Adoption of Cereal–Legume Double Cropping toward More Sustainable Organic Systems in the Mediterranean Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trial Set-Up
2.2. Measurements
2.3. Statistical Analysis
3. Results
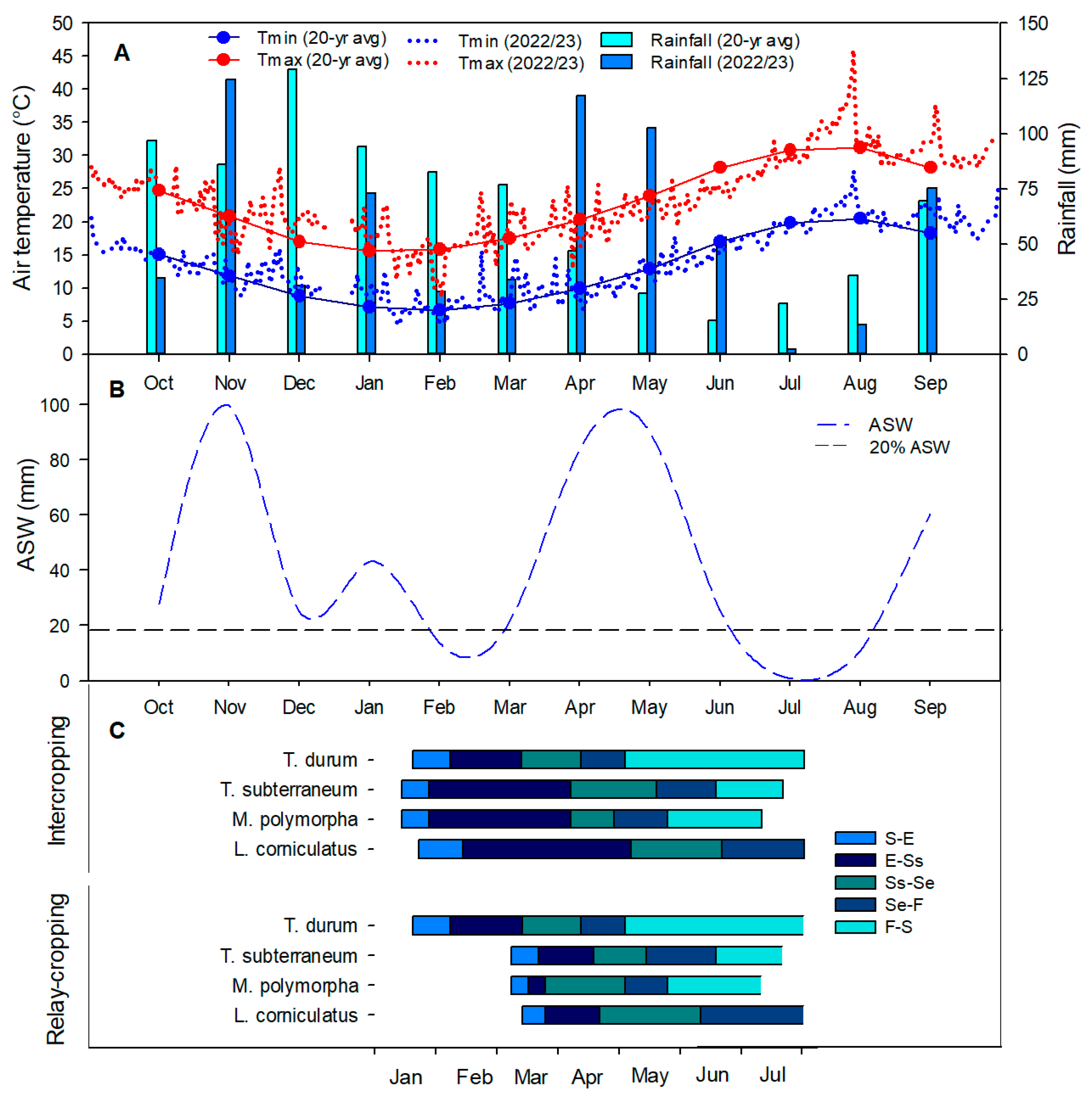
3.1. Meteorological Trend and Crop Phenology
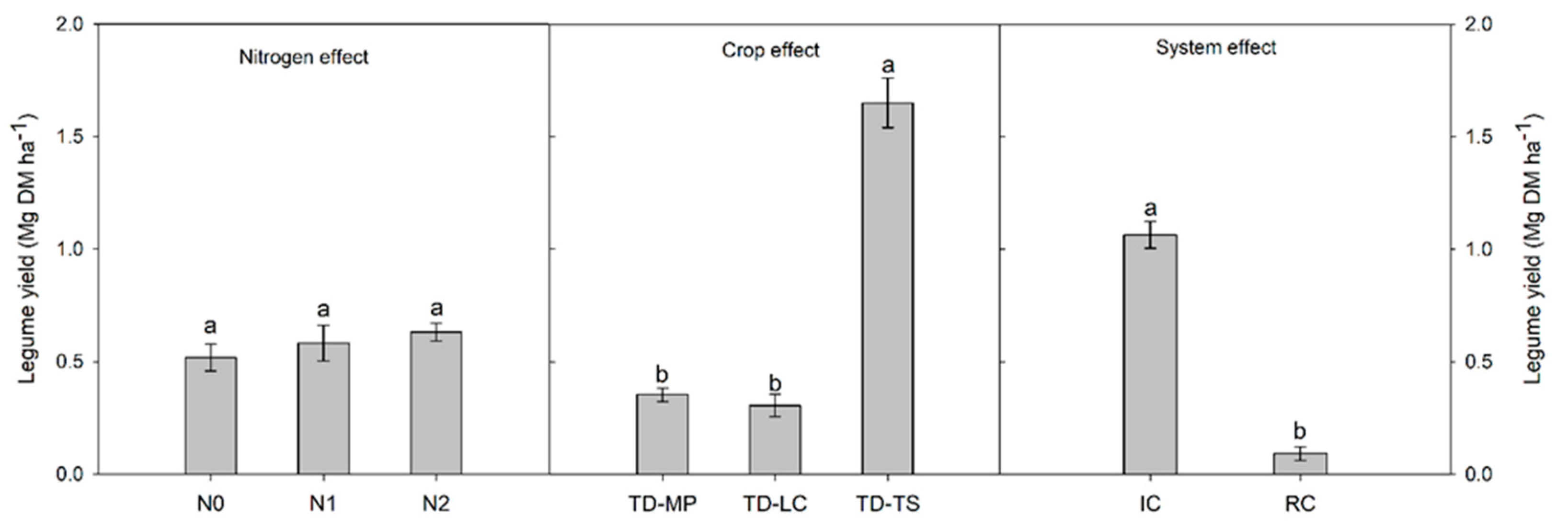
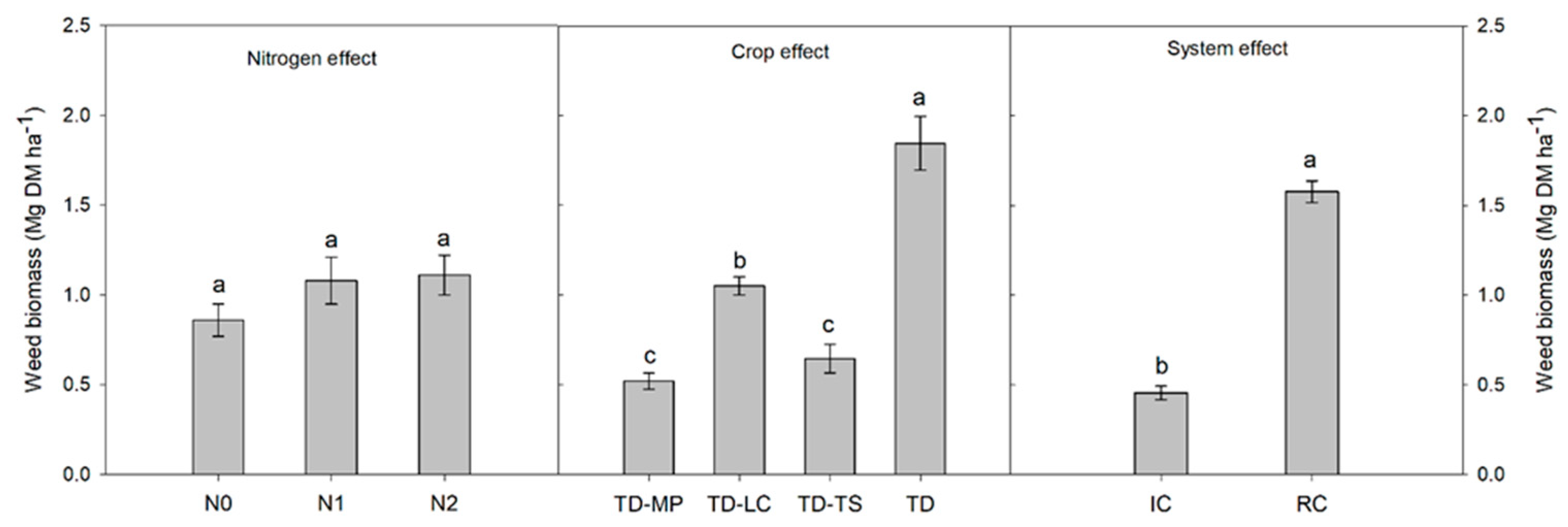
