Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Measurement Items and Methods
2.3.1. Determination of Chemical Composition and Quality Evaluation of Tobacco Leaves after Flue-Curing
2.3.2. Soil Biological Shape Determination
2.4. Data Processing
3. Results
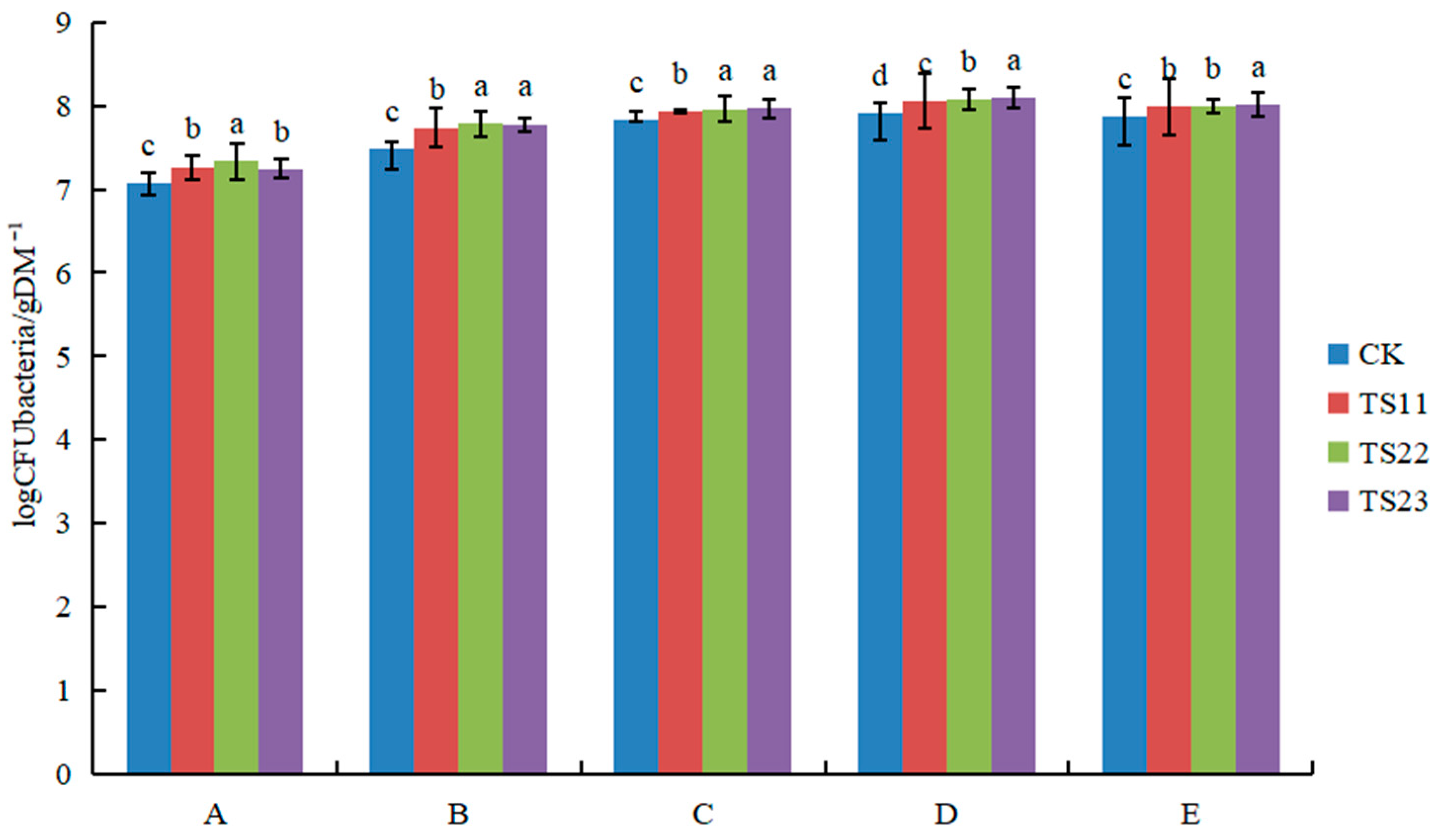
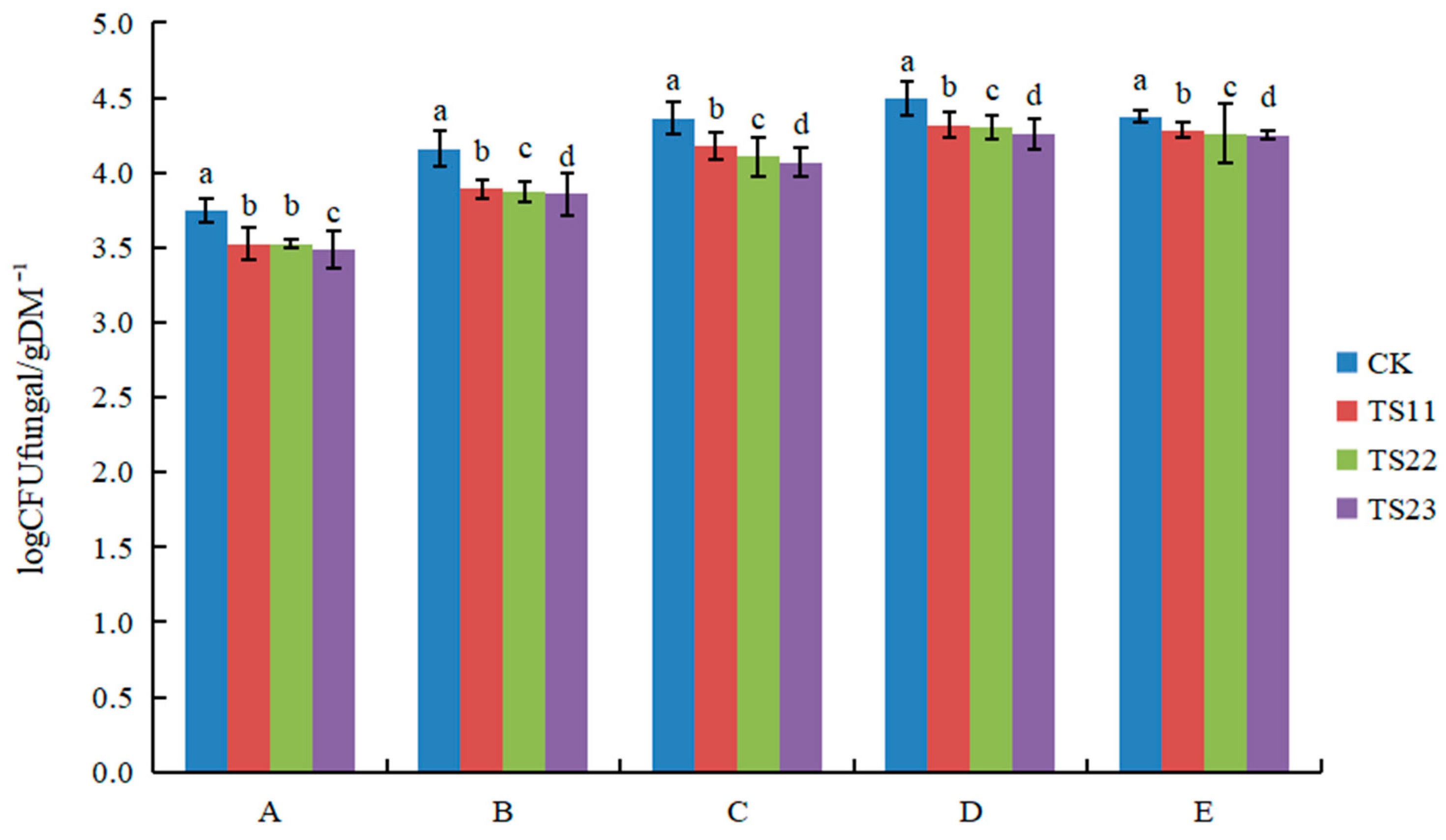
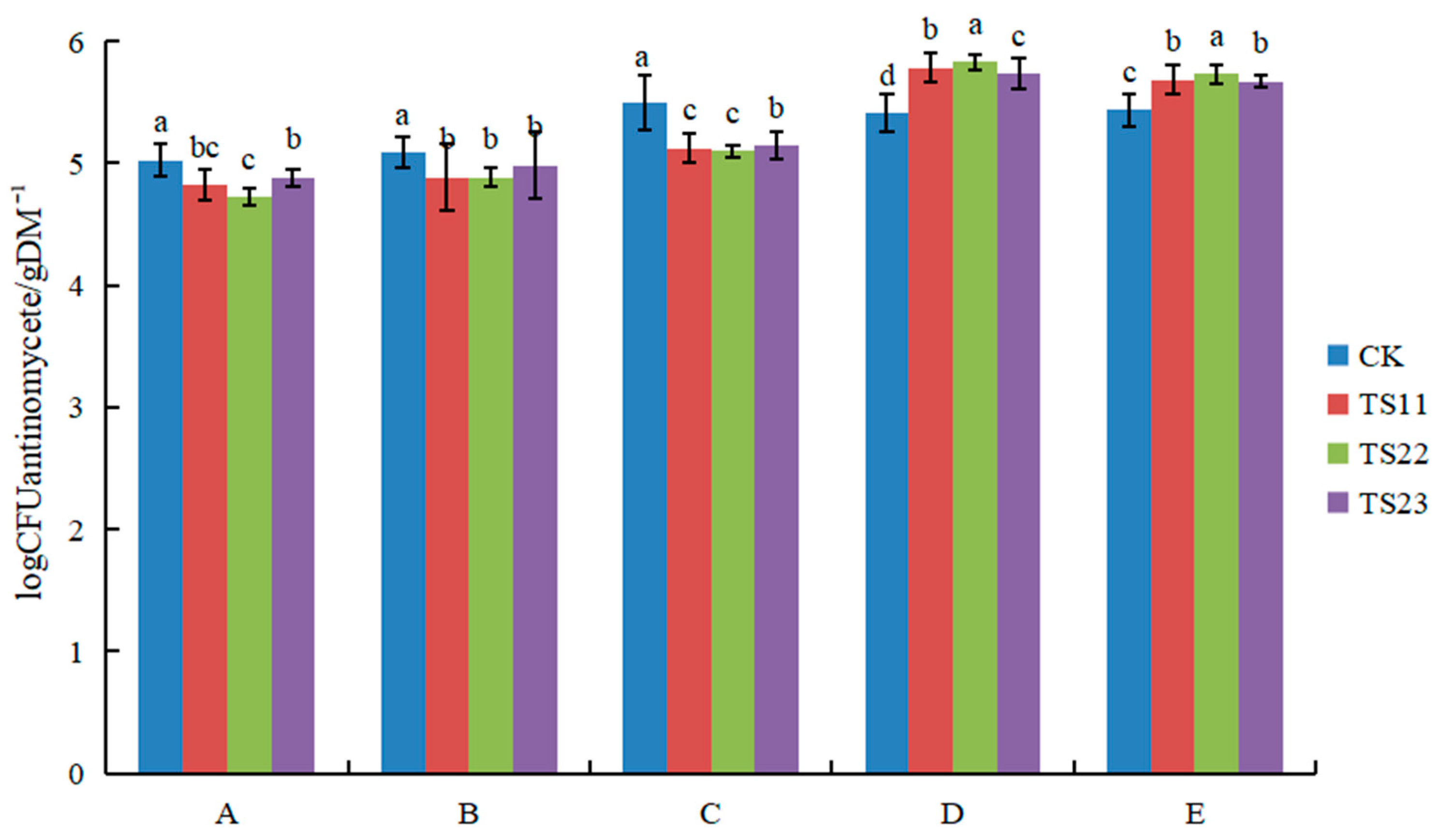
3.1. Effect of Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza on Soil Microbial Population
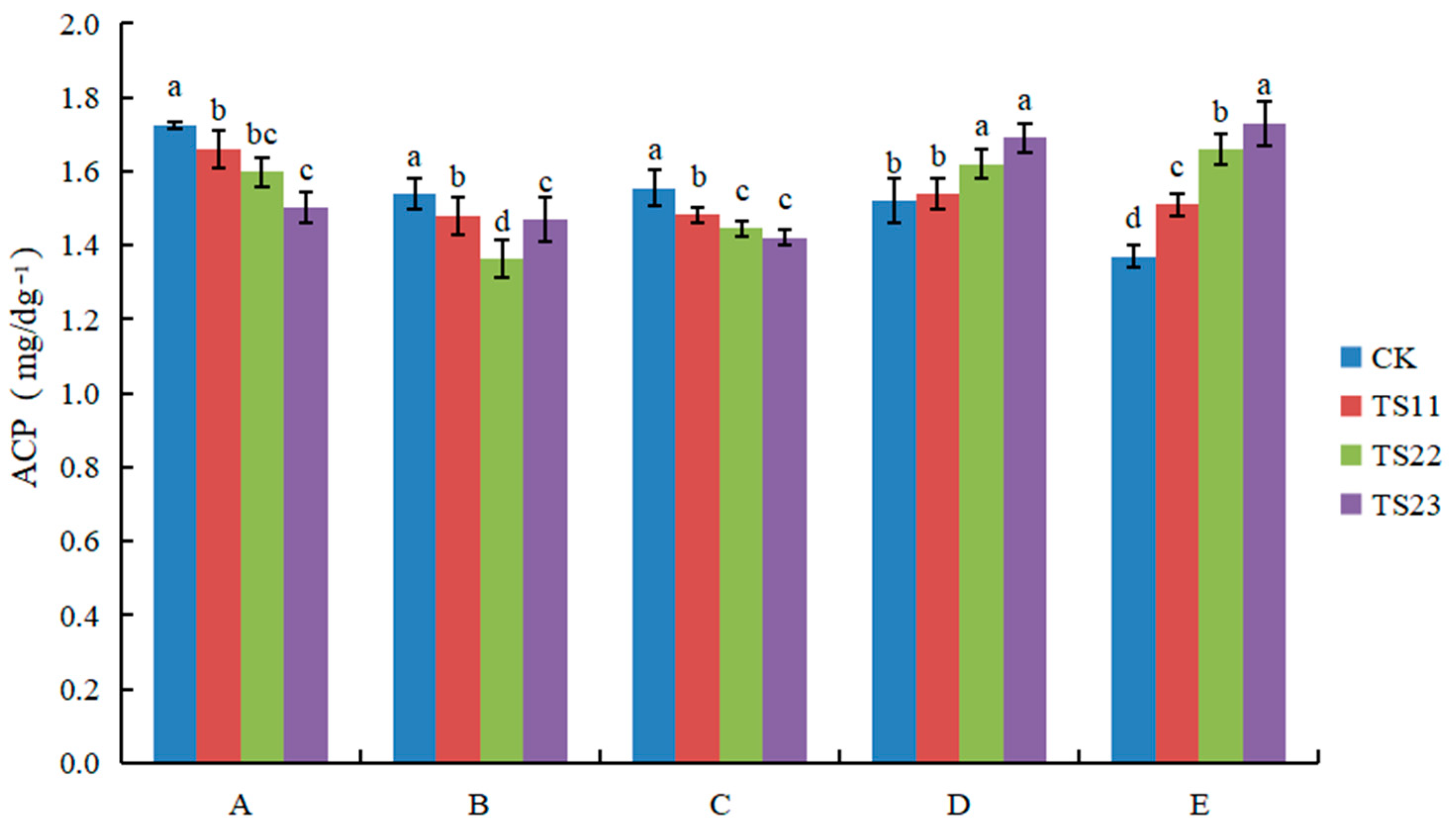
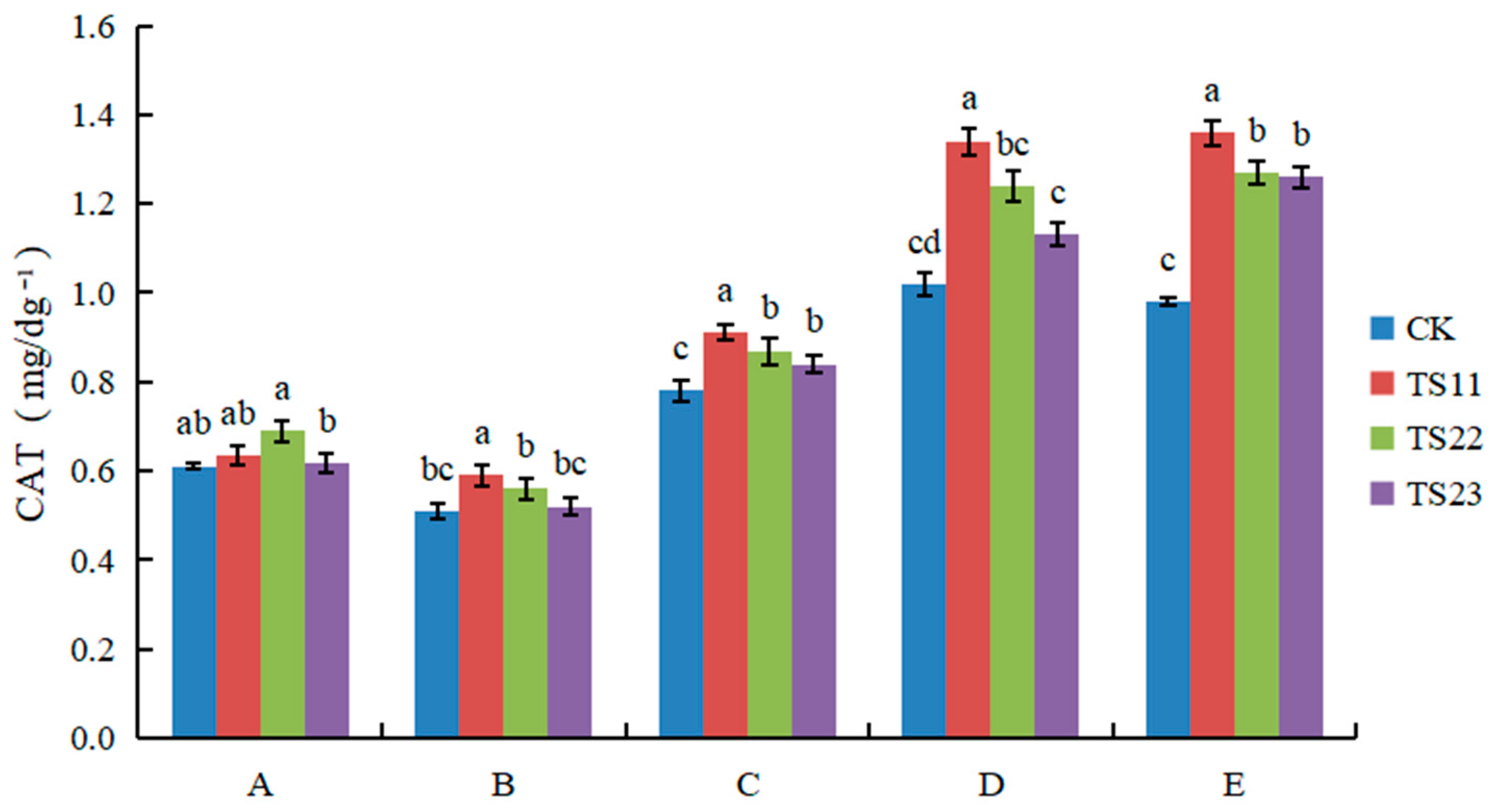
3.2. Effect of Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza on Soil Enzyme Activities
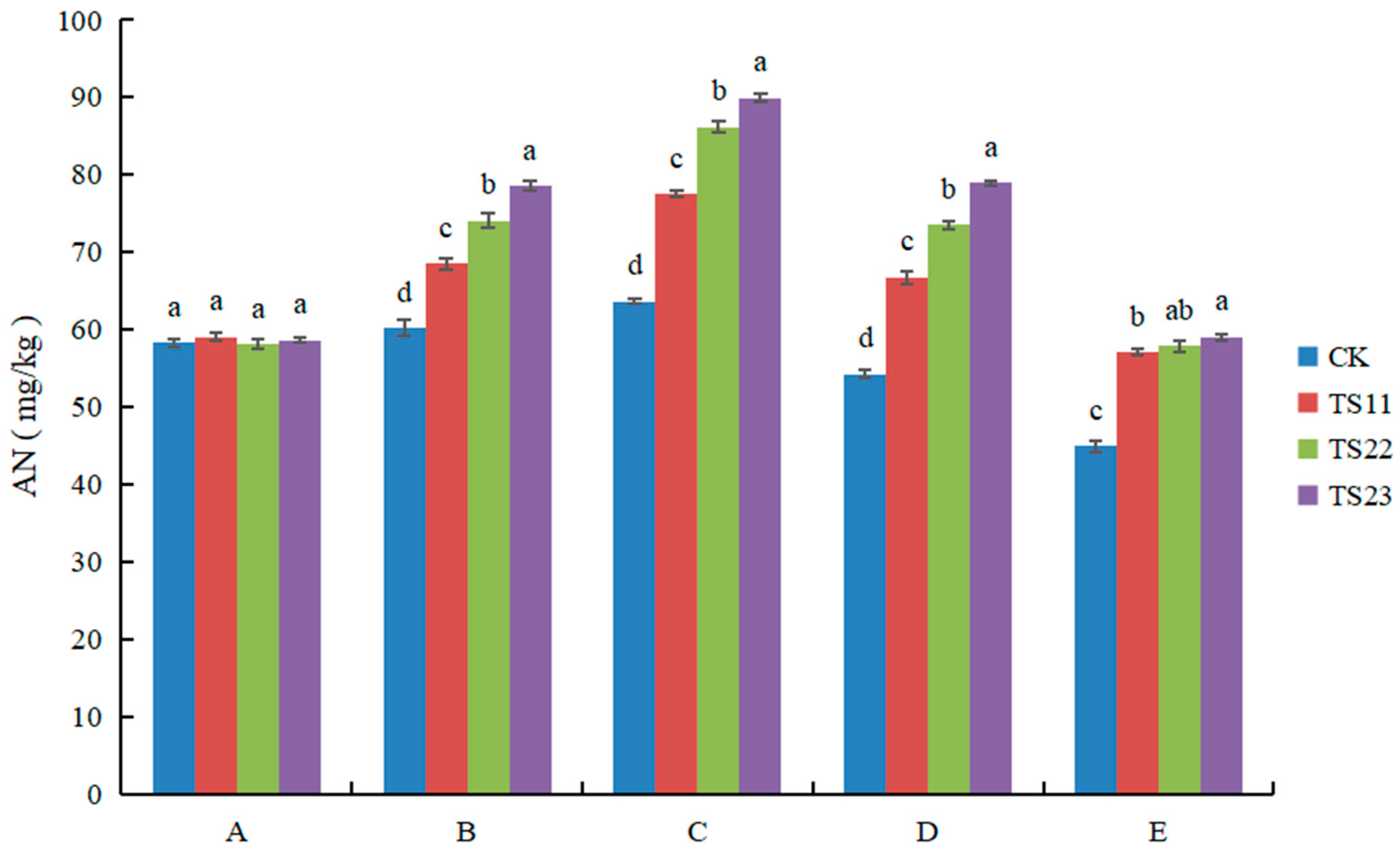
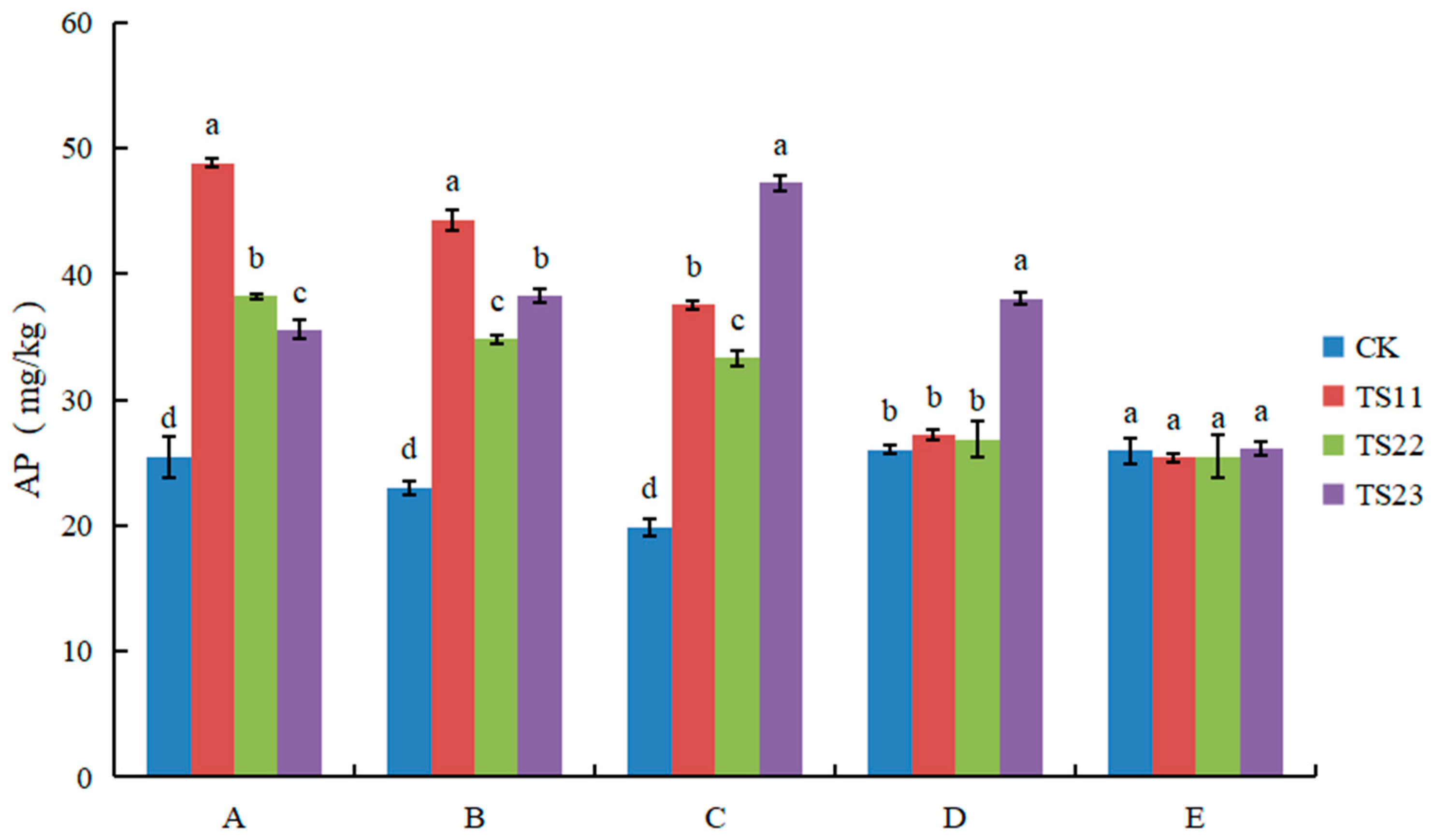
3.3. Effect of Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza on Available Nitrogen, Phosphorus and Potassium Content of Soil
