Assessment of Drought Responses of Wild Soybean Accessions at Different Growth Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experiment 1: Drought Response at the Vegetative Stage
2.2.1. Measurement of LWS
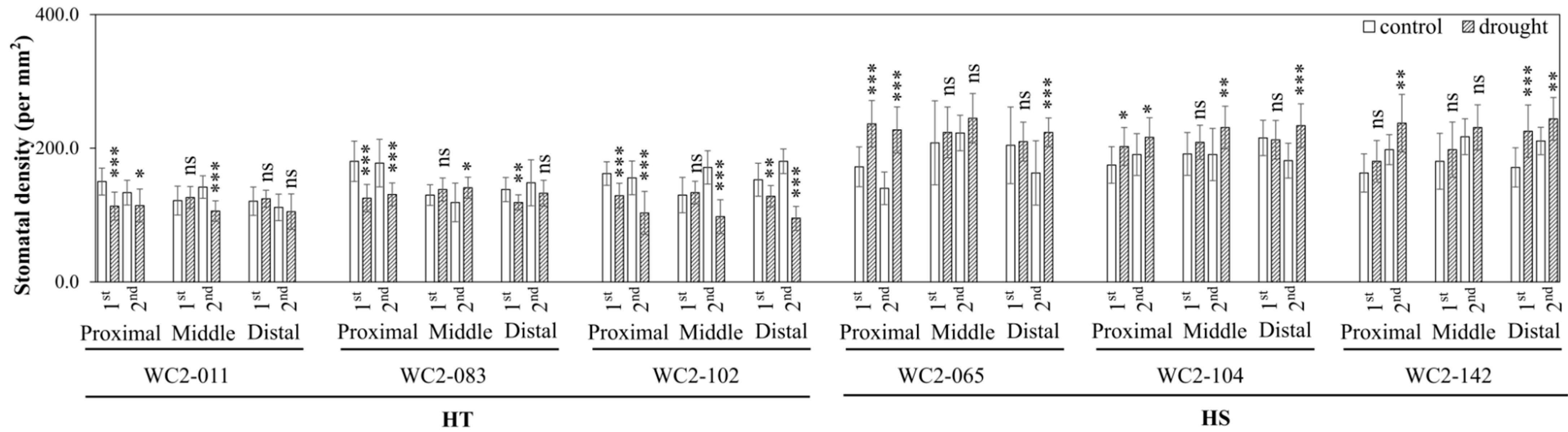
2.2.2. Stomatal Density
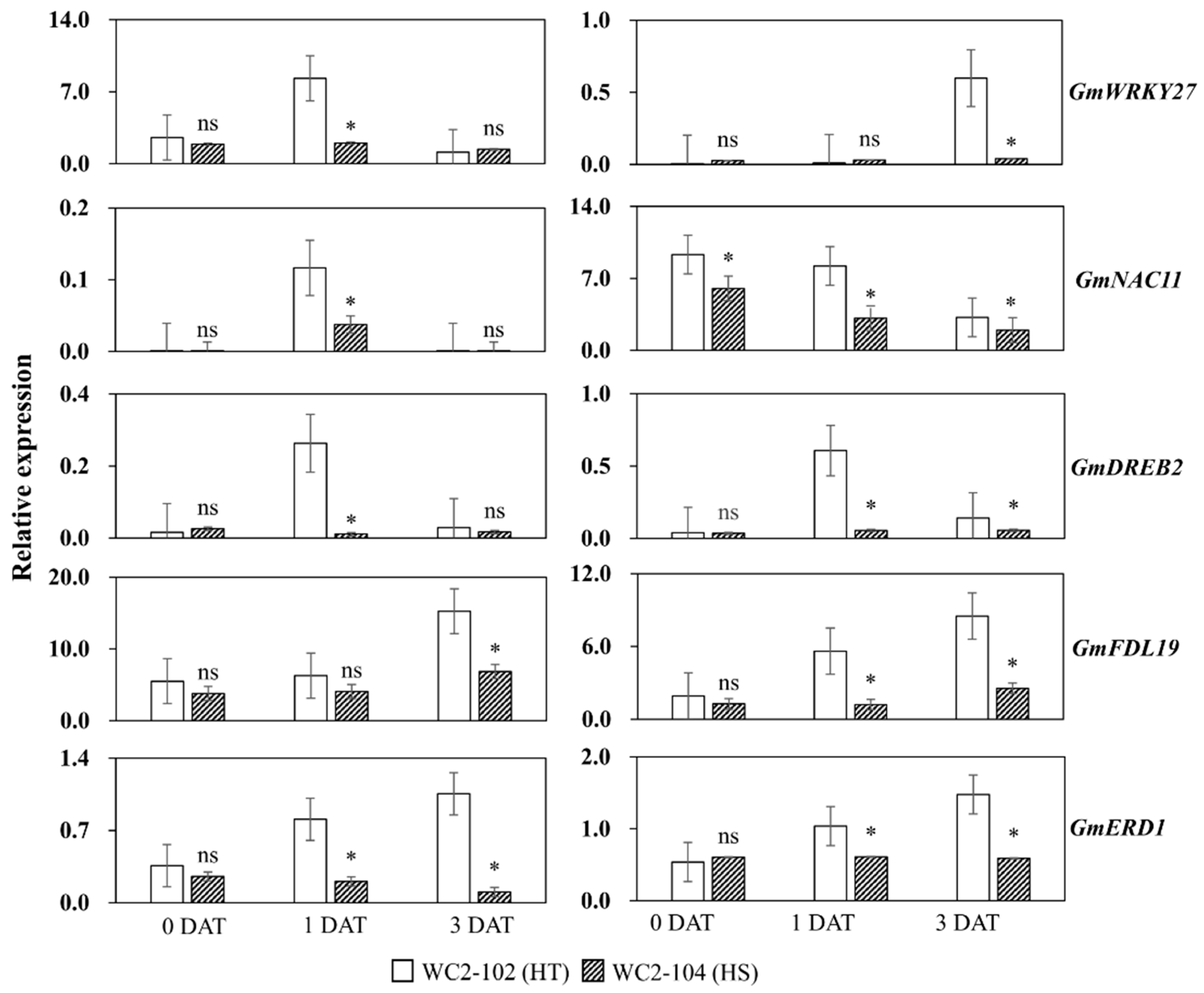
2.2.3. Isolation of RNA and qRT-PCR Analysis
2.3. Experiment 2: Drought Response at the Reproductive Stage
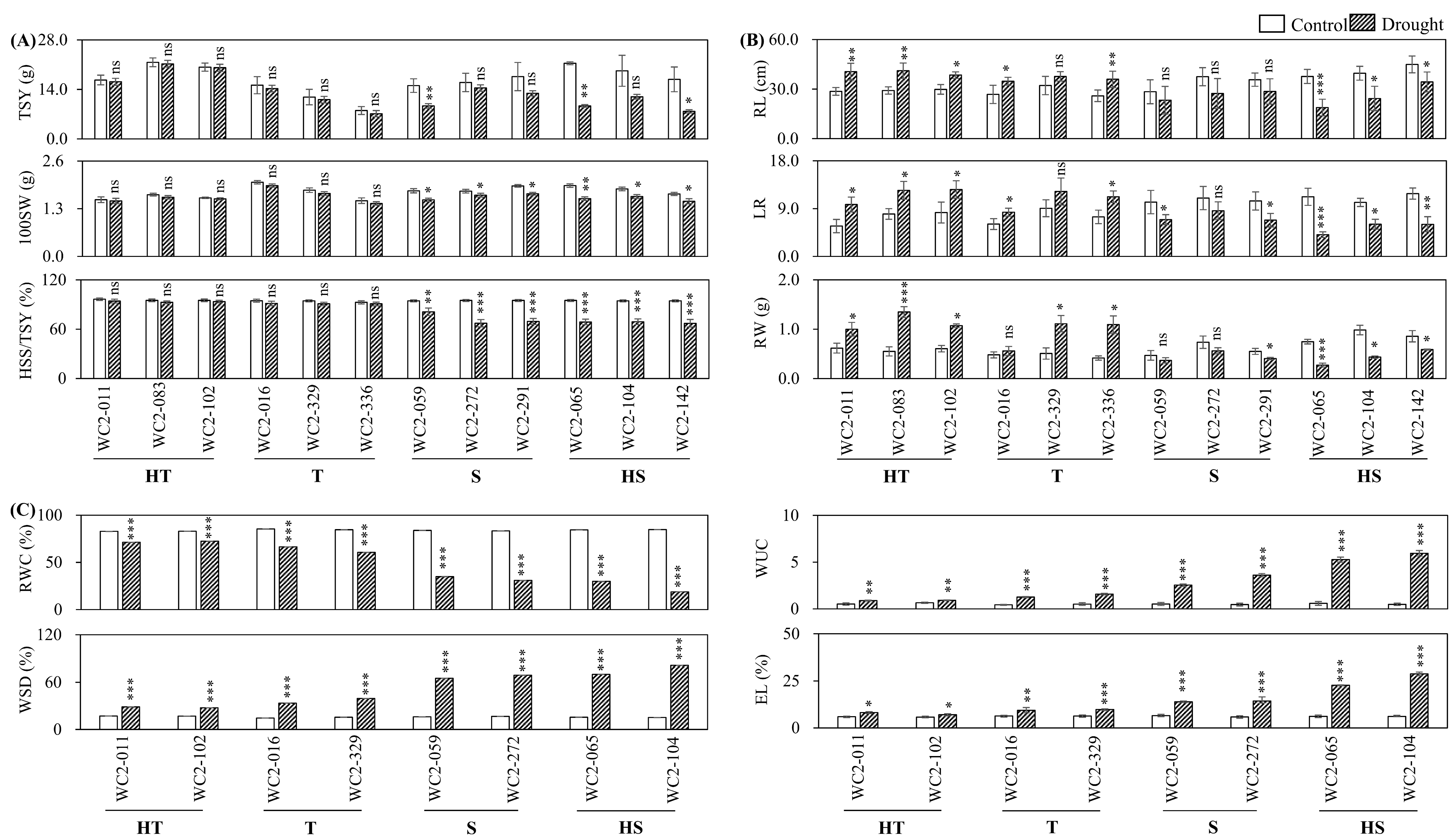
2.3.1. Seed Yield
2.3.2. Root System Architecture
2.3.3. Physiological Indicators
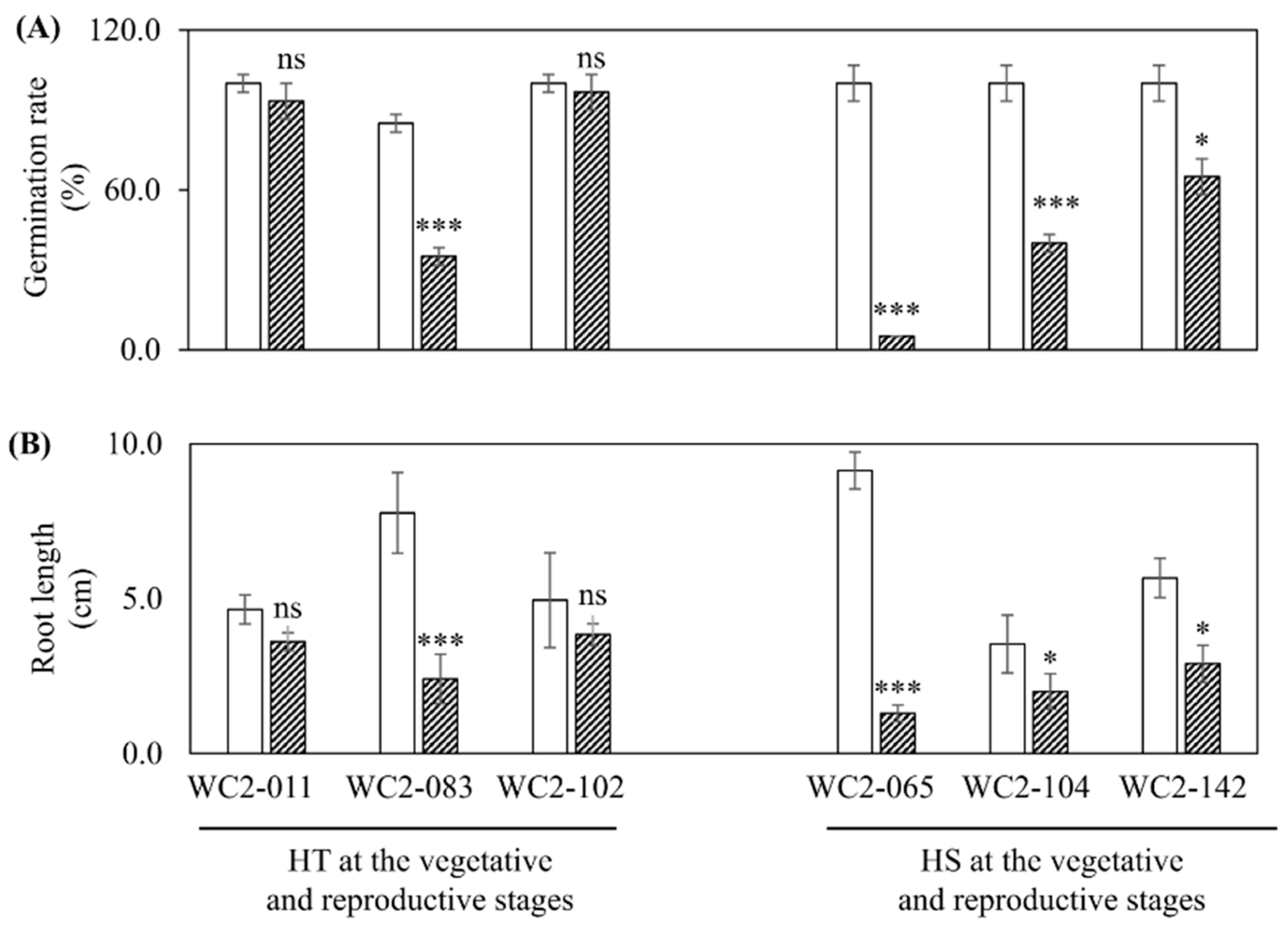
2.4. Experiment 3: Drought Response at the Germination Stage
2.5. Statistical Analysis
3. Results
3.1. Drought Tolerance at the Vegetative Stage (Experiment 1)
3.1.1. Stomatal Density
3.1.2. Expression Patterns of Drought-Related Genes
3.2. Drought Tolerance at the Reproductive Stage (Experiment 2)
3.2.1. Seed Yield
3.2.2. Root System Architecture
3.2.3. Physiological Indicators
3.3. Drought Tolerance at the Germination Stage (Experiment 3)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jane, A.B.; George, D.H.; Coppola, D.P. Hazards. In Homeland Security, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 45–66. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Khamidov, M.; Ishchanov, J.; Hamidov, A.; Shermatov, E.; Gafurov, Z. Impact of Soil Surface Temperature on Changes in the Groundwater Level. Water 2023, 15, 3865. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Hymowitz, T. On the domestication of the soybean. Econ. Bot. 1970, 23, 408–421. [Google Scholar] [CrossRef]
- Li, L.F.; Olsen, K.M. To have and to hold: Selection for seed and fruit retention during crop domestication. Curr. Top. Dev. Biol. 2016, 119, 63–109. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Lin, X.; Chan, T.-F.; Ham, J.; Shin, T.-S.; Ercisli, S.; Golokhvast, K.S.; Lam, H.-M.; Chung, G. Korean Wild Soybeans (Glycine soja Sieb & Zucc.): Geographic Distribution and Germplasm Conservation. Agronomy 2020, 10, 214. [Google Scholar] [CrossRef]
- Hobbs, E.H.; Muendel, H.H. Water requirements of irrigated soybeans in Southern Alberta. Can. J. Plant Sci. 1993, 63, 855–860. [Google Scholar] [CrossRef]