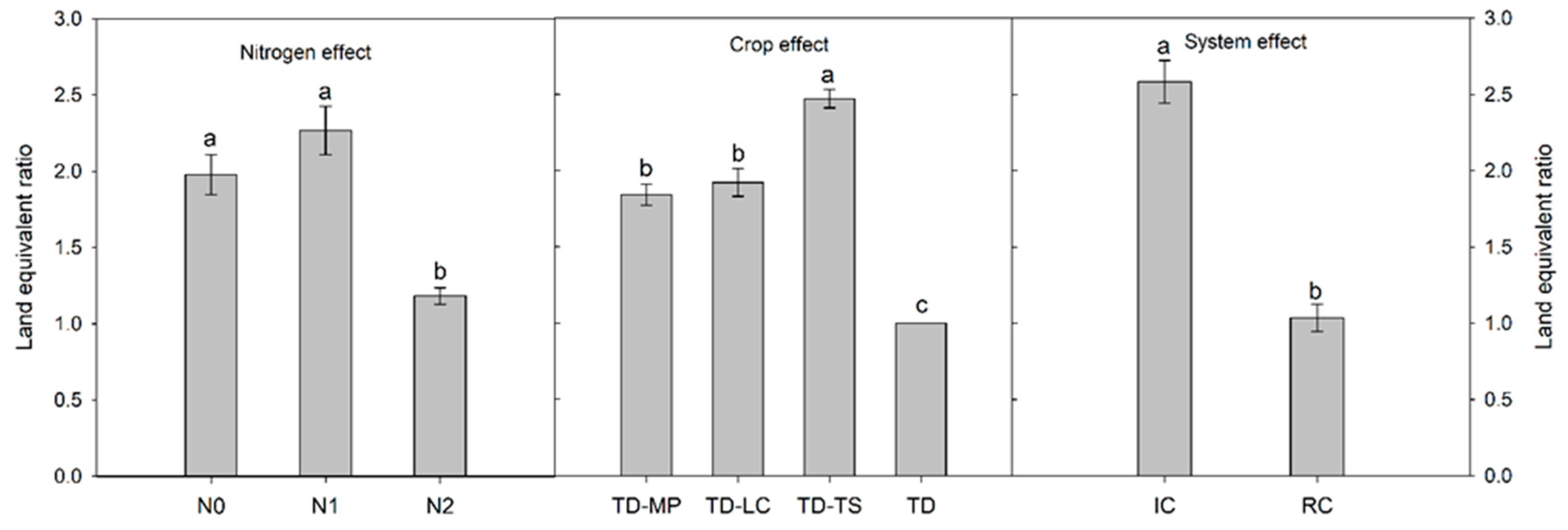
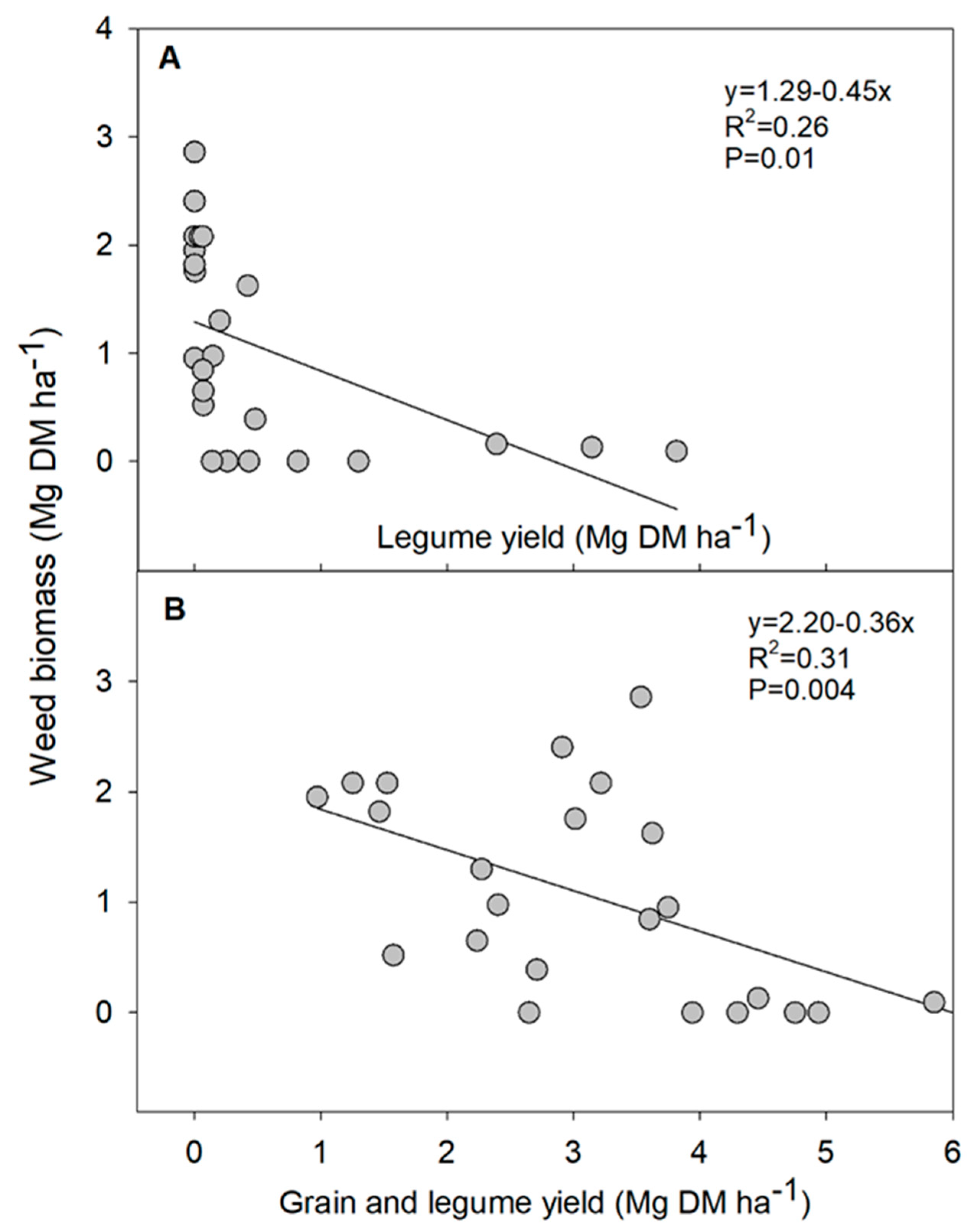
3.2. Crop Yield, Weed Biomass and Grain Protein Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission (EC), Directorate-General for Agriculture and Rural Development. List of Potential Agricultural Practices That Eco-Schemes Could Support. January 2021 #EUGreenDeal. 2021. Available online: https://agriculture.ec.europa.eu/system/files/2021-01/factsheet-agri-practices-under-ecoscheme_en_0.pdf (accessed on 31 May 2023).
- Mondelaers, K.; Aertsens, J.; Van Huylenbroeck, G. A meta-analysis of the differences in environmental impacts between organic and conventional farming. Brit. Food J. 2009, 111, 1098–1119. [Google Scholar] [CrossRef]
- Smith, L.G.; Williams, A.G.; Pearce, B.D. The energy efficiency of organic agriculture: A review. Renew. Agric. Food Syst. 2015, 30, 280–301. [Google Scholar] [CrossRef]
- Istituto Nazionale di Statistica (ISTAT). 2016. Available online: http://www.istat.it (accessed on 31 May 2023).
- Sistema d’Informazione Nazionale sull’Agricoltura Biologica (SINAB). Bio in Cifre. 2019. Available online: http://www.sinab.it/ (accessed on 31 May 2023).