3.4. Effects of Tobacco-Tansy Intercropping on Yield and Quality of Flue-Cured Tobacco
4. Discussion
4.1. Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza Changed Soil Microflora
4.2. Intercropping of Flue-Cured Tobacco and Salvia miltiorrhiza Enhanced the Absorption of Soil Nutrients
4.3. Intercropping Salvia miltiorrhiza Increased Total Output Value and Improved the Quality of Flue-Cured Tobacco
4.4. Intercropping Salvia miltiorrhiza Alleviates Continuous Cropping Obstacles and Reflects Intercropping Advantages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, C.; Gao, F.; Wang, F.; Tang, S.; Gu, L.; Zhang, T.; Xu, Z.; Zhang, Z.; Institute of Tobacco Science and Technology; Fujian Agriculture and Forestry University; et al. Effect of continuous cropping on rhizosphere microecology and tobacco production quality of flue-cured tobacco in Yunnan. J. Chin. Tob. 2015, 21, 60–67. [Google Scholar]
- Chen, D. Ecological Regulation Mechanism of Tobacco Continuous Cropping Obstacle by Crop Diversity Cultivation. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2010. [Google Scholar]
- Yan, J.; Yan, Y.; Duan, Y.; Long, Y.; Ye, C. Preliminary report on the influence of flue-cured tobacco on tobacco yield and quality. Tob. Technol. 2002, 41–45. [Google Scholar]
- Jiang, P. Preliminary study on the influence of different continuous cropping years on soil nutrients. Hubei Today 2015, 10, 92–93. [Google Scholar]
- Wang, Y.; Yu, H.; Li, X.; Lu, Z. Progress on continuous cropping disorders of Chinese medicinal materials. New Agric. 2023, 5, 27–28. [Google Scholar]
- Daniela, C.; Andrew, B.; Peter, H.; Thrall, G.B. Plantgenus (Acacia and Eucalyptus) alters soil microbial community structure and relative abundance within revegetated shelterbelts. Appl. Soil Ecol. 2019, 133, 1–11. [Google Scholar]