- Kpoghomou, B.K.; Sapra, V.T.; Beyl, C.A. Screening for Drought Tolerance: Soybean Germination and its Relationship to Seedling Responses. J. Agric. Crop Sci. 1990, 164, 153–159. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Guttikonda, S.K.; Phan Tran, L.S.; Nguyen, H.T. Physiological and Molecular Approaches to Improve Drought Resistance in Soybean. Plant Cell Physiol. 2009, 50, 1260–1276. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Fleury, D.J.; Stephen, K.; Haydn, L.P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yu, D.; Fu, S. Detection of quantitative trait loci for yield and drought tolerance traits in soybean using a recombinant inbred line population. J. Integr. Plant Biol. 2009, 51, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007, 143, 78–87. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A. Evolution of the stomatal regulation of plant water content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef]
- Ye, H.; Song, L.; Schapaugh, W.T.; Ali, M.L.; Sinclair, T.R.; Riar, M.K.; Mutava, R.N.; Li, Y.; Vuong, T.; Valliyodan, B.; et al. The importance of slow canopy wilting in drought tolerance in soybean. J. Exp. Bot. 2019, 71, 457–460. [Google Scholar] [CrossRef]
- Fromm, H. Root Plasticity in the Pursuit of Water. Plants 2019, 8, 236. [Google Scholar] [CrossRef]
- Mwenye, O.J.; Rensburg, L.V.; Van der Merwe, R. Seedling Shoot and Root Growth Responses among Soybean (Glycine max) Genotypes to Drought Stress. In Soybean-Biomass, Yield and Productivity; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Zhang, W.; Qin, M.; Li, S.; Qiao, M.; Liu, Z.; Xiang, F. Drought tolerance conferred in soybean (Glycine max. L.) by GmMYB84, a novel R2R3-MYB transcription factor. Plant Cell Physiol. 2017, 58, 1764–1776. [Google Scholar] [CrossRef]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Van, N.T. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Manna, M.; Thakur, T.; Chirom, O.; Mandlik, R.; Deshmukh, R.; Salvi, P. Transcription factors as key molecular target to strengthen the drought stress tolerance in plants. Physiol. Plant. 2021, 172, 847–868. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, A.G.; Soltani, Z.; Tahmasebi, A.; Poczai, P. Integrative system biology analysis of transcriptomic responses to drought stress in soybean (Glycine max L.). Genes 2022, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Sintaha, M.; Man, C.K.; Yung, W.S.; Duan, S.; Li, M.W.; Lam, H.M. Drought Stress Priming Improved the Drought Tolerance of Soybean. Plants 2022, 11, 2954. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, H.W.; Li, Q.T.; Wei, W.; Li, W.; Zhang, W.K.; Ma, B.; Bi, Y.D.; Lai, Y.C.; Liu, X.L.; et al. GmWRKY27 interacts with GmMYB174 to reduce expression of GmNAC29 for stress tolerance in soybean plants. Plant J. 2015, 83, 224–236. [Google Scholar] [CrossRef]