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Asseng, S.; Zhu, Y.; Basso, B.; Wilson, T.; Cammarano, D. Simulation Modeling: Applications in Cropping Systems. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Cambridge, UK, 2014; pp. 102–212. [Google Scholar] [CrossRef]
- Hariharan, G.; Abhayapala, R.; Karunarathna, B. Efficient utilization of rice fallow through pulse cultivation. In Advances in Legumes for Sustainable Intensification; Meena, R.S., Kuma, S., Eds.; Academic Press (Elsevier): Amsterdam, The Netherlands, 2022; pp. 71–92. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Seguin, P. Forage legumes for sustainable cropping systems. J. Crop Prod. 2003, 8, 187–216. [Google Scholar] [CrossRef]
- Scholberg, J.M.S.; Dogliotti, S.; Leoni, C.; Cherr, C.M.; Zotarelli, L.; Rossing, W.A.H. Cover crops for sustainable agrosystems in the Americas. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming. Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2010; Volume 4, pp. 23–58. [Google Scholar] [CrossRef]
- Raza, M.A.; Zhiqi, W.; Yasin, H.S.; Gul, H.; Qin, R.; Rehman, S.U.; Mahmood, A.; Iqbal, Z.; Ahmed, Z.; Luo, S.; et al. Effect of crop combination on yield performance, nutrient uptake, and land use advantage of cereal/legume intercropping systems. Field Crops Res. 2023, 304, 109144. [Google Scholar] [CrossRef]
- Melander, B.; Rasmussen, I.A.; Bàrberi, P. Integrating physical and cultural methods of weed control-examples from European research. Weed Sci. 2005, 53, 369–381. [Google Scholar] [CrossRef]
- Mariotti, M.; Masoni, A.; Ercoli, L.; Arduini, I. Optimizing forage yield of durum wheat/field bean intercropping through N fertilization and row ratio. Grass Forage Sci. 2012, 67, 243–254. [Google Scholar] [CrossRef]
- Amossé, C.; Jeuffroy, M.H.; Celette, F.; David, C. Relay-intercropped forage legumes help to control weeds in organic grain production. Eur. J. Agron. 2013, 49, 158–167. [Google Scholar] [CrossRef]
- Gecaitė, V.; Arlauskienė, A.; Cesevičienė, J. Competition effects and productivity in oat–forage legume relay intercropping systems under organic farming conditions. Agriculture 2021, 11, 99. [Google Scholar] [CrossRef]
- Leoni, F.; Lazzaro, M.; Ruggeri, M.; Carlesi, S.; Meriggi, P.; Moonen, A.C. Relay intercropping can efficiently support weed management in cereal-based cropping systems when appropriate legume species are chosen. Agron. Sustain. Dev. 2022, 42, 75. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen fixation of red clover interseeded with winter cereals across a management-induced fertility gradient. Nutr. Cycling Agroecosyst. 2011, 90, 105–119. [Google Scholar] [CrossRef]
- De Notaris, C.; Rasmussen, J.; Sørensen, P.; Melander, B.; Olesena, J.E. Manipulating cover crop growth by adjusting sowing time and cereal interrow spacing to enhance residual nitrogen effects. Field Crops Res. 2019, 234, 15–25. [Google Scholar] [CrossRef]
- Ross, S.M.; King, J.R.; Izaurralde, R.C.; O‘Donovan, J.T. Weed suppression by seven clover species. Agron. J. 2001, 93, 820–827. [Google Scholar] [CrossRef]
- Uchino, H.; Iwama, K.; Jitsuyama, Y.; Ichiyama, K.; Sugiura, E.; Yudate, T. Stable characteristics of cover crops for weed suppression in organic farming systems. Plant Prod. Sci. 2011, 14, 75–85. [Google Scholar] [CrossRef]
