Effect of Water Retainer® During Seedling Period on Bioactive Components of Tomato (Solanum lycopersicum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Fielding
2.1.1. Seedling
2.1.2. Open Fielding
2.2. Chemicals
2.3. Analytical Methods
2.3.1. Sample Preparation of Tomato
2.3.2. Measurement of Polyphenol Content
2.3.3. DPPH Radical Scavenging Activity
2.3.4. Ascorbic Acid Determination
2.3.5. Determinations of Carotenoids
2.3.6. Determination of Tocopherols
2.3.7. Statistical Analysis
3. Results
3.1. Harvesting Results
3.2. Bioactive Component of Tomato Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Statistical Databases; Food and Agriculture Organization of the United Nations, FAOSTAT: Rome, Italy, 2020; Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 December 2022).
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retino in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Mahakun, N.; Leeper, P.W.; Burns, E.E. Acidic constituents of various tomato fruit types. J. Food Sci. 1979, 44, 1241–1244. [Google Scholar] [CrossRef]
- Warner, J.; Tan, C.S.; Zhang, T.Q. Water management strategies to enhance fruit solids and yield of drip irrigated processing tomato. Can. J. Plant Sci. 2007, 87, 345–353. [Google Scholar] [CrossRef]
- Ragab, M.E.; Arafa, Y.E.; Sawan, O.M.; Fawzy, Z.F.; El-Sawy, S.M. Effect of irrigation systems on vegetative growth, fruit yield, quality and irrigation water use efficiency of tomato plants (Solanum lycopersicum L.) grown under water stress conditions. Acta Sci. Agric. 2019, 3, 172–183. [Google Scholar]
- Macua, J.I.; Lahoz, I.; Arzoz, A.; Garnica, J. The influence of irrigation cut-off time on the yield and quality of processing tomatoes. Acta Hortic. 2003, 613, 151–153. [Google Scholar] [CrossRef]