- Chen, C.T.; Lu, C.T.; Tzen, J.T.; Yang, C.Y. Physiological Properties and Molecular Regulation in Different Edamame Cultivars under Drought Stress. Agronomy 2021, 11, 939. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.Y.; Cheng, X.G.; Xu, Z.S.; Li, L.C.; Ye, X.G.; Ma, Y.Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Nan, H.; Li, X.; Lu, S.; Zhao, X.; Liu, B.; Guo, C.; Kong, F.; Cao, D. Overexpression of GmFDL19 enhances tolerance to drought and salt stresses in soybean. PLoS ONE 2017, 22, e0179554. [Google Scholar] [CrossRef]
- Hao, Y.J.; Wei, W.; Song, Q.X.; Chen, H.W.; Zhang, Y.Q.; Wang, F.; Chen, S.Y. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J. 2011, 68, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Moon, J.K.; Park, S.K.; Kim, M.S.; Lee, K.; Lee, S.R.; Park, E. Genetic diversity patterns and domestication origin of soybean. Theor. Appl. Genet. 2019, 132, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.V.; Le, D.T.; Nishiyama, R. Characterization of the newly developed soybean cultivar DT2008 in relation to the model variety W82 reveals a new genetic resource for comparative and functional genomics for improved drought tolerance. BioMed Res. Int. 2013, 2013, 759657. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Van Ha, C.; Nasr Esfahani, M.; Watanabe, Y.; Nishiyama, R.; Pham, C.T.; Van Nguyen, D.; Tran, L.S. DT2008: A promising new genetic resource for improved drought tolerance in soybean when solely dependent on symbiotic N2 fixation. BioMed Res. Int. 2015, 2015, 687213. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.; Van, T.K. Study on concentrations of drought- induced sucrose and dynamics of enzyme amylase, protease at germination stage of soybean varieties in artificial drought condition. VNU J. Sci Nat. Sci. Technol. 2017, 33. [Google Scholar] [CrossRef]
- Sharma, N. Leaf Clearing Protocol to Observe Stomata and Other Cells on Leaf Surface. Bio-Protocol 2017, 7, e2538. [Google Scholar] [CrossRef]
- Libault, M.A.R.C.; Thibivilliers, S.A.N.D.R.A.; Bilgin, D.D.; Radwan, O.; Benitez, M.; Clough, S.J.; Stacey, G. Identification of four soybean reference genes for gene expression normalization. Plant Genome 2008, 1, 44–54. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Singh, D.P.; Singh, H.; Sharma, H.C. Differences in osmoregulation in Brassica species. Ann. Bot. 1984, 54, 537–541. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 594–597. [Google Scholar]
- Shu, Y.; Zhou, Y.; Shi, X.; Hu, N.; Shao, Q.; Du, J. Screening of appropriate PEG-6000 concentration for the identification of soybean drought tolerance at germination stage. Soybean Sci. 2015, 1, 56–59. [Google Scholar]
