Apple Growth and Yield in Replant Soils Supplemented by Organic Soil Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Parameters
2.2. Pot Trials—For Testing ARD and Additives in Soils
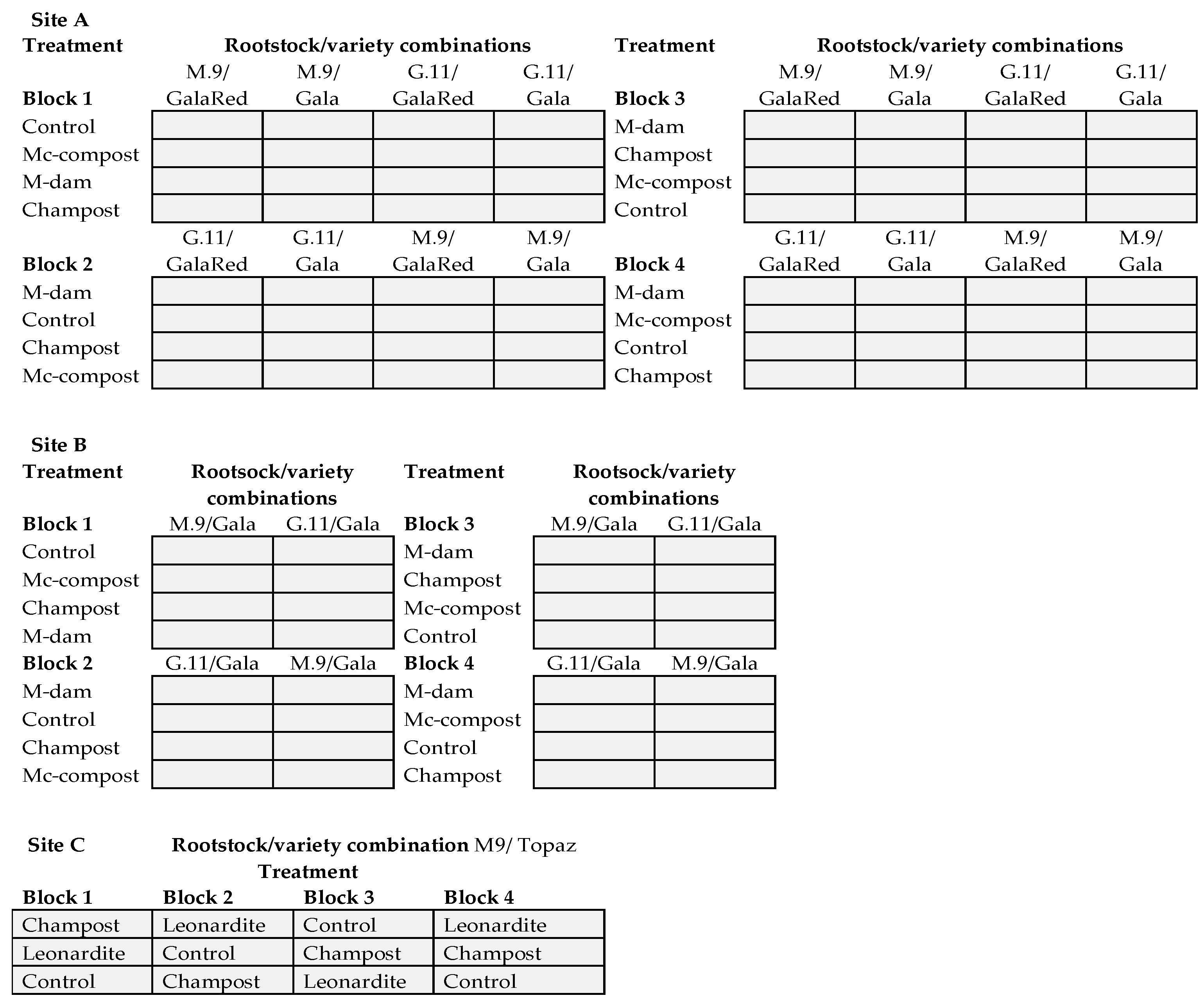
2.3. Field Trials
2.4. Statistical Analyses
3. Results
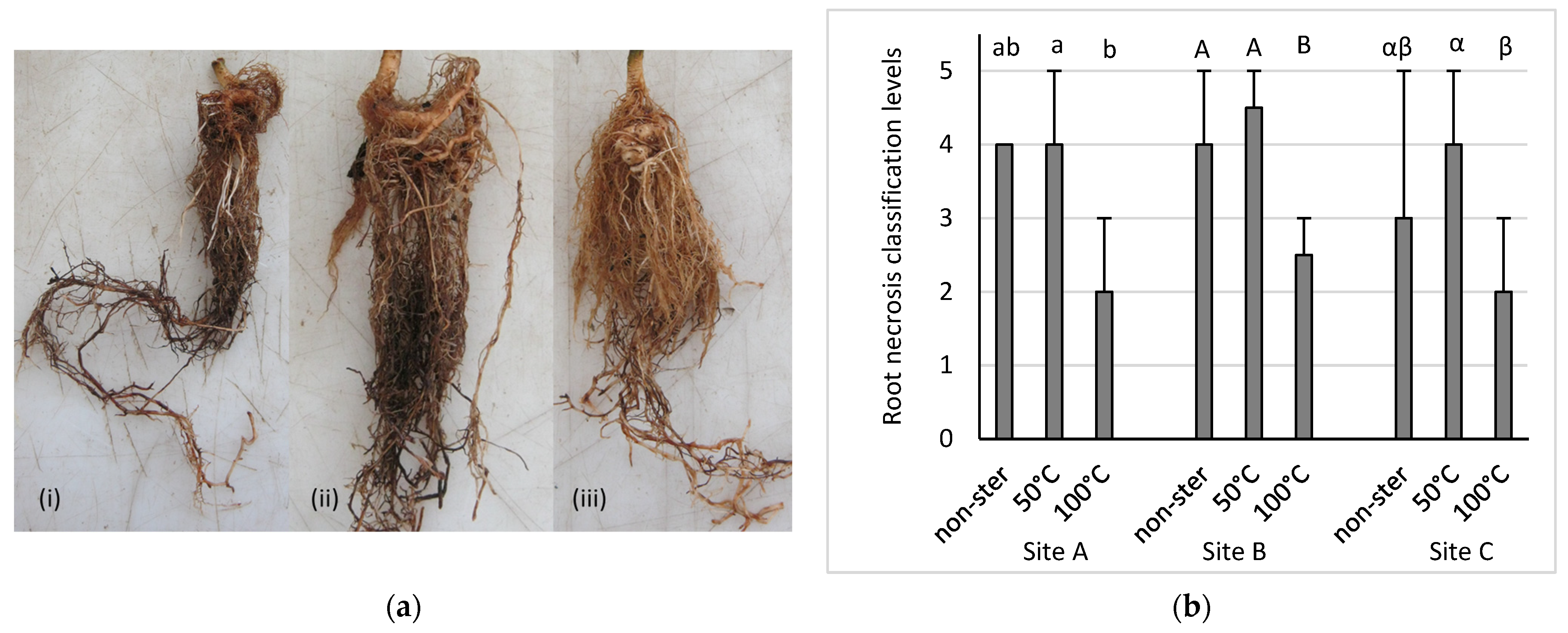
3.1. Pot Trials
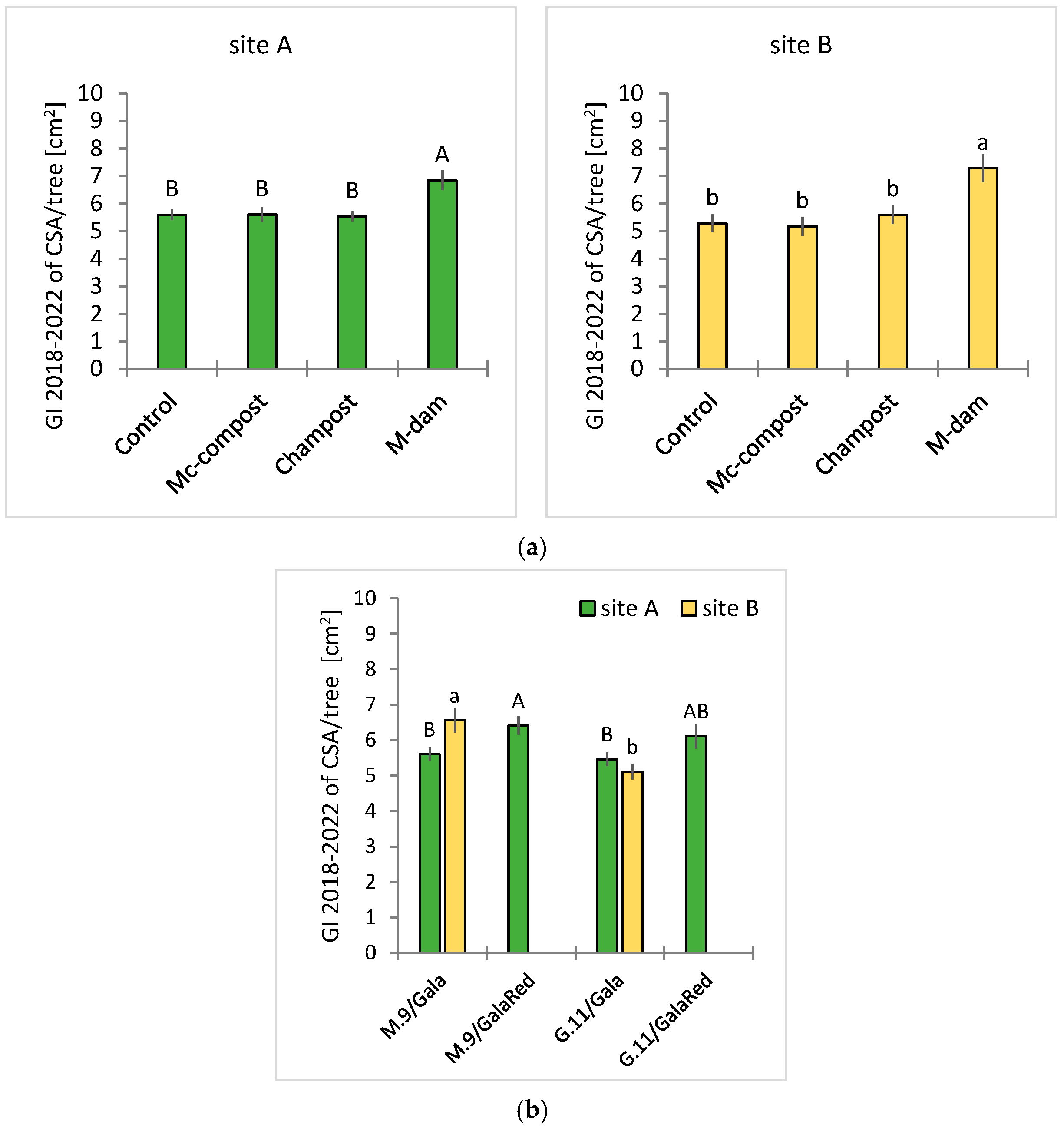