- Nuruddin, M.M.; Madramootoo, C.A.; Dodds, G.T. Effects of Water Stress at Different Growth Stages on Greenhouse Tomato Yield and Quality. HortScience 2003, 38, 1389–1393. [Google Scholar] [CrossRef]
- Kumar, R.; Solankey, S.; Singh, M. Breeding for drought tolerance in vegetables. Veg. Sci. 2012, 39, 1–15. [Google Scholar]
- Zdravković, J.; Jovanović, Z.; Djordjević, M.; Girek, Z.; Zdravković, M.; Stikić, R. Application of stress susceptibility index for drought tolerance screening of tomato populations. Genetika 2013, 45, 679–689. [Google Scholar] [CrossRef]
- Helyes, L.; Bőcs, A.; Pék, Z. Effect of water supply on canopy temperature, stomatal conductance and yield quantity of processing tomato (Lycopersicon esculentum Mill.). Int. J. Hortic. Sci. 2010, 16, 13–15. [Google Scholar] [CrossRef]
- Nahar, K.; Gretzmacher, R. Effect of water stress on nutrient uptake, yield and quality of tomato (Lycopersicon esculentum Mill.) under subtropical conditions. Bodenkultur 2002, 53, 45–51. [Google Scholar]
- Ghanem, K.h.M.; Abou-Shleel, S.M.; El-Saka, Z.I. Evaluating Different Tomato Genotypes for Drought. Egypt. J. Plant Breed. 2016, 20, 995–1008. [Google Scholar] [CrossRef]
- Shamim, F.; Farooq, K.; Waheed, A. Effect of different water regimes on biometric traits of some tolerant and sensitive tomato genotypes. J. Anim. Plant Sci. 2014, 24, 1178–1182. [Google Scholar]
- Giuliani, M.; Nardella, E.; Gagliardi, A.; Gatta, G. Deficit Irrigation and Partial Root-Zone Drying Techniques in Processing Tomato Cultivated under Mediterranean Climate Conditions. Sustainability 2017, 9, 2197. [Google Scholar] [CrossRef]
- Urlić, B.; Runjić, M.; Mandušić, M.; Žanić, K.; Selak, G.V.; Matešković, A.; Dumičić, G. Partial root-zone drying and deficit irrigation effect on growth, yield, water use and quality of greenhouse grown grafted tomato. Agronomy 2020, 10, 1297. [Google Scholar] [CrossRef]