- Talebi, R.; Fayaz, F.; Naji, N. Effective selection criteria for assessing drought stress tolerance in durum wheat (Triticum durum Desf.). Gen. Appl. Plant Physiol. 2009, 35, 64–74. [Google Scholar]
- Sloane, R.J.; Patterson, R.P.; Carter, T.E., Jr. Field drought tolerance of a soybean plant introduction. Crop Sci. 1990, 30, 118–123. [Google Scholar] [CrossRef]
- Kunert, K.; Vorster, B.J. In search for drought-tolerant soybean: Is the slow-wilting phenotype more than just a curiosity? J. Exp. Bot. 2020, 71, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Inamullah; Isoda, A. Adaptive Responses of Soybean and Cotton to Water Stress: I. Transpiration Changes in Relation to Stomatal Area and Stomatal Conductance. Plant Prod. Sci. 2005, 8, 16–26. [Google Scholar] [CrossRef]
- Tripathi, P.; Rabara, R.C.; Reese, R.N.; Miller, M.A.; Rohila, J.S.; Subramanian, S.; Shen, Q.J.; Morandi, D.; Bücking, K.; Rushton, P.J.; et al. A toolbox of genes, proteins, metabolites and promoters for improving drought tolerance in soybean includes the metabolite coumestrol and stomatal development genes. BMC Genom. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.T.; de Castro, S.A.Q.; de Andrade, J.F.; Politano, A.A.; Meneghetti, E.C.; Favarin, J.L.; de Almeida, M.; Mazzafera, P. Drought stress induces changes in the physiology and root system of soybean plants. Braz. J. Bot. 2021, 44, 779–789. [Google Scholar] [CrossRef]
- Buttery, B.R.; Tan, C.S.; Buzzell, R.I.; Gaynor, J.D.; MacTavish, D.C. Stomatal numbers of soybean and response to water stress. Plant Soil 1993, 149, 283–288. [Google Scholar] [CrossRef]
- Mano, N.A.; Madore, B.; Mickelbart, M.V. Different Leaf Anatomical Responses to Water Deficit in Maize and Soybean. Life 2023, 13, 290. [Google Scholar] [CrossRef]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Morales-Navarro, S.; Pérez-Díaz, R.; Ortega, A.; De Marcos, A.; Mena, M.; Fenoll, C.; Ruiz-Lara, S. Overexpression of a SDD1-like gene from wild tomato decreases stomatal density and enhances dehydration avoidance in Arabidopsis and cultivated tomato. Front. Plant Sci. 2018, 9, 940. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Hamanishi, E.T.; Thomas, B.R.; Campbell, M.M. Drought induces alterations in the stomatal development program in Populus. J. Exp. Bot. 2012, 63, 4959–4971. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Struik, P.C.; Yin, X.; Yang, J. Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 2017, 68, 5191–5205. [Google Scholar] [CrossRef]