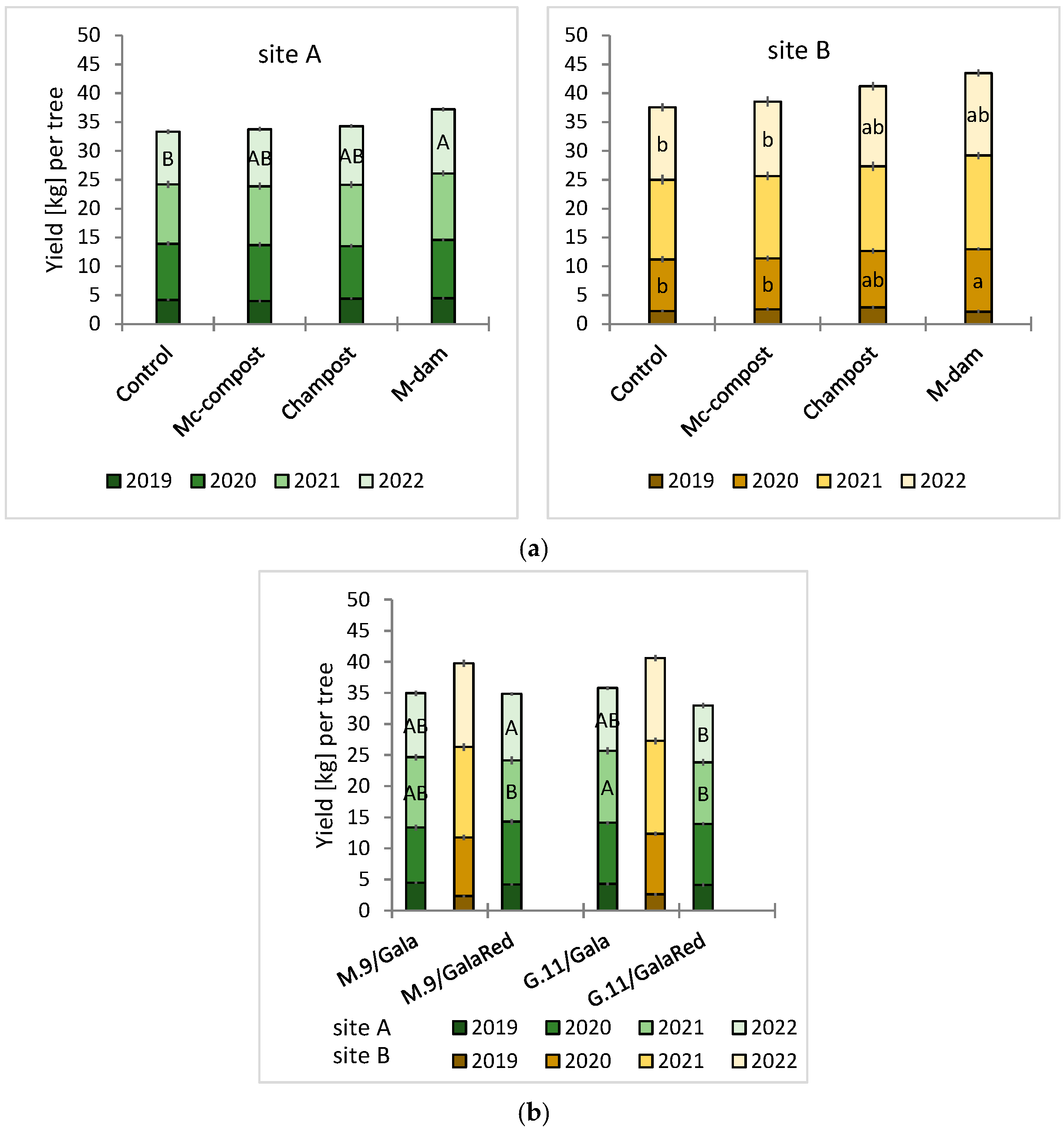
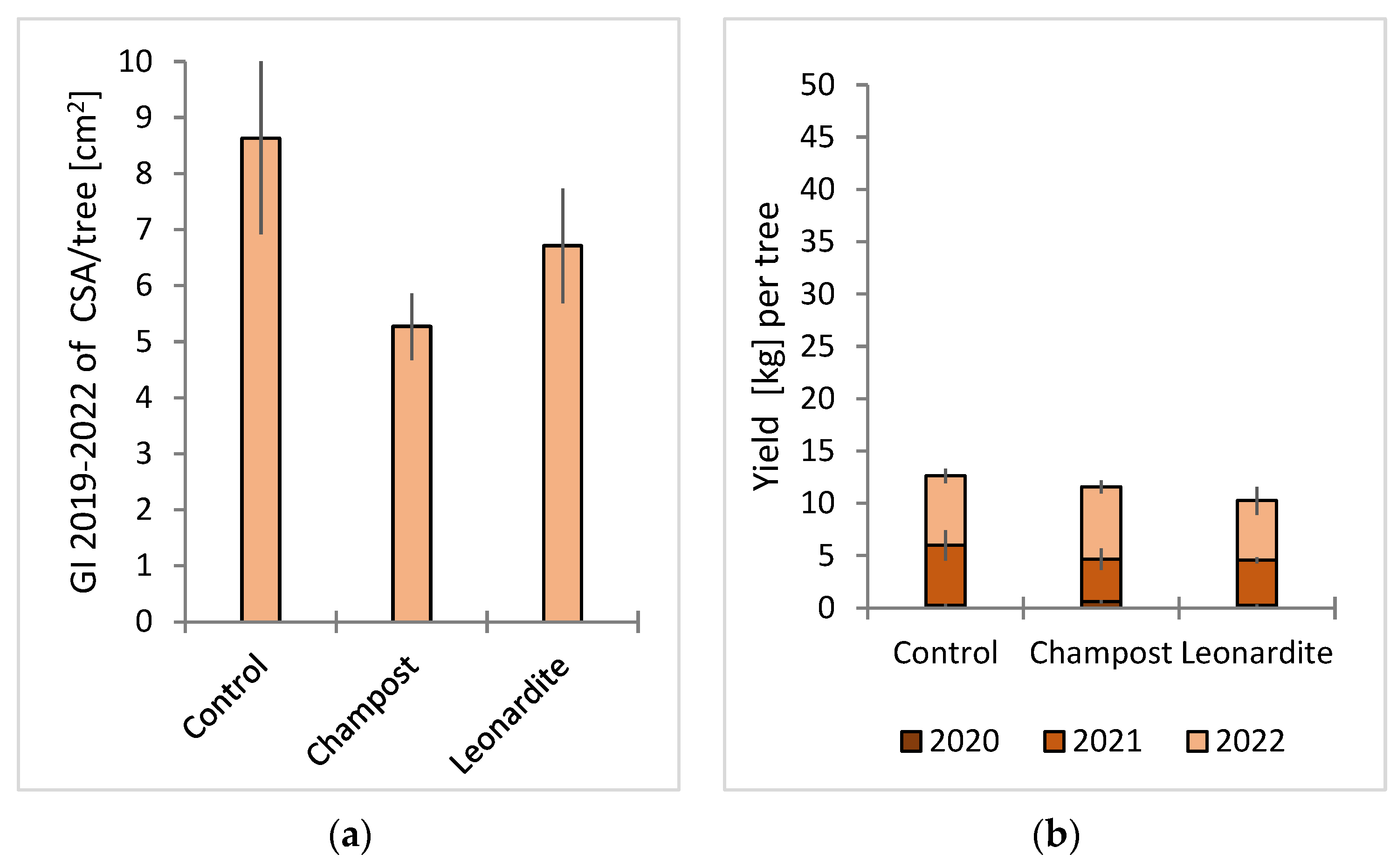
3.2. Field Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoestra, H. Replant Diseases of Apple in the Netherlands. Ph.D. Thesis, Mededelingen Landbouwhogeschool Wageningen, Wageningen, The Netherlands, 1968; p. 108. Available online: https://edepot.wur.nl/285366 (accessed on 6 February 2024).