- Casa, R.; Rouphael, Y. Effects of partial root-zone drying irrigation on yield, fruit quality, and water-use efficiency in processing tomato. J. Hortic. Sci. Biotechnol. 2014, 89, 389–396. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Al-Selwey, W.A.; Alsadon, A.A.; Al-Doss, A.A.; Solieman, T.H.; Dewir, Y.H.; Ibrahim, A.A. Effect of deficit irrigation on total yield, fruit physical characteristics, and nutritional value of four drought tolerant tomato (Solanum lycopersicum L.) genotypes. J. Agric. Sci. Technol. 2021, 23, 1105–1118. [Google Scholar]
- Medyouni, I.; Zouaoui, R.; Rubio, E.; Serino, S.; Ahmed, H.B.; Bertin, N. Effects of water deficit on leaves and fruit quality during the development period in tomato plant. Food Sci. Nutr. 2021, 9, 1949–1960. [Google Scholar] [CrossRef]
- El-Hady, O.A.; Abd El-Kader, A.A.; Shafi, A.M. Physico-bio-chemical properties of sandy soil conditioned with acrylamide hydrogels after cucumber plantation. Aust. J. Basic Appl. Sci. 2009, 3, 3145–3151. [Google Scholar]
- Chen, F.; Lu, X. Effects of Soil Texture and Water Retaining Agent on the Emergence of Processing Tomatoes. J. Agric. Sci. 2009, 1, 148. [Google Scholar] [CrossRef]
- Carmen, B.; Sumalan, R.; Schmidt, B.; Sumalan, R.L. Researches Concerning the Effects of Incorporation Into the Soil of Water-Retainer Polymers on Morpho-Physiological Traits in Bean (Phaseolus sativum L.) plantlets Subjected to Drought. J. Hortic. For. Biotechnol. 2018, 22, 35. [Google Scholar]
- Sultana, S.; Shariff, M.A.; Hossain, M.A.; Khatun, A.; Huque, R. Effect of Super water absorbent (SWA) hydrogel on productivity and quality of Tomato. Arch. Appl. Sci. Res. 2016, 8, 5–9. [Google Scholar]
- Helyes, L.; Varga, G. Irrigation demand of tomato according to the results of three decades. Acta Hortic. 1994, 376, 323–328. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nagy, Z.; Daood, H.; Ambrózy, Z.; Helyes, L. Determination of Polyphenols, Capsaicinoids, and Vitamin C in New Hybrids of Chili Peppers. J. Anal. Methods Chem. 2015, 2015, 102125. [Google Scholar] [CrossRef]