- Liu, W.; Zhou, B.; Wang, X.; Lu, H.; Guo, L.; Li, F. Effects of efficient ecological intercropping mode on the growth and active ingredient content of Salvia miltiorrhiza. Chin. Herb. Med. 2018, 41, 1027–1030. [Google Scholar]
- Deng, G.; Li, X.; Zhang, H.; Ren, W.; Zhang, J.; Wang, X.; Shan, C. Effects of intercropping maize on the growth of Salvia miltiorrhiza. Anhui Agric. Sci. 2017, 45, 122–123. [Google Scholar]
- He, X.; Xiao, C.; Ma, G.; Xu, A.; Dai, X. Effects of soybean in tobacco field on soil microorganisms and antagonistic microbial communities. In Proceedings of the Annual Conference of the Chinese Plant Protection Society, Beijing, China, 16–22 August 2009. [Google Scholar]
- Zhu, H. Tobacco sweet potato intercropping set-planting high-yield cultivation technology. Grassroots Agric. Technol. Promot. 2018, 10, 2. [Google Scholar]
- Jing, Y.; Guo, X.; Wang, X.; Niu, H.; Han, D.; Xu, Z. Effect of intercropping ginger on production quality, soil bacterial quantity and physical and chemical properties of flue-cured tobacco. Shandong Agric. Sci. 2022, 54, 86–94. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, X.; Xu, R.; Wang, X.; Niu, H.; Han, D.; Shao, H. Effect of flue-cured tobacco intercropping on the growth and yield and quality of flue-cured tobacco. China Agric. Sci. Technol. Her. 2023, 25, 161–169. [Google Scholar] [CrossRef]
- State Meteorological Administration. The China Meteorological Yearbook; Meteorological Publishing House: Beijing, China, 2019.
- Zhang, G.; Gong, Z. Methods for Laboratory Analysis of Soil Survey; Science Press: Beijing, China, 2012. [Google Scholar]
- World Reference Base for Soil Resources. World Reference Base for Soil Resources 2014; Version 3.03; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- GB2635-92; Flue-Cured Tobacco. China Standards Press: Beijing, China, 2003.
- Jia, M.; Jia, L.; Fu, W.; Chen, B.; Zhao, Q.; Liang, H.; Wang, J.; Liu, J.; Zhou, Q.; Wang, G. Effects of different well cellar depths on soil temperature and humidity and quality of roasted tobacco production. South. J. Agric. 2019, 50, 2141–2148. [Google Scholar]
- YC/T 159-2002; Determination of Water-Soluble Sugar in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- YC/T 160-2002; Determination of Total Alkaloid in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- YC/T 161-2002; Determination of Total Nitrogen in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- YC/T 217-2007; Determination of Potassium in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2007.
- YC/T 162-2002; Determination of Chlorine in Tobacco and Tobacco Products Was Done by Continuous Flow Method. Standard Publishing House: Beijing, China, 2002.