- Savory, B.M. Specific replant diseases causing root necrosis and growth depression in perennial fruit and plantation crops. In Commonwealth Bureau of Horticulture and Plantation Crops East Malling [Eng.]; Research review no. 1; Commonwealth Agricultural Bureaux: Kent, UK, 1966; p. 64. [Google Scholar]
- Mazzola, M. Elucidation of the Microbial Complex Having a Causal Role in the Development of Apple Replant Disease in Washington. Phytopathology 1989, 88, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Van Schoor, L.; Denman, S.; Cook, N.C. Characterisation of apple replant disease under South African conditions and potential biological management strategies. Sci. Hortic. 2009, 119, 153–162. [Google Scholar] [CrossRef]
- Tewoldemedhin, Y.T.; Mazzola, M.; Labuschagne, I.; McLeod, A. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol. Biochem. 2011, 43, 1917–1927. [Google Scholar] [CrossRef]
- Mazzola, M.; Manici, L.M. Apple Replant Disease: Role of Microbial Ecology in Cause and Control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Schmitz, M.; Vetterlein, D.; et al. Apple replant disease: Causes and mitigation strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Henfrey, J.L.; Baab, G.; Schmitz, M. Physiological stress responses in apple under replant conditions. Sci. Hortic. 2015, 194, 111–117. [Google Scholar] [CrossRef]
- Mai, W.F.; Abawi, G.S. Controlling Replant Diseases of Pome and Stone Fruits in Northeastern United States by Replant Fumigation. Plant Dis. 1981, 65, 859–864. [Google Scholar] [CrossRef]
- Otto, G.; Winkler, H.; Szabo, K. Proof of actinomycetes in rootlets of species of Rosaceae from SARD soil—A contribution to the specificity of replant diseases. Acta Hortic. 1994, 363, 43–48. [Google Scholar] [CrossRef]
- Uthkede, R.S. Soil sickness, replant problem or replant disease and its integrated control. Allelopath. J. 2006, 18, 23–38. [Google Scholar]
- Yim, B.; Smalla, K.; Winkelmann, T. Evaluation of apple replant problems based on different soil disinfection treatments-links to soil microbial community structure? Plant Soil 2013, 366, 617–631. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Manici, L.M.; Insam, H.; Stres, B. Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 2015, 395, 317–333. [Google Scholar] [CrossRef]
- Spath, M.; Insam, H.; Peintner, U.; Kelderer, M.; Kuhnert-Finkernagel, R.; Franke-Whittle, I.H. Linking soil biotic and abiotic factors to apple replant disease: A greenhouse approach. J. Phytopathol. 2015, 163, 287–299. [Google Scholar] [CrossRef]
- Jaffee, B.A.; Abawi, G.S.; Mai, W.F. Role of soil microflora and Pratylenchus penetrans in an apple replant disease. Phytopathology 1982, 72, 247–251. [Google Scholar] [CrossRef]
- Cesarano, G.; Zotti, M.; Antignani, V.; Marra, R.; Scala, F.; Bonanomi, G. Soil sickness and negative plant-soil feedback: A reappraisal of Hypotheses. J. Plant Pathol. 2017, 99, 545–570. [Google Scholar]
- Fazio, G.; Chang, L.; Grusak, M.A.; Robinson, T.L. Apple rootstocks influence mineral nutrient concentration of leaves and fruit. N. Y. Fruit Q. 2015, 23, 11–15. [Google Scholar]
- Mahnkopp, F.; Simon, M.; Lehndorff, E.; Pätzold, S.; Wrede, A.; Winkelmann, T. Induction and diagnosis of apple replant disease (ARD): A matter of heterogeneous soil properties? Sci. Hortic. 2018, 241, 167–177. [Google Scholar] [CrossRef]
- Jonkers, H.; Hoestra, H.; Borsboom, O.; Power, A. Soil pH in fruit trees in relation to specific apple replant disorder (SARD). II: The first five years at the Wageningen research plot. Sci. Hortic. 1980, 13, 149–154. [Google Scholar] [CrossRef]