- Daood, H.G.; Bencze, G.; Palotás, G.; Pék, Z.; Sidikov, A.; Helyes, L. HPLC analysis of carotenoids from tomatoes using cross-linked C18 column and MS detection. J. Chromatogr. Sci. 2014, 52, 985–991. [Google Scholar] [CrossRef]
- Abushita, A.A.; Hebshi, E.A.; Daood, H.G.; Biacs, P.A. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997, 60, 207–212. [Google Scholar] [CrossRef]
- Dobrescu, A.; Scorza, L.C.T.; Tsaftaris, S.A.; McCormick, A.J. A “Do-It-Yourself” phenotyping system: Measuring growth and morphology throughout the diel cycle in rosette shaped plants. Plant Methods 2017, 13, 95. [Google Scholar] [CrossRef]
- Schmidt-Szantner, B.; Gasztonyi, M.; Milotay, P.; Tömösközi-Farkas, R. Effect of irrigation and fertilisation on the biologically active components of tomato. Acta Aliment. 2022, 51, 270–281. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Du, T.; Qiu, R.; Guo, P.; Chen, R. Quantitative response of greenhouse tomato yield and quality to water deficit at different growth stages. Agric. Water Manag. 2013, 129, 152–162. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E Content and Composition in Tomato, Fruits: Beneficial, Roles and Bio-Fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef] [PubMed]
- Zanfini, A.; Franchi, G.G.; Massarelli, P.; Corbini, G.; Dreassi, E. Phenolic compounds, carotenoids and antioxidant activity in five tomato (Lycopersium esculentum Mill.) cultivars. Ital. J. Food Sci. 2017, 29, 90–99. [Google Scholar]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian Tomato Cultivars under Drought Stress Show Different Content of Bioactives in Pulp and Peel of Fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef]