- Guan, S.-Y. Soil Enzymes and Their Research Methods; Agricultural Press: Beijing, China, 1982; pp. 168–171. [Google Scholar]
- Xu, G.; Zheng, H. Handbook of Soil Microbiological Methods; Agricultural Press: Beijing, China, 1986; pp. 102–110. [Google Scholar]
- Rukun, L. Methods of Soil Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Liu, S.; Xia, X.; Chen, G.; Mao, D.; Che, S.; Li, Y. Progress in the study of soil enzymes. China Agric. Bull. 2011, 27, 1–7. [Google Scholar]
- Chen, W.; Jiang, Z.; Hu, Y.; Shu, H. The ecological characteristics of soil microorganisms in apple orchards. J. Soil Water Conserv. 2008, 3, 168–171. [Google Scholar]
- Zhang, D.; Wang, J.; Yang, S.; Zhang, X.; Liu, J.; Zhao, J.; He, D.; Yang, H.; Mo, J.; Gou, J.; et al. Effects of genotypes involved in tobacco intercropping on soil bacterial community structure. J. Grass Ind. 2017, 26, 120–130. [Google Scholar]
- Lai, R. Impacts of Planting Garlic on Tobacco Biotopes. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2011. [Google Scholar]
- Zhan, J.; Chen, Z. Tobacco Cultivation; Yunnan Science and Technology Press: Kunming, China, 1998; pp. 67–69. [Google Scholar]
- Meng, P.; Wang, J.; Song, Z.; Dong, L.; Zhang, J.; Yang, S. Characteristics of nitrogen, phosphorus and potassium accumulation and distribution in Salvia miltiorrhiza and its relationship with dry matter and salvinorin B accumulation. J. Plant Nutr. Fertil. 2013, 19, 940–945. [Google Scholar]
- Wang, P.; Zhu, L.; Chen, X.; Feng, H.; Sun, W.; Qin, N. Effects of intercropping Eustoma and shallot on soil nutrients, microbiota and enzyme activities. J. Plant Nutr. Fertil. 2018, 24, 668–675. [Google Scholar]
- Tofinger, M.P.; Snaydom, R.W. The root activity of cereals and peas when grown in pure stands and mixtures. Plant Soil 1992, 142, 281–285. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, M.; Liang, Z.; Ru, M.; Liu, Y.; Liu, F. Logistic modelling of root growth pattern of Salvia miltiorrhiza. Hubei Agric. Sci. 2014, 53, 1583–1589. [Google Scholar]
- Fan, F.; Zhang, F.; Lu, Y. Linking plant identity and interspecific competitionto soil nitrogen cycling through ammonia oxidiser communities. Soil Biol. Biochem. 2011, 43, 46–54. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, G.; Sun, C.; Jiang, Y.; Jiang, Y.; Liu, X. Dynamics of soil microbial nitrogen and its bioavailability. J. Plant Nutr. Fertil. 2003, 9, 87–90. [Google Scholar]
- Wang, H.; Wang, H.; Zhao, Q.; Zhuang, H.; Song, Y.; Zhu, Z. Effects of intercropping betel nut with vanilla orchid at different spacing on soil nutrients and microorganisms. J. Plant Nutr. Fertil. 2013, 19, 988–994. [Google Scholar]
- Sun, H.; Zhang, F. Acid phosphatase activity in wheat roots under phosphorus deficiency. J. Appl. Ecol. 2002, 13, 379–381. [Google Scholar]
- Shi, A.-D.; Li, J.-W.; Yuan, L. Effects of rotational intercropping system on yield, quality and soil nutrients of roasted tobacco. J. Plant Nutr. Fertil. 2011, 17, 411–418. [Google Scholar]
- Zhang, D. Effects of Crop Rotation and Intercropping on the Growth Status and Yield Quality of Roasted Tobacco KRK26. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2012. [Google Scholar]
- Li, T.; Ma, G. Effects of seed amaranth-tobacco intercropping on the content and quality of some mineral elements in tobacco leaves. J. Soil Water Conserv. 2004, 18, 138–141. [Google Scholar]
- Yang, T.; Xia, W.; Fan, J. Study on the characteristics of potassium accumulation in tobacco under different levels of potassium supply. Henan Sci. 2005, 23, 375–378. [Google Scholar]
- Mulchi, C.L. Chloride effects on agronomic and physical properties of Maryland tobacco. Tob. Sci. 1982, 26, 13–16. [Google Scholar]
- Wang, P. Progress of research on continuous cropping obstacles and control of tobacco. Anhui Agric. Bull. 2023, 29, 24–26+32. [Google Scholar] [CrossRef]
- Hao, W.Y.; Ren, L.X.; Ran, W.; Shen, Q. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Janvier, C.; Villeneuve, F.; Alabouvette, C.; Edel-Hermann, V.; Mateille, T.; Steinberg, C. Soil health through soil disease suppression: Which strategy from descriptors to indicators? Soil Biol. Biochem. 2007, 39, 12–23. [Google Scholar] [CrossRef]
- Insam, H.; Mitchell, C.C.; Dormaar, J.F. Relationship of soil microbial biomass and activity with fertilization practice and crop yield of three Ultisols. Soil Biol. Biochem. 1991, 23, 459–464. [Google Scholar] [CrossRef]
