou, Yibiao Gu, Chenchen Shu, Kuanyu Zhu, Weiyang Zhang, Hao Zhang, Lijun Liu, Zhiqin Wang, Junfei Gu, and et al. 2024. "γ-Aminobutyric Acid Alleviates Salinity-Induced Impairments in Rice Plants by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants" Agronomy 14, no. 11: 2524. https://doi.org/10.3390/agronomy14112524
APA StyleFeng, J., Zhou, T., Gu, Y., Shu, C., Zhu, K., Zhang, W., Zhang, H., Liu, L., Wang, Z., Gu, J., & Yang, J. (2024). γ-Aminobutyric Acid Alleviates Salinity-Induced Impairments in Rice Plants by Improving Photosynthesis and Upregulating Osmoprotectants and Antioxidants. Agronomy, 14(11), 2524. https://doi.org/10.3390/agronomy14112524