- Riggi, E.; Patanè, C.; Ruberto, G. Content of carotenoids a different ripening stages in processing tomato in relation to soil water availability. Aust. J. Agric. Res. 2008, 59, 348–353. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Dew, T.P.; Orfila, C.; Urwin, P.E. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum L.). J. Agric. Food Chem. 2011, 59, 9673–9682. [Google Scholar] [CrossRef]
- Theobald, J.C.; Bacon, M.A.; Davies, W.J. Delivering enhanced fruit quality to the UK tomato industry through implementation of partial root-zone drying. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, S241. [Google Scholar] [CrossRef]
- Valšíková-Frey, M.; Komár, P.; Rehuš, M. The effect of varieties and degree of ripeness to vitamin C content in tomato fruits. Acta Hortic. Regiotect. 2017, 2, 44–48. [Google Scholar] [CrossRef]
- Pellegrini, N.; Riso, P.; Porrini, M. Tomato consumption does not affect the total antioxidant capacity of plasma. Nutrition 2000, 16, 268–271. [Google Scholar] [CrossRef]
- Klunklin, W.; Savag, G. Effect on Quality Characteristics of Tomatoes Grown Under Well-Watered and Drought Stress Conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L, cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, B.; Gao, S.; Xu, K. Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma 2019, 256, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.D.M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Cao, H.; Wang, H.; Pan, X. The physiological responses of tomato to water stress and re-water in different growth periods. Sci. Hortic. 2019, 249, 143–154. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L. Effects of soil water deficit on yield and quality of processing tomato under a Mediterranean climate. Agric. Water Manag. 2010, 97, 131–138. [Google Scholar] [CrossRef]
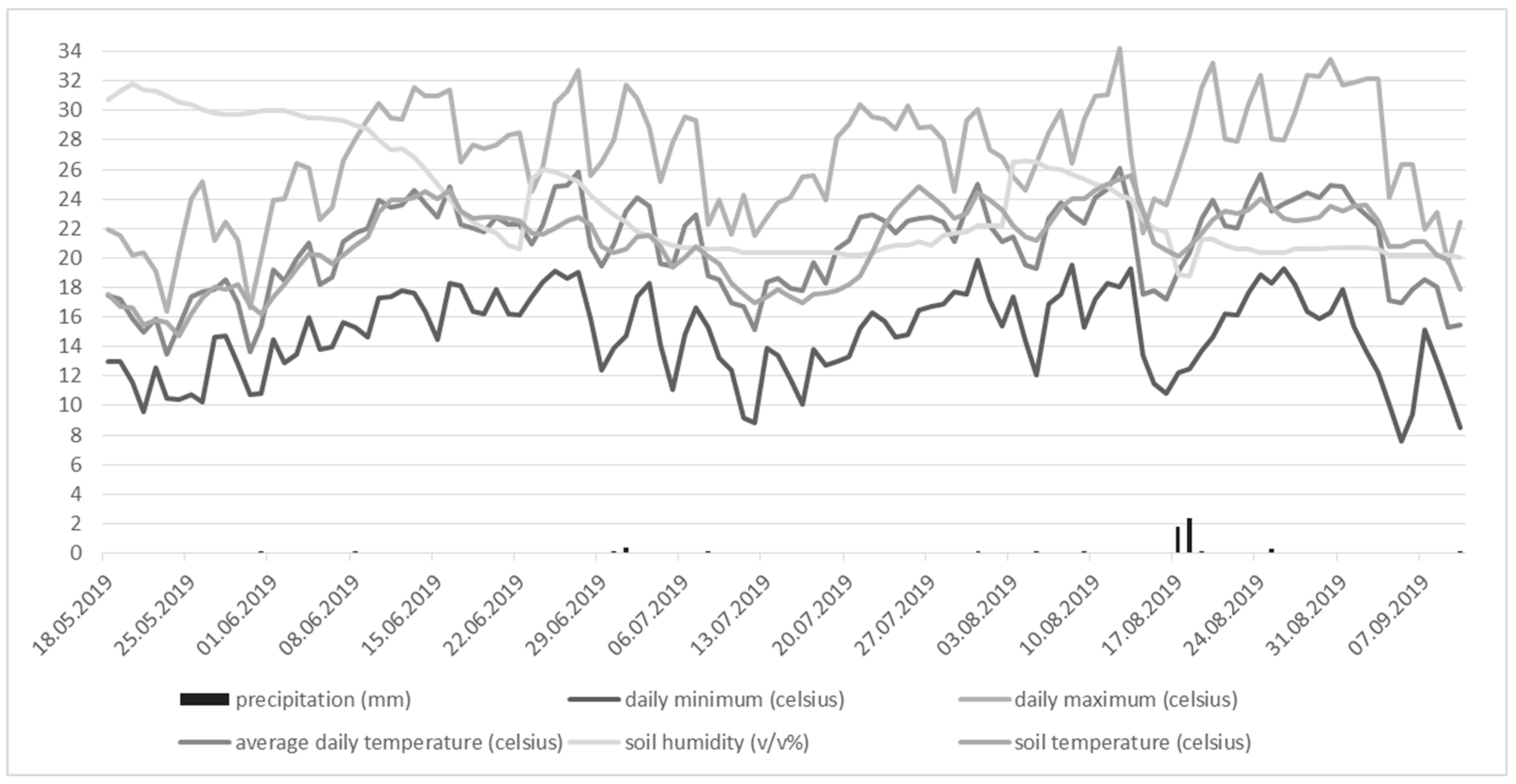