- Li, L.; Li, S.M.; Sun, J.H.; Zhou, L.L.; Bao, X.G.; Zhang, H.G.; Zhang, F.S. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [PubMed]
- Li, F.D.; Meng, P.; Fu, D.L.; Wang, B. Light distribution, photosynthetic rate and yield in a paulownia- wheat intercropping system in China. Agrofor. Syst. 2008, 74, 163–174. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, S.; Xie, J.; Yan, B.; Hou, J.; Wang, W.; Song, Y.; Zhang, X.; Li, J. Changing law of biomass accumulation and active components of Salvia miltiorrhiza in different growth periods. Chin. Mod. Tradit. Med. 2015, 25, 1171–1176. [Google Scholar]




| Year | Month | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|
| 2018 | monthly average temperature (°C) | 15.1 | 20.2 | 24.6 | 26.5 |
| monthly precipitation (mm) | 57 | 63 | 132 | 246 | |
| Month | 8 | 9 | 10 | 11 | |
| monthly average temperature (°C) | 26.2 | 20.3 | 13.9 | 7.6 | |
| monthly precipitation (mm) | 180 | 76 | 12 | 34 | |
| 2019 | Month | 4 | 5 | 6 | 7 |
| monthly average temperature (°C) | 14.5 | 22 | 25.5 | 28 | |
| monthly precipitation (mm) | 50 | 43 | 120 | 231 | |
| Month | 8 | 9 | 10 | 11 | |
| monthly average temperature (°C) | 25.5 | 22 | 15 | 10.5 | |
| monthly precipitation (mm) | 153 | 66 | 13 | 34 |
| Treatment | Type | Top-Quality Tobacco (%) | Production (kg·ha−1) | Production Value (CNY·ha−1) | Total Production Value (CNY·ha−1) | |
|---|---|---|---|---|---|---|
| 2018 | CK | T | 48.05 | 2178.41 | 48,343.99 | 48,469.64 |
| TS11 | T | 49.41 | 1991.2 | 44,572.46 | 52,561.59 | |
| S | 826.44 | 7989.13 | ||||
| TS22 | T | 49.12 | 1969.47 | 44,769.57 | 58,799.49 | |
| S | 1451.52 | 14,029.92 | ||||
| TS23 | T | 49.59 | 1841.94 | 41,369.97 | 56,650.36 | |
| S | 1591.71 | 15,280.39 | ||||
| 2019 | CK | T | 48.22 | 2140.93 | 47,635.68 | 47,635.68 |
| TS11 | T | 49.88 | 2106.46 | 47,152.13 | 54,367.72 | |
| S | 751.62 | 7215.59 | ||||
| TS22 | T | 49.58 | 2092.95 | 46,850.08 | 58,315.15 | |
| S | 1177.91 | 11,465.07 | ||||
| TS23 | T | 49.51 | 1870.87 | 42,019.64 | 54,985.44 | |
| S | 1177.91 | 12,965.8 |
| Treatment | Grade | Total Sugar | Reducing Sugar | Total Nicotine | Total Nitrogen | K | CI | K/CI | Glycemic Ratio | Ratio of Nitrogen to Alkali |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |||||
| CK | B2F | 22.71 a | 16.31 b | 3.03 c | 2.32 a | 2.06 c | 0.15 a | 13.73 c | 7.50 b | 0.77 a |
| TS11 | B2F | 24.02 b | 17.74 a | 3.11 b | 2.28 ab | 2.21 a | 0.13 ab | 16.46 a | 7.72 a | 0.72 b |
| TS22 | B2F | 21.72 c | 15.95 c | 3.14 b | 2.21 b | 1.85 d | 0.12 b | 15.42 b | 6.92 c | 0.70 b |
| TS23 | B2F | 22.15 ab | 15.46 c | 3.21 a | 2.18 b | 2.14 b | 0.14 a | 15.79 b | 6.90 c | 0.68 b |
| CK | C3F | 24.53 b | 17.02 b | 3.19 b | 2.07 b | 1.58 d | 0.18 a | 8.78 d | 7.69 c | 0.65 a |
| TS11 | C3F | 26.06 a | 19.03 a | 3.12 b | 2.04 b | 2.32 a | 0.17 b | 13.65 a | 8.35 a | 0.65 a |
| TS22 | C3F | 24.52 b | 17.59 b | 3.04 c | 2.01 bc | 1.91 b | 0.16 c | 11.94 b | 8.07 b | 0.66 a |
| TS23 | C3F | 23.86 c | 16.47 c | 3.39 a | 2.12 a | 1.91 b | 0.18 a | 10.61 c | 7.04 d | 0.63 a |
| CK | X2F | 33.55 a | 26.30 a | 1.24 d | 1.55 c | 1.42 d | 0.15 a | 9.47 d | 27.06 a | 1.25 a |
| TS11 | X2F | 30.92 b | 23.09 b | 1.40 c | 1.58 c | 2.32 a | 0.14 a | 16.57 a | 22.09 b | 0.68 d |
| TS22 | X2F | 25.79 c | 19.26 c | 2.74 a | 1.87 a | 1.64 c | 0.15 a | 10.93 c | 9.41 d | 1.13 b |
| TS23 | X2F | 27.78 bc | 20.68 c | 2.12 b | 1.75 b | 1.83 b | 0.13 ab | 14.08 a | 13.10 c | 0.83 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Guo, X.; Chen, Q.; Sun, Z.; Shang, X.; Gao, Y.; Yu, T.; Zhang, L.; Yang, L.; Hou, X. Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value. Agronomy 2024, 14, 598. https://doi.org/10.3390/agronomy14030598
Su X, Guo X, Chen Q, Sun Z, Shang X, Gao Y, Yu T, Zhang L, Yang L, Hou X. Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value. Agronomy. 2024; 14(3):598. https://doi.org/10.3390/agronomy14030598
Chicago/Turabian StyleSu, Xueqi, Xiaomeng Guo, Qian Chen, Zheng Sun, Xianchao Shang, Yun Gao, Tao Yu, Li Zhang, Long Yang, and Xin Hou. 2024. "Tobacco/Salvia miltiorrhiza Intercropping Improves Soil Quality and Increases Total Production Value" Agronomy 14, no. 3: 598. https://doi.org/10.3390/agronomy14030598