- Clifford, S.C.; Black, C.R.; Roberts, J.A.; Stronach, I.M.; Singleton-Jones, P.R.; Mohamed, A.D.; Azam-Ali, S.N. The effect of elevated atmospheric CO2 and drought on stomatal frequency in groundnut (Arachis hypogaea (L.)). J. Exp. Bot. 1995, 46, 847–852. [Google Scholar] [CrossRef]
- Bosabalidis, A.M.; Kofidis, G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002, 163, 375–379. [Google Scholar] [CrossRef]
- Yang, H.M.; Wang, G.X. Leaf Stomatal Densities and Distribution in Triticum aestivum under Drought and CO2 Enrichment. Chin. J. Plant Ecol. 2001, 25, 312–316. Available online: https://www.plant-ecology.com/EN/Y2001/V25/I3/312 (accessed on 1 February 2024).
- Zhang, Y.P.; Wang, Z.M.; Wu, Y.C.; Zhang, X. Stomatal characteristics of different green organs in wheat under different irrigation regimes. Acta Agron. Sin. 2006, 32, 70–75. [Google Scholar]
- Gan, Y.; Zhou, L.; Shen, Z.J.; Shen, Z.X.; Zhang, Y.Q.; Wang, G.X. Stomatal clustering, a new marker for environmental perception and adaptation in terrestrial plants. Bot. Stud. 2010, 51, 325–336. [Google Scholar]
- Orsini, F.; Alnayef, M.; Bona, S.; Maggio, A.; Gianquinto, G. Low stomatal density and reduced transpiration facilitate strawberry adaptation to salinity. Environ. Exp. Bot. 2012, 81, 1–10. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Neves-Borges, A.C.; Guimarães-Dias, F.; Cruz, F. Expression pattern of drought stress marker genes in soybean roots under two water deficit systems. Genet. Mol. Biol. 2012, 35, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xia, Z.; Cai, Z.; Li, L.; Cheng, Y.; Liu, J.; Nian, H. GmWRKY16 Enhances Drought and Salt Tolerance Through an ABA-Mediated Pathway in Arabidopsis thaliana. Front. Plant Sci. 2019, 9, 1979. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Stolf-Moreira, R.; Lemos, E.G.; Carareto-Alves, L.; Marcondes, J.; Pereira, S.S.; Rolla, A.A.; Nepomuceno, A.L. Transcriptional profiles of roots of different soybean genotypes subjected to drought stress. Plant Mol. Biol. Rep. 2011, 29, 19–34. [Google Scholar] [CrossRef]
- Alves, M.S.; Fontes, E.P.; Fietto, L.G. Early responsive to dehydration 15, a new transcription factor that integrates stress signaling pathways. Plant Signal. Behav. 2011, 6, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.H.; Mostofa, M.G.; Li, W.; Van Ha, C.; Watanabe, Y.; Le, D.T.; Tran, L.S.P. The soybean transcription factor GmNAC085 enhances drought tolerance in Arabidopsis. Environ. Exp. Bot. 2018, 151, 12–20. [Google Scholar] [CrossRef]