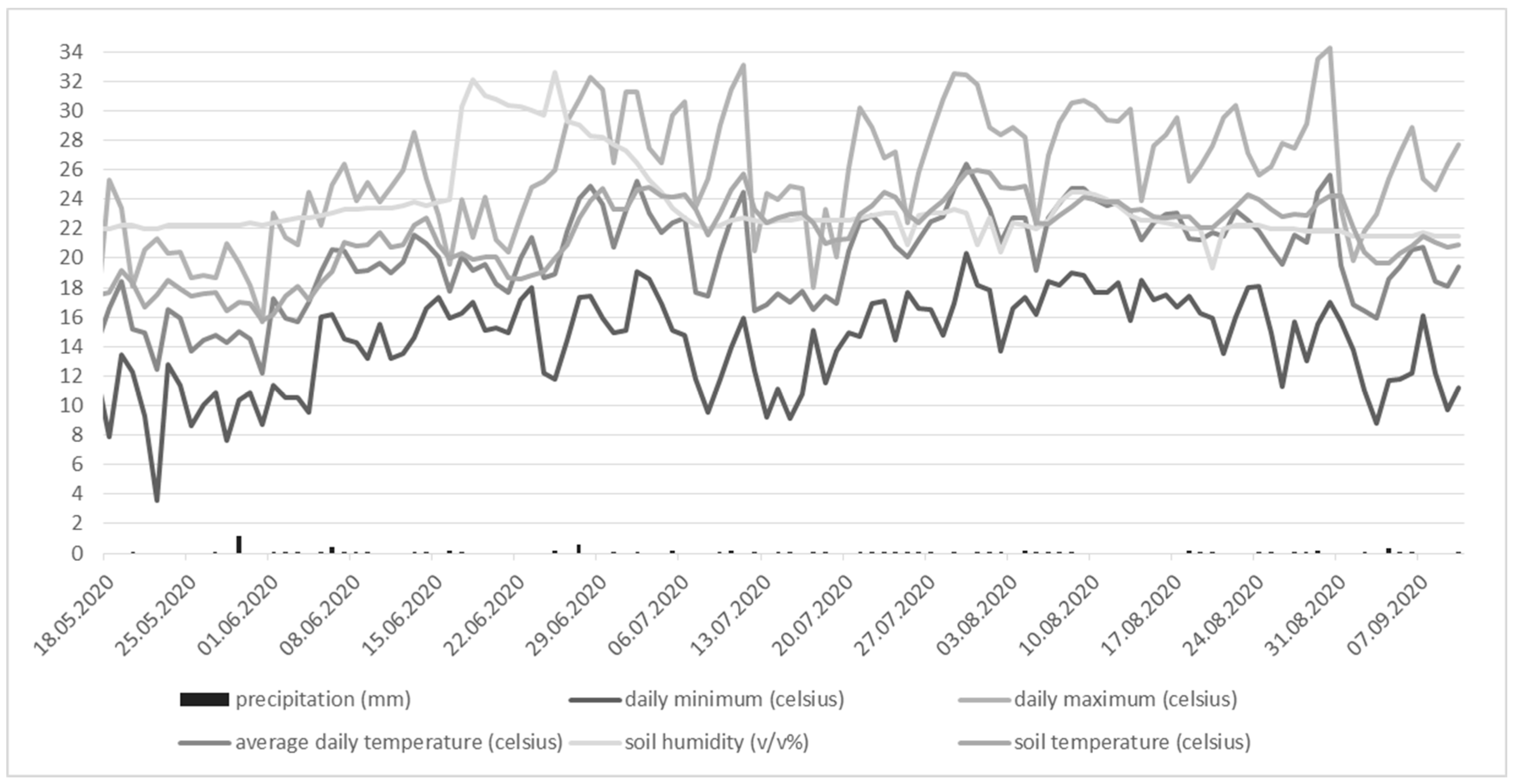
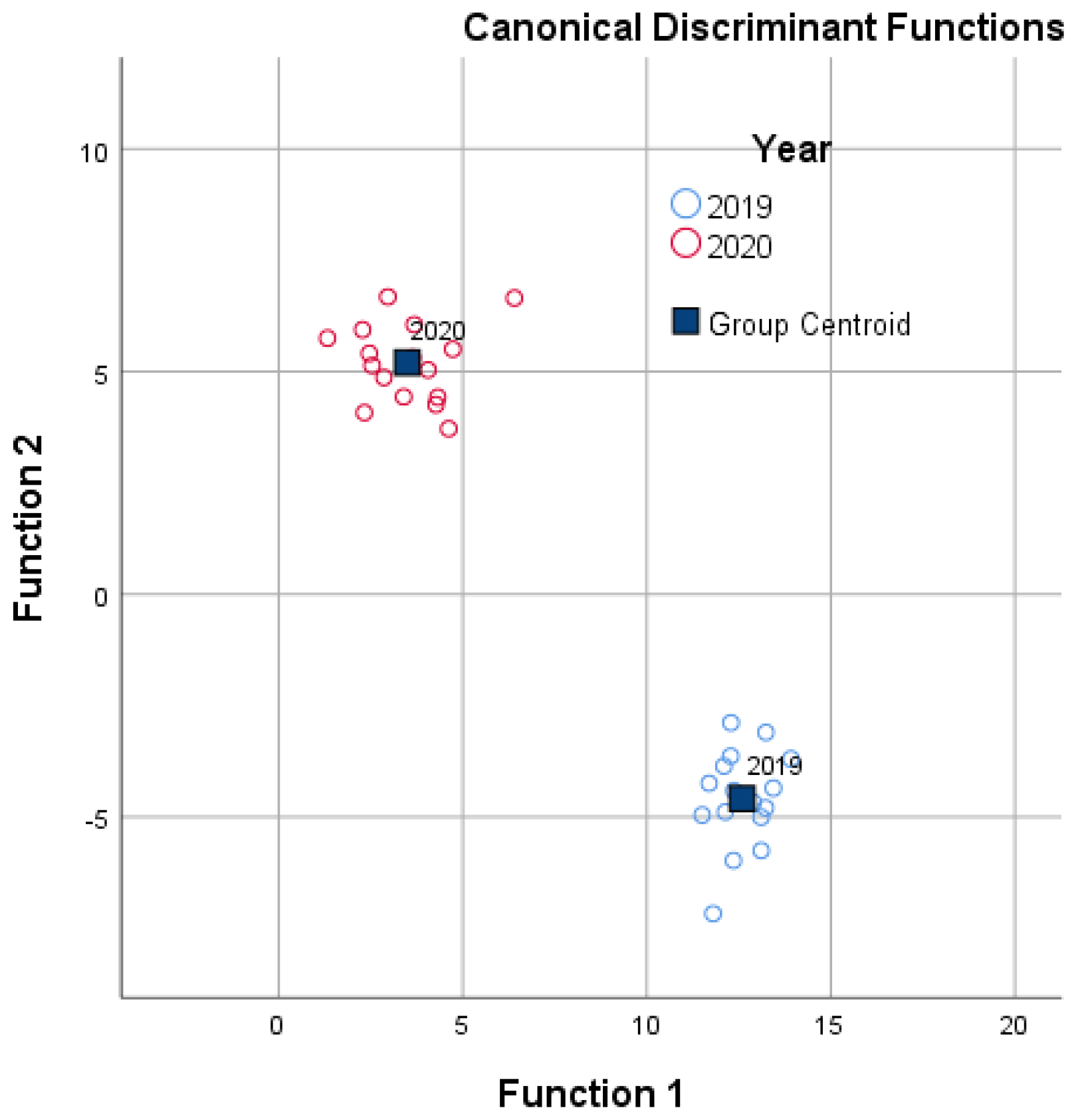
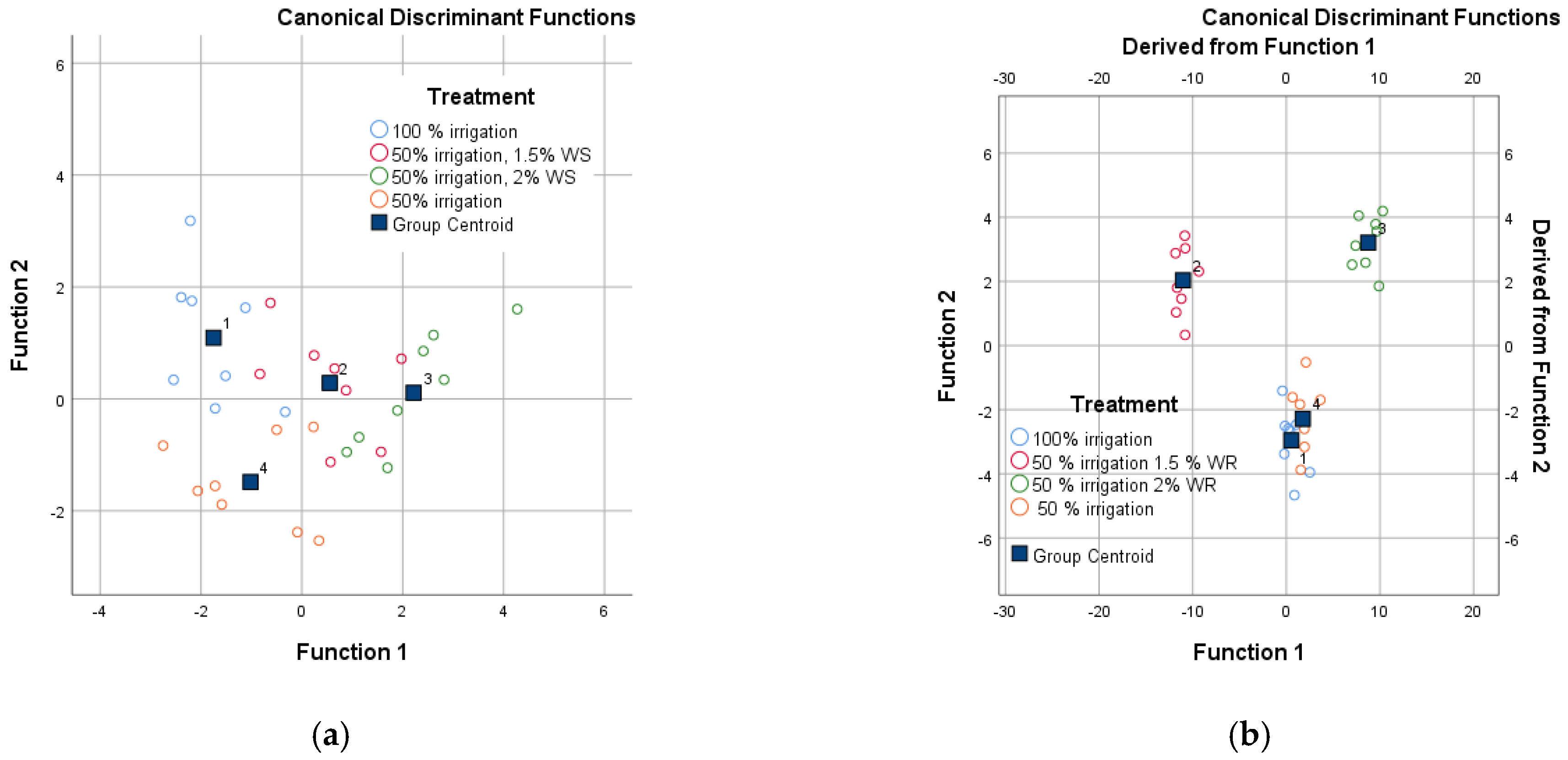
| Amount of the irrifated water | ||||
| 2019 | ||||
| 100% irrigation | 50% irrigation | 50% irrigation + 1.5 mL/m2 WR | 50% irrigation + 2 mL/m2 WR | |
| amount (L/m2) | 94 | 56.25 | 47.05 | 47.05 |
| percentage (%) | 100 | 59.84 | 50.05 | 50.05 |
| 2020 | ||||
| 100% irrigation | 50% irrigation | 50% irrigation + 1.5 mL/m2 WR | 50% irrigation + 2 mL/m2 WR | |
| amount (L/m2) | 113.00 | 56.50 | 56.50 | 61.50 |
| percentage (%) | 100.00 | 59.84 | 50.05 | 50.05 |
| Year | Treatment | Plant Height [cm] | Number of True Leaves | Stem Diameter [mm] |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| 2019 | 50% irrigation | 10.59 ± 0.88 b | 3.05 ± 0.38 a | 3.54 ± 0.15 a |
| 50% irrigation + 1.5 mL/m2 WR® | 10.07 ± 1.00 b | 3.2 ± 0.16 a | 3.55 ± 0.02 a | |
| 50% irrigation + 2 mL/m2 WR® | 11.87 ± 1.62 a,b | 3.2 ± 0.37 a | 3.6 ± 0.19 a | |
| 100% irrigation | 13.87 ± 1.58 a | 3.65 ± 0.3 a | 3.59 ± 0.05 a | |
| 2020 | 50% irrigation | 14.24 ± 1.29 a | 4.88 ± 0.05 b | 4.31 ± 0.05 b |
| 50% irrigation + 1.5 mL/m2 WR® | 11.88 ± 0.28 b | 4.45 ± 0.34 c | 4.10 ± 0.10 b,c | |
| 50% irrigation + 2 mL/m2 WR® | 11.92 ± 1.41 b | 4.33 ± 0.17 c | 3.85 ± 0.23 c | |
| 100% irrigation | 15.26 ± 0.74 a | 5.39 ± 0.08 a | 4.59 ± 0.12 a |
| Year | Treatment | Ripened Berries (kg/m2) | Half-Ripe Berries (kg/m2) | Green Berries (kg/m2) | Unhealty Berries (kg/m2) | Yield (kg/m2) | Brix% | L | a | b | a/b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| 2019 | 50% irrigation | 11.26 ± 0.36 a | 3.11 ± 0.54 a | 1.06 ± 0.28 a | 1.18 ± 0.13 a | 16.61 ± 0.43 a | 4.60 ± 0.23 a | 27.16 ± 1.17 a | 31.49 ± 1.47 a | 12.91 ± 0.66 a | 2.44 ± 0.04 b |
| 50% irrigation + 1.5 mL/m2 WR® | 12.27 ± 1.33 a | 2.72 ± 0.56 a | 1.48 ± 0.29 a | 0.90 ± 0.21 a | 17.37 ± 1.73 a | 5.05 ± 0.42 a | 26.25 ± 0.75 ab | 32.86 ± 0.82 a | 12.46 ± 0.51 a | 2.64 ± 0.06 a | |