- Willett, M.; Smith, T.J.; Peterson, A.B.; Hinman, H.; Stevens, R.G.; Ley, T.; Tvergyak, P.; Williams, K.M.; Maib, K.M.; Williams, J.W. Growing Profitable Apple Orchards in Replant Sites: An Interdisciplinary Team Approach in Washington State. HortTechnology 1994, 4, 175–181. [Google Scholar] [CrossRef]
- Kome, G.K.; Enang, R.K.; Tabi, F.O.; Yerima, B.P.K. Influence of Clay Minerals on Some Soil Fertility Attributes: A Review. Open J. Soil Sci. 2019, 9, 155–188. [Google Scholar] [CrossRef]
- Schimmel, J.; Gentsch, N.; Boy, J.; Uteau, D.; Rohr, A.-D.; Winkelmann, T.; Busnena, B.; Liu, B.; Krueger, J.; Kaufhold, S.; et al. Alleviation of apple replant disease in sandy soils by clay amendments increasing plant available silicon. Res. Sq. 2023. [Google Scholar] [CrossRef]
- De Ceuster, T.J.J.; Hoitink, H.A.J. Prospects for composts and biocontrol agents as substitutes for methyl bromide in biological control of plant diseases. Compost. Sci. Util. 1999, 7, 6–15. [Google Scholar] [CrossRef]
- Thompson, A.A.; Williams, M.A.; Peck, G.M. Compost and Geneva® series rootstocks increase young ‘Gala’ apple tree growth and change root-zone microbial communities. Sci. Hortic. 2019, 256, 108573. [Google Scholar] [CrossRef]
- Rumberger, A.; Yao, S.; Merwin, I.A.; Nelson, E.B.; Thies, J.E. Rootstock genotype and orchard replant position rather than soil fumigation or compost amendment determine tree growth and rhizosphere bacterial community composition in an apple replant soil. Plant Soil 2004, 264, 247–260. [Google Scholar] [CrossRef]
- Spornberger, A.; Schüller, E.; Noll, D. The Influence of Geneva Rootstocks on the Vegetative and Generative Characteristics of the Apple Cultivar ‘Topaz’ in an Organically Managed Replanted Orchard. Int. J. Fruit Sci. 2020, 20, 1436–1444. [Google Scholar] [CrossRef]
- Schwärzel, H.; Schubert, P. Versuchsberichte Obstbau. Bestands- und Ertragsicherheit von Heidelbeeren in Substratkultur. 2005. Available online: https://www.hortigate.de/publikation/12833/Bestands-und-Ertragsicherheit-von-Heidelbeeren-in-Substratkultur/ (accessed on 6 June 2023).
- Diehl, K.; Cavael, U.; Schwärzel, H.; Lentzsch, P. Orchard floor management strategy to maintain performance of apple trees in replant soils. Act. Hortic. 2020, 1270, 267–279. [Google Scholar] [CrossRef]
- Otto, G.; Winkler, H. Nachweis der Bodenmüdigkeit in wachsenden Apfelanlagen—Proof of soil sickness in growing apple plantations. Zentralblatt Für Bakteriol. II 1976, 131, 730–735. [Google Scholar]
- Witte, W. Die Mikrobielle Carbonisierung Teil 1; “Das ist Humus—So Sieht Er Aus”; MC—Kompostierung im Kontext von Klimaentwicklung, Umwelt und Nachhaltiger Landwirtschaft; Jürgen Kannemann Verlag: Halberstadt, Germany, 2013; p. 96. ISBN 978-3-942975-11-7. [Google Scholar]
- Berns, A.; Philipp, H.; Narres, H.D.; Burauel, P.; Vereecken, H.; Tappe, W. Effect of gamma-sterilization and autoclaving on soil organic matter structure as studied by solid state NMR, UV and fluorescence spectroscopy. Eur. J. Soil Sci. 2008, 59, 540–550. [Google Scholar] [CrossRef]
- Dietrich, P.; Cesarz, S.; Eisenhauer, N.; Roscher, C. Effects of steam sterilization on soil abiotic and biotic properties. Soil Org. 2020, 92, 99–108. [Google Scholar] [CrossRef]
- Sullmann, J.; Gravalon, P.; Perren, S.; Hollenstein, R. Feuerbrandrobuste Unterlagen in der Praxis. Besseres Obst. 2023, 68, 16–19. Available online: https://www.besseres-obst.at/fachartikel/produktion/2023/feuerbrandrobuste-unterlagen-in-der-praxis.html (accessed on 28 June 2023).