- Thao, N.P.; Thu, N.B.A.; Hoang, X.L.T.; Ha, C.V.; Tran, L.S.P. Differential expression analysis of a subset of drought-responsive GmNAC genes in two soybean cultivars differing in drought tolerance. Int. J. Mol. Sci. 2013, 14, 23828–23841. [Google Scholar] [CrossRef]
- Pham, T.T.N.; Nguyen, H.Q.; Nguyen, T.N.L.; Dao, X.T.; Sy, D.T.; Van Son, L.; Hoang Mau, C. Overexpression of the “GmDREB2” gene increases proline accumulation and tolerance to drought stress in soybean plants. Aust. J. Crop Sci. 2020, 14, 495–503. [Google Scholar] [CrossRef]
- Chen, K.; Tang, W.; Zhou, Y.; Chen, J.; Xu, Z.; Ma, R.; Dong, Y.; Ma, Y.; Chen, M. AP2/ERF transcription factor GmDREB1 confers drought tolerance in transgenic soybean by interacting with GmERFs. Plant Physiol. Biochem. 2022, 170, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Marinho, J.P.; Pagliarini, R.F.; Molinari, M.D.C.; Marcolino-Gomes, J.; Caranhoto, A.L.H.; Marin, S.R.R.; Oliveira, M.C.N.; Foloni, J.S.S.; Melo, C.L.P.; Mertz-Henning, L.M.; et al. Overexpression of full-length and partial DREB2A enhances soybean drought tolerance. Agron. Sci. Biotechnol. 2022, 8, 1–21. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms, and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar] [CrossRef]
- Arya, H.; Singh, M.B.; Bhalla, P.L. Towards developing drought-smart soybeans. Front. Plant Sci. 2021, 12, 750664. [Google Scholar] [CrossRef] [PubMed]
- Kocheva, K.; Nenova, V.; Karceva, T.; Petrov, P.; Georgiev, G.I.; Börner, A.; Landjeva, S. Changes in water status, membrane stability, and antioxidant capacity of wheat seedlings carrying different Rht-B1 dwarfing alleles under drought stress. J. Agric. Crop Sci. 2014, 200, 83–91. [Google Scholar] [CrossRef]
- Fatema, M.K.; Mamun, M.A.A.; Sarker, U.; Hossain, M.S.; Mia, M.A.B.; Roychowdhury, R.; Ercisli, S.; Marc, R.A.; Babalola, O.O.; Karim, M.A. Assessing Morpho-Physiological and Biochemical Markers of Soybean for Drought Tolerance Potential. Sustainability 2023, 15, 1427. [Google Scholar] [CrossRef]
- Lobato, A.K.S.; Costa, R.C.L.; Oliveira, N.C.F.; Santos, F.B.G.; Cruz, F.J.R.; Freitas, J.M.N.; Cordeiro, F.C. Morphological changes in soybean under progressive water stress. J. Exp. Bot. 2008, 4, 231–235. [Google Scholar] [CrossRef]
- Hossain, M.M.; Liu, X.; Qi, X.; Lam, H.M.; Zhang, J. Differences between soybean genotypes in physiological response to sequential soil drying and rewetting. Crop J. 2014, 2, 366–380. [Google Scholar] [CrossRef]