| 50% irrigation + 2 mL/m2 WR® | 10.81 ± 0.66 a | 3.20 ± 0.84 a | 1.61 ± 0.43 a | 0.87 ± 0.25 a | 16.49 ± 1.27 a | 4.57 ± 0.17 a | 26.85 ± 0.41 a | 31.98 ± 1.02 a | 12.64 ± 0.53 a | 2.53 ± 0.13 ab | |
| 100% irrigation | 11.98 ± 0.85 a | 3.11 ± 0.70 a | 1.46 ± 0.46 a | 1.17 ± 0.25 a | 17.73 ± 1.66 a | 4.48 ± 0.19 a | 25.03 ± 0.91 b | 27.97 ± 1.31 b | 11.23 ± 0.33 b | 2.49 ± 0.10 ab | |
| 2020 | 50% irrigation | 5.01 ± 0.90 a | 1.68 ± 0.30 a | 1.28 ± 0.42 a,b | 0.68 ± 0.28 a | 8.65 ± 1.11 a | 4.13 ± 0.17 a | 24.42 ± 0.59 a | 30.93 ± 1.71 b | 12.01 ± 0.56 b | 2.58 ± 0.06 a |
| 50% irrigation + 1.5 mL/m2 WR® | 4.53 ± 0.99 a | 1.71 ± 0.38 a | 1.35 ± 0.28 a,c | 0.84 ± 0.21 a | 8.43 ± 1.37 a | 4.08 ± 0.22 a | 25.05 ± 0.19 a | 31.06 ± 1.31 b | 12.21 ± 0.66 b | 2.54 ± 0.03 a | |
| 50% irrigation + 2 mL/m2 WR® | 4.38 ± 0.86 a | 1.55 ± 0.27 a | 1.94 ± 0.61 a | 0.74 ± 0.06 a | 8.59 ± 0.24 a | 4.05 ± 1.17 a | 26.2 ± 0.93 a | 31.96 ± 0.70 ab | 12.73 ± 0.44 b | 2.51 ± 0.04 a | |
| 100% irrigation | 6.15 ± 1.55 a | 1.15 ± 0.35 a | 0.97 ± 0.30 b | 0.72 ± 0.12 a | 8.99 ± 0.60 a | 4.53 ± 0.48 a | 27.29 ± 1.14 a | 33.96 ± 1.02 a | 13.48 ± 0.55 a | 2.52 ± 0.08 a |
| Year | Treatment | α-Toco Pherol (μg/g) | γ-Toco Pherol (μg/g) | Lycopene (μg/g) | β-Carotene (μg/g) | Vitamin C (μg/g) | DPPH (mMTr/kg) | Total Polyphenol (mg GAE/kg) |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| 2019 | 50% irrigation | 9.95 ± 0.26 d | 0.35 ± 0.02 c | 66.65 ± 1.34 c | 7.93 ± 0.53 a | 262.20 ± 10.60 b | 0.85 ± 0.13 b | 405 ± 31 b |
| 50% irrigation + 1.5 mL/m2 WR® | 13.13 ± 0.42 a | 2.37 ± 0.06 a | 89.19 ± 2.94 a | 5.18 ± 0.37 b | 338.10 ± 13.70 a | 1.54 ± 0.17 a | 498 ± 27 a | |
| 50% irrigation + 2 mL/m2 WR® | 10.55 ± 0.23 c | 0.72 ± 0.06 b | 71.77 ± 1.66 b | 3.75 ± 0.10 c | 192.70 ± 6.70 c | 0.91 ± 0.11 b | 496 ± 22 a | |