- Enriquez-Hidalgo, D.; Cruz, T.; Teixeira, D.L.; Steinfort, U. Phenological stages of Mediterranean forage legumes, based on the BBCH scale. Ann. Appl. Biol. 2020, 176, 357–368. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Lombardo, S.; Fontanazza, S.; Abbate, C.; Pandino, G.; Anastasi, U.; Onofri, A.; Mauromicale, G. Improving soil health, weed management and nitrogen dynamics by Trifolium subterraneum cover cropping. Agron. Sustain. Dev. 2020, 40, 18. [Google Scholar] [CrossRef]
- Blackwell, M.S.A.; Jarvis, S.C.; Wilkins, R.J. Chapter Four - The importance of sustained grassland and environmental research: A case study from North Wyke Research station, UK, 1982–2017. Adv. Agron. 2018, 149, 161–235. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Copani, V.; Patanè, C.; Cosentino, S.L. Lignocellulosic biomass production of Mediterranean wild accessions (Oryzopsis miliacea, Cymbopogon hirtus, Sorghum halepense and Saccharum spontaneum) in a semi-arid environment. Field Crops Res. 2017, 214, 56–65. [Google Scholar] [CrossRef]
- Mason, S.C.; Leihner, D.E.; Vorst, J.J. Cassava–cowpea and cassava–peanut intercropping. I. Yield and land use efficiency. Agron. J. 1986, 78, 43–46. [Google Scholar] [CrossRef]
- Deb, D.; Dutta, S. The robustness of land equivalent ratio as a measure of yield advantage of multi-crop systems over monocultures. Exp. Res. 2022, 3, e2. [Google Scholar] [CrossRef]
- Scordia, D.; Corinzia, S.A.; Coello, J.; Ventura, R.V.; Jiménez-De-Santiago, D.E.; Just, B.S.; Castaño-Sánchez, O.; Arcarons, C.C.; Tchamitchian, M.; Garreau, L.; et al. Are agroforestry systems more productive than monocultures in Mediterranean countries? A meta-analysis. Agron. Sustain. Dev. 2023, 43, 73. [Google Scholar] [CrossRef]
- Tuel, A.; Eltahir, E.A.B. Why Is the Mediterranean a Climate Change Hot Spot? J. Clim. 2020, 33, 5829–5843. [Google Scholar] [CrossRef]
- Anastasi, U.; Corinzia, S.A.; Cosentino, S.L.; Scordia, D. Performances of durum wheat varieties under conventional and no-chemical input management systems in a semiarid Mediterranean environment. Agronomy 2019, 9, 788. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Sanzone, E.; Testa, G.; Patanè, C.; Anastasi, U.; Scordia, D. Does post-anthesis heat stress affect plant phenology, physiology, grain yield and protein content of durum wheat in a semi-arid Mediterranean environment? J. Agron. Crop Sci. 2018, 205, 309–323. [Google Scholar] [CrossRef]
- Bergkvist, G.; Stenberg, M.; Wetterlind, J.; Båth, B.; Elfstrand, S. Clover cover crops under-sown in winter wheat increase yield of subsequent spring barley—Effect of N dose and companion grass. Field Crops Res. 2011, 120, 292–298. [Google Scholar] [CrossRef]
- Amossé, C.; Jeuffroy, M.H.; David, C. Relay intercropping of legume cover crops in organic winter wheat: Effects on performance and resource availability. Field Crops Res. 2013, 145, 78–87. [Google Scholar] [CrossRef]
- Gu, C.; Bastiaans, L.; Anten, N.P.R.; Makowski, D.; van der Werf, W. Annual intercropping suppresses weeds: A meta-analysis. Agric. Ecosyst. Environ. 2021, 322, 107658. [Google Scholar] [CrossRef]
- Nelson, A.G.; Pswarayi, A.; Quideau, S.; Frick, B.; Spaner, D. Yield and weed suppression of crop mixtures in organic and conventional systems of the western Canadian prairie. Agron. J. 2012, 104, 756–762. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Mahmoud, R.; Casadebaig, P.; Hilgert, N.; Alletto, L.; Freschet, G.T.; de Mazancourt, C.; Gaudio, N. Species choice and N fertilization influence yield gains through complementarity and selection effects in cereal-legume intercrops. Agron. Sustain. Dev. 2022, 42, 12. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carlsson, G.; Englund, J.-E.; Flöhr, A.; Pelzer, E.; Jeuffroy, M.-H.; Makowski, D.; Jensen, E.S. Grain legume-cereal intercropping enhances the use of soil-derived and biologically fixed nitrogen in temperate agroecosystems. A meta-analysis. Eur. J. Agron. 2020, 118, 126077. [Google Scholar] [CrossRef]