| Site | Clay (%) | Soil Class | OS (%) | pH | C/N Ratio | N Total | C Total | S Total | P | K | Mg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/100 g Soil Dry Matter) | |||||||||||
| A | 8.0 | lS | 1.3 | 6.0 | 10.1 | 78.8 | 800 | 13.2 | 9.3 | 12.2 | 9.3 |
| B | 5.9 | lS | 2.0 | 5.7 | 11.1 | 111.7 | 1235 | 15.8 | 5.0 | 12.1 | 10.3 |
| C | 7.0 | lS | 1.5 | 6.0 | 11.8 | 82.3 | 973 | 20.3 | 7.1 | 10.3 | 6.5 |
| 1. Pot Trial | 2. Pot Trial | |
|---|---|---|
| Treatments | Nonster soil; Ster soil, 100 °C, 1 h; Nonster: ster = 2:1 (v/v); Nonster: ster = 1:2 (v/v) | Nonster soil; Ster soil 50 °C; Ster soil 100 °C |
| Root stock | M.26 | ‘Bittenfelder Sämling’ |
| Site | A, B, C | A, B, C |
| Test specimen | 10 | 8 |
| Measurements | GI, shoot, and root DM | GI, shoot, and root DM |
| Model | two-factorial | two-factorial |
| Experiment duration | 8 weeks | 8 weeks |
| Organic Supplement | pH | C/N Ratio | N Total | C Total | S Total | P | K | Mg |
|---|---|---|---|---|---|---|---|---|
| (g/kg Dry Substance) | ||||||||
| Champost | 6.8 | 13.8 | 20.70 | 285.97 | 17.95 | 2.50 | 20.95 | 1.45 |
| Mc-compost | 6.9 | 10.7 | 14.19 | 152.50 | 3.16 | 2.82 | 16.09 | 0.77 |
| M-dam | 5.2 | 75.0 | 5.53 | 414.50 | 2.05 | 9.25 | 17.75 | 0.86 |
| Leonardite | 7.2 | 56.0 | 6.20 | 347.50 | 40.10 | 0.02 | 0.05 | 2.82 |
| Shoot DM | Root DM | Total Biomass | Growth Increase | |
|---|---|---|---|---|
| Pot trial 1 | ||||
| Treatment | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Site | 0.032 * | 0.377 | 0.046 * | 0.005 * |
| Site × treatment | 0.008 * | 0.066 | 0.008 * | 0.060 |
| Pot trial 2 | ||||
| Treatment | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Site | 0.003 * | 0.081 | 0.004 * | 0.063 |
| Site × treatment | 0.701 | 0.188 | 0.631 | 0.424 |
| Effect | Shoots (g/Plant) | Roots (g/Plant) | Total Biomass (g/Plant) | Growth Increase (cm/Plant) |
|---|---|---|---|---|
| Soil treatment | ||||
| Nonster | 4.82 ± 0.29 | 0.79 ± 0.06 b | 5.60 ± 0.33 | 15.48 ± 0.96 b |
| Ster | 7.49 ± 0.23 | 1.17 ± 0.04 a | 8.66 ± 0.27 | 23.12 ± 0.45 a |
| 1 nonster: 2 ster | 6.18 ± 0.35 | 1.07 ± 0.07 ab | 7.25 ± 0.41 | 19.94 ± 1.02 a |
| 2 nonster: 1 ster | 5.02 ± 0.38 | 0.86 ± 0.07 b | 5.88 ± 0.44 | 16.34 ± 1.24 b |
| HSD (p = 0.05) | 1.07 | 0.22 | 1.25 | 3.19 |
| Source site of soil | ||||
| A | 6.12 ± 0.21 | 0.97 ± 0.05 a | 7.10 ± 0.25 | 20.06 ± 0.64 a |
| B | 5.54 ± 0.39 | 0.94 ± 0.06 a | 6.48 ± 0.44 | 17.41 ± 1.10 b |
| C | 6.21 ± 0.39 | 1.04 ± 0.07 a | 7.24 ± 0.45 | 19.20 ± 1.14 ab |
| HSD (p = 0.05) | 0.84 | 0.17 | 0.97 | 2.49 |
| Site/soil treatment | ||||
| A/nonster | 5.64 ± 0.40 a | 0.87 ± 0.10 | 6.50 ± 0.48 a | 18.09 ± 1.30 |
| A/ster | 6.99 ± 0.25 a | 1.08 ± 0.06 | 8.07 ± 0.30 a | 23.13 ± 0.65 |
| A/1 nonster: 2 ster | 6.09 ± 0.43 a | 1.00 ± 0.12 | 7.09 ± 0.53 a | 20.28 ± 1.04 |
| A/2 nonster: 1 ster | 5.86 ± 0.47 a | 0.97 ± 0.11 | 6.84 ± 0.54 a | 19.08 ± 1.39 |
| HSD (p = 0.05) | 2.47 | 0.52 | 2.87 | 7.36 |