- Coutinho, F.S.; Rodrigues, J.M.; Lima, L.L.; Mesquita, R.O.; Carpinetti, P.A.; Machado, J.P.B.; Vital, C.E.; Vidigal, P.M.; Ramos, M.E.S.; de Oliveira Ramos, H.J.; et al. Remodeling of the cell wall as a drought-tolerance mechanism of a soybean genotype revealed by global gene expression analysis. Abiotech 2021, 2, 14–31. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2007, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Specht, J.E.; Chase, K.; Macrander, M.; Graef, G.L.; Chung, J.; Markwell, J.P.; Germann, M.; Orf, J.H.; Lark, K.G. Soybean response to water. A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Mohammadian, R.; Moghaddam, M.; Ramanian, H.; Sadeghian, S.Y. Effect of early season drought stress on growth characteristics of sugar beet genotypes. Turk. J. Agric. For. 2005, 29, 357–368. [Google Scholar]
- Lafitte, H.R.; Yongsheng, G.; Yan, S.; Li, Z.K. Whole plant responses, key processes, and adaptation to drought stress: The case of rice. J. Exp. Bot. 2007, 58, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zdzislaw, A.T.; Sanoubar, R.; Pluhár, Z.; Mancarella, S.; Orsini, F.; Gianquinto, G. Morphological and physiological plant responses to drought stress in Thymus citriodorus. Int. J. Agron. 2016, 2016, 4165750. [Google Scholar] [CrossRef]
- Xiong, R.; Liu, S.; Considine, M.J.; Siddique, K.H.M.; Lam, H.M.; Chen, Y. Root system architecture, physiological and transcriptional traits of soybean (Glycine max L.) in response to water deficit: A review. Physiol. Plant 2020, 172, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.G.; Jubery, T.Z.; O’Rourke, J.A.; Singh, A.; Sarkar, S.; Ganapathysubramanian, B.; Singh, A.K. Soybean root system architecture trait study through genotypic, phenotypic, and shape-based clusters. Plant Phenomics 2020, 2020, 1925495. [Google Scholar] [CrossRef]
- Türkan, I.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius and drought-sensitive P. vulgaris subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Landjeva, S.; Ganeva, G. Changes in the seedling growth parameters in three common wheat (Triticum aestivum. L) cultivars subjected to drought stress and subsequent re-hydration. Field Crop Stud. 2006, 3, 185–190. [Google Scholar]
- Kocheva, K.; Georgiev, G. Evaluation of the reaction of two contrasting barley (Hordeum vulgare L.) cultivars in response to osmotic stress with PEG 6000. Bulg. J. Plant Physiol. 2003, 49, 290–294. [Google Scholar]
- Turner, N.C. Further progress in crop water relations. Adv. Agron. 1996, 58, 293–338. [Google Scholar] [CrossRef]
- Abd, A.A.; Badawy, S.A.; Zayed, B.A.; El-Gohary, A.A. The role of root system traits in the drought tolerance of rice (Oryza sativa L.). J. Plant Prod. 2010, 1, 621–631. [Google Scholar] [CrossRef]