- Pellegrini, F.; Carlesi, S.; Nardi, G.; Bàrberi, P. Wheat–clover temporary intercropping under Mediterranean conditions affects wheat biomass, plant nitrogen dynamics and grain quality. Eur. J. Agron. 2021, 130, 126347. [Google Scholar] [CrossRef]


| Source | df | S-E | E-Ss | Ss-Se | Se-F | F-S |
|---|---|---|---|---|---|---|
| MS | ||||||
| Blocks | 2 | 5.79 ns | 48.1 ns | 3.76 ns | 76.2 ns | 358.3 ns |
| Nitrogen (N) | 2 | 0.09 ns | 3.45 ns | 1.09 ns | 82.2 ns | 197.7 ns |
| Main-plot error | 4 | 2.85 | 17.7 | 1.14 | 47.1 | 142.8 |
| Crop (C) | 3 | 192.7 *** | 1798.2 *** | 2590.8 *** | 5288.2 *** | 11996.7 *** |
| N × C | 6 | 5.51 ns | 29.4 ns | 5.63 ns | 32.8 ns | 83.3 ns |
| Sub-plot error | 18 | 2.31 | 16.0 | 2.62 | 47.7 | 110.1 |
| Cropping system (S) | 1 | 176.9 *** | 12954.3 *** | 1930.1 *** | 728.9 *** | 274.3 ns |
| N × S | 2 | 0.10 ns | 7.22 ns | 2.39 ns | 98.1 ns | 252.4 ns |
| C × S | 3 | 80.3 ** | 3960.5 *** | 3889.1 *** | 1051.4 *** | 1310.4 *** |
| N × C × S | 6 | 5.07 ns | 31.5 ns | 5.24 ns | 52.4 ns | 101.1 ns |
| Error | 24 | 2.71 | 18.9 | 2.49 | 50.9 | 139.2 |
| Source | df | GY | GP | LY | WB | LER |
|---|---|---|---|---|---|---|
| MS | ||||||
| Blocks | 2 | 0.58 ns | 0.84 ns | 0.09 ns | 0.76 ns | 0.77 ns |
| Nitrogen (N) | 2 | 20.4 ** | 12.4 * | 0.07 ns | 0.45 ns | 7.57 * |
| Main-plot error | 4 | 0.44 | 0.73 | 0.03 | 0.30 | 2.02 |
| Crop (C) | 3 | 2.43 ** | 9.48 *** | 9.65 *** | 6.42 ** | 6.64 ** |
| N × C | 6 | 0.86 ns | 0.03 ns | 0.31 ns | 0.50 ns | 1.27 ns |
| Sub-plot error | 18 | 0.32 | 0.25 | 0.11 | 0.99 | 0.42 |
| Cropping system (S) | 1 | 0.79 ns | 0.56 ns | 17.1 *** | 22.6 *** | 48.9 *** |
| N × S | 2 | 0.16 ns | 0.03 ns | 0.02 ns | 0.85 ns | 5.04 ns |
| C × S | 3 | 1.96 * | 0.07 ns | 7.87 ** | 1.25 ns | 6.96 * |
| N × C × S | 6 | 0.88 ns | 0.64 ns | 0.66 ns | 0.34 ns | 1.33 ns |
| Error | 24 | 0.64 | 0.46 | 0.09 | 0.61 | 0.88 |
| Effects | CPw (%) | |
|---|---|---|
| Legume (L) | M. polymorpha | 90.1 a |
| T. subterraneum | 59.8 b | |
| L. corniculatus | 94.0 a | |
| Cropping system (S) | Relay cropping | 93.8 a |
| Intercropping | 59.6 b | |
| Significance | L | * |
| S | ** | |
| L × S | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scordia, D.; Guarnaccia, P.; Calderone, F.; Maio, A.; La Malfa, T.; Scavo, A.; Gresta, F. Adoption of Cereal–Legume Double Cropping toward More Sustainable Organic Systems in the Mediterranean Area. Agronomy 2024, 14, 772. https://doi.org/10.3390/agronomy14040772
Scordia D, Guarnaccia P, Calderone F, Maio A, La Malfa T, Scavo A, Gresta F. Adoption of Cereal–Legume Double Cropping toward More Sustainable Organic Systems in the Mediterranean Area. Agronomy. 2024; 14(4):772. https://doi.org/10.3390/agronomy14040772
Chicago/Turabian StyleScordia, Danilo, Paolo Guarnaccia, Francesca Calderone, Aurora Maio, Tommaso La Malfa, Aurelio Scavo, and Fabio Gresta. 2024. "Adoption of Cereal–Legume Double Cropping toward More Sustainable Organic Systems in the Mediterranean Area" Agronomy 14, no. 4: 772. https://doi.org/10.3390/agronomy14040772