| B/nonster | 4.12 ± 0.30 b | 0.74 ± 0.06 | 4.85 ± 0.34 b | 13.29 ± 1.29 |
| B/ster | 7.89 ± 0.51 a | 1.24 ± 0.10 | 9.13 ± 0.58 a | 23.50 ± 0.86 |
| B/1 nonster: 2 ster | 5.25 ± 0.56 b | 0.92 ± 0.09 | 6.17 ± 0.63 b | 17.06 ± 1.91 |
| B/2 nonster: 1 ster | 3.72 ± 0.58 b | 0.71 ± 0.11 | 4.42 ± 0.66 b | 12.63 ± 2.38 |
| HSD (p = 0.05) | 2.47 | 0.52 | 2.87 | 7.36 |
| C/ nonster | 4.59 ± 0.63 b | 0.75 ± 0.12 | 5.34 ± 0.73 b | 14.75 ± 2.02 |
| C/ster | 7.52 ± 0.37 a | 1.19 ± 0.06 | 8.71 ± 0.41 a | 22.72 ± 0.85 |
| C/1 nonster: 2 ster | 7.37 ± 0.70 a | 1.33 ± 0.14 | 8.70 ± 0.81 a | 22.79 ± 1.99 |
| C/2 nonster: 1 ster | 4.43 ± 0.62 b | 0.78 ± 0.15 | 5.20 ± 0.76 b | 13.88 ± 2.36 |
| HSD (p = 0.05) | 2.47 | 0.52 | 2.87 | 7.36 |
| Effect | Shoot DM (g/Plant) | Root DM (g/Plant) | Total Biomass (g/Plant) | Growth Increase (cm/Plant) |
|---|---|---|---|---|
| Treatment | ||||
| Nonster | 4.21 ± 0.37 b | 1.45 ± 0.09 b | 5.65 ± 0.43 b | 29.64 ± 1.96 b |
| Ster 50 °C | 4.67 ± 0.34 b | 1.60 ± 0.09 b | 6.27 ± 0.42 b | 30.29 ± 1.88 b |
| Ster 100 °C | 6.66 ± 0.29 a | 2.08 ± 0.11 a | 8.74 ± 0.37 a | 42.28 ± 1.36 a |
| HSD (p = 0.05) | 1.09 | 0.32 | 1.32 | 5.89 |
| Source site of soil | ||||
| A | 6.06 ± 0.43 a | 1.87 ± 0.11 a | 7.93 ± 0.53 a | 37.10 ± 2.07 a |
| B | 5.02 ± 0.39 ab | 1.66 ± 0.12 a | 6.68 ± 0.48 ab | 33.57 ± 2.21 a |
| C | 4.59 ± 0.34 b | 1.63 ± 0.10 a | 6.22 ± 0.42 b | 31.90 ± 2.02 a |
| HSD (p = 0.05) | 1.09 | 0.32 | 1.32 | 5.89 |
| GI-TCSA | Yield/Tree | ||||
|---|---|---|---|---|---|
| 2018/19–2022 | 2019 | 2020 | 2021 | 2022 | |
| Site A | |||||
| Treatment | 0.000 * | 0.341 | 0.268 | 0.126 | 0.001 * |
| R/v-combi | 0.006 * | 0.689 | 0.123 | 0.009 * | 0.012 * |
| Treatment × r/v-combi | 0.826 | 0.919 | 0.851 | 0.852 | 0.925 |
| Site B | |||||
| Treatment | 0.000 * | 0.236 | 0.012 * | 0.060 | 0.360 |
| R/v-combi | 0.000 * | 0.300 | 0.487 | 0.511 | 0.848 |
| Treatment × r/v-combi | 0.133 | 0.543 | 0.538 | 0.318 | 0.904 |
| Site C | |||||
| Treatment | 0.619 | 0.144 | 0.443 | 0.597 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djalali Farahani-Kofoet, R.; Schneider, D.; Feller, C. Apple Growth and Yield in Replant Soils Supplemented by Organic Soil Additives. Agronomy 2024, 14, 678. https://doi.org/10.3390/agronomy14040678
Djalali Farahani-Kofoet R, Schneider D, Feller C. Apple Growth and Yield in Replant Soils Supplemented by Organic Soil Additives. Agronomy. 2024; 14(4):678. https://doi.org/10.3390/agronomy14040678
Chicago/Turabian StyleDjalali Farahani-Kofoet, Roxana, Daniel Schneider, and Carmen Feller. 2024. "Apple Growth and Yield in Replant Soils Supplemented by Organic Soil Additives" Agronomy 14, no. 4: 678. https://doi.org/10.3390/agronomy14040678
APA StyleDjalali Farahani-Kofoet, R., Schneider, D., & Feller, C. (2024). Apple Growth and Yield in Replant Soils Supplemented by Organic Soil Additives. Agronomy, 14(4), 678. https://doi.org/10.3390/agronomy14040678