- Fraser, T.E.; Silk, W.K.; Rost, T.L. Effects of low water potential on cortical cell length in growing regions of maize roots. Plant Physiol. 1990, 93, 648–651. [Google Scholar] [CrossRef]
- Kosturkova, G.; Todorova, R.; Sakthivelu, G.; Akitha Devi, M.K.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Response of Bulgarian and Indian soybean genotypes to drought and water deficiency in field and laboratory conditions. Gen. Appl. Plant Physiol. 2008, 34, 239–250. [Google Scholar]

| Drought Condition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | RL | LR | RW | RWC | WSD | WUC | EL | 100SW | HSW/TSY | TSY | |
| Control condition | RL | 1 | 0.83 ** | 0.89 *** | 0.92 ** | −0.92 ** | −0.86 ** | −0.81 * | −0.02 | 0.77 ** | 0.50 |
| LR | 0.85 ** | 1 | 0.94 *** | 0.77 * | −0.77 * | −0.80 * | −0.79 * | −0.02 | 0.82 ** | 0.55 | |
| RW | 0.86 ** | 0.62 * | 1 | 0.81 * | −0.81 * | −0.75 * | −0.72 * | −0.21 | 0.80 ** | 0.50 | |
| RWC | 0.15 | 0.10 | −0.00 | 1 | −1.00 *** | −0.93 ** | −0.89 ** | 0.19 | 0.95 ** | 0.63 | |
| WSD | −0.15 | −0.10 | 0.00 | −1.00 ** | 1 | 0.93 ** | 0.89 ** | −0.19 | −0.95 ** | −0.63 | |
| WUC | −0.09 | 0.08 | −0.01 | −0.49 | 0.49 | 1 | 0.98 ** | −0.17 | −0.93 ** | −0.53 | |
| EL | −0.34 | −0.03 | −0.50 | 0.59 | −0.59 | −0.38 | 1 | −0.16 | −0.84 ** | −0.53 | |
| 100SW | 0.27 | 0.33 | 0.09 | 0.92 ** | −0.92 ** | −0.51 | 0.56 | 1 | 0.03 | 0.25 | |
| HSW/TSY | 0.01 | −0.28 | 0.16 | −0.53 | 0.53 | −0.13 | −0.18 | −0.08 | 1 | 0.45 | |
| TSY | 0.35 | 0.27 | 0.50 | −0.15 | 0.15 | 0.57 | −0.52 | 0.22 | 0.32 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.C.; Jo, H.; Tran, H.A.; Lee, J.; Lee, J.-D.; Kim, J.H.; Seo, H.S.; Song, J.T. Assessment of Drought Responses of Wild Soybean Accessions at Different Growth Stages. Agronomy 2024, 14, 471. https://doi.org/10.3390/agronomy14030471
Nguyen TC, Jo H, Tran HA, Lee J, Lee J-D, Kim JH, Seo HS, Song JT. Assessment of Drought Responses of Wild Soybean Accessions at Different Growth Stages. Agronomy. 2024; 14(3):471. https://doi.org/10.3390/agronomy14030471
Chicago/Turabian StyleNguyen, Thi Cuc, Hyun Jo, Hai Anh Tran, Jinwon Lee, Jeong-Dong Lee, Jeong Hoe Kim, Hak Soo Seo, and Jong Tae Song. 2024. "Assessment of Drought Responses of Wild Soybean Accessions at Different Growth Stages" Agronomy 14, no. 3: 471. https://doi.org/10.3390/agronomy14030471
APA StyleNguyen, T. C., Jo, H., Tran, H. A., Lee, J., Lee, J.-D., Kim, J. H., Seo, H. S., & Song, J. T. (2024). Assessment of Drought Responses of Wild Soybean Accessions at Different Growth Stages. Agronomy, 14(3), 471. https://doi.org/10.3390/agronomy14030471