| 100% irrigation | 12.35 ± 0.27 b | 0.65 ± 0.03 b | 69.51 ± 1.73 c | 5.40 ± 0.42 b | 267.27 ± 5.60 b | 1.04 ± 0.16 b | 377 ± 33 b | |
| 2020 | 50% irrigation | 9.82 ± 0.37 c | 4.31 ± 0.17 a | 71.83 ± 1.93 c | 5.23 ± 0.08 a | 169.20 ± 7.50 b | 0.78 ± 0.07 b | 433 ± 24 b |
| 50% irrigation + 1.5 mL/m2 WR® | 11.04 ± 0.3 b | 4.23 ± 0.19 a | 114.30 ± 3.18 a | 2.90 ± 0.11 d | 221.50 ± 11.10 a | 0.91 ± 0.07 a | 504 ± 44 a | |
| 50% irrigation + 2 mL/m2 WR® | 12.09 ± 0.55 a | 4.13 ± 0.13 a | 91.91 ± 2.85 b | 4.02 ± 0.09 b | 202.50 ± 8.20 a | 0.99 ± 0.07 a | 382 ± 33 b | |
| 100% irrigation | 9.99 ± 0.29 c | 2.40 ± 0.04 b | 83.37 ± 1.73 c | 3.54 ± 0.08 c | 168.10 ± 6.60 b | 0.98 ± 0.14 b | 409 ± 29 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tömösközi-Farkas, R.A.; Molnár-Mondovics, Á.; Schmidtné Szantner, B.I. Effect of Water Retainer® During Seedling Period on Bioactive Components of Tomato (Solanum lycopersicum). Agronomy 2024, 14, 2799. https://doi.org/10.3390/agronomy14122799
Tömösközi-Farkas RA, Molnár-Mondovics Á, Schmidtné Szantner BI. Effect of Water Retainer® During Seedling Period on Bioactive Components of Tomato (Solanum lycopersicum). Agronomy. 2024; 14(12):2799. https://doi.org/10.3390/agronomy14122799
Chicago/Turabian StyleTömösközi-Farkas, Rita Adél, Ágnes Molnár-Mondovics, and Barbara Ildikó Schmidtné Szantner. 2024. "Effect of Water Retainer® During Seedling Period on Bioactive Components of Tomato (Solanum lycopersicum)" Agronomy 14, no. 12: 2799. https://doi.org/10.3390/agronomy14122799
APA StyleTömösközi-Farkas, R. A., Molnár-Mondovics, Á., & Schmidtné Szantner, B. I. (2024). Effect of Water Retainer® During Seedling Period on Bioactive Components of Tomato (Solanum lycopersicum). Agronomy, 14(12), 2799. https://doi.org/10.3390/agronomy14122799







