Uptake of Thallium(I) by Rice Seedlings Grown in Different Soils: Key Soil Properties Determining Soil Thallium Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Characterization
2.2. Preparation of Rice Seedlings
2.3. Growth of Rice Seedlings in Tl-Spiked Soils
2.4. Analysis of Rice Plant
2.5. Statistical Analysis of Data
3. Results and Discussion
3.1. Basic Properties of the Tested Soils
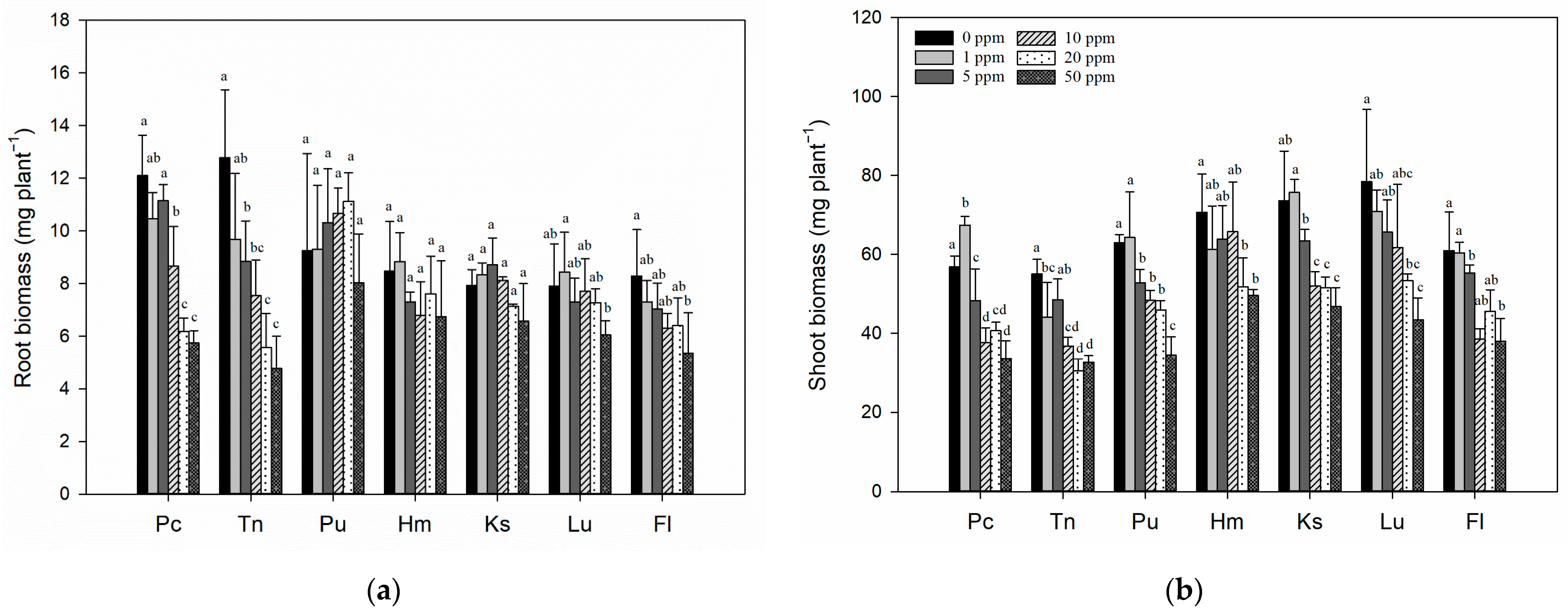
3.2. Effects of Tl Treatments on the Growth of Rice Seedlings in Different Soils
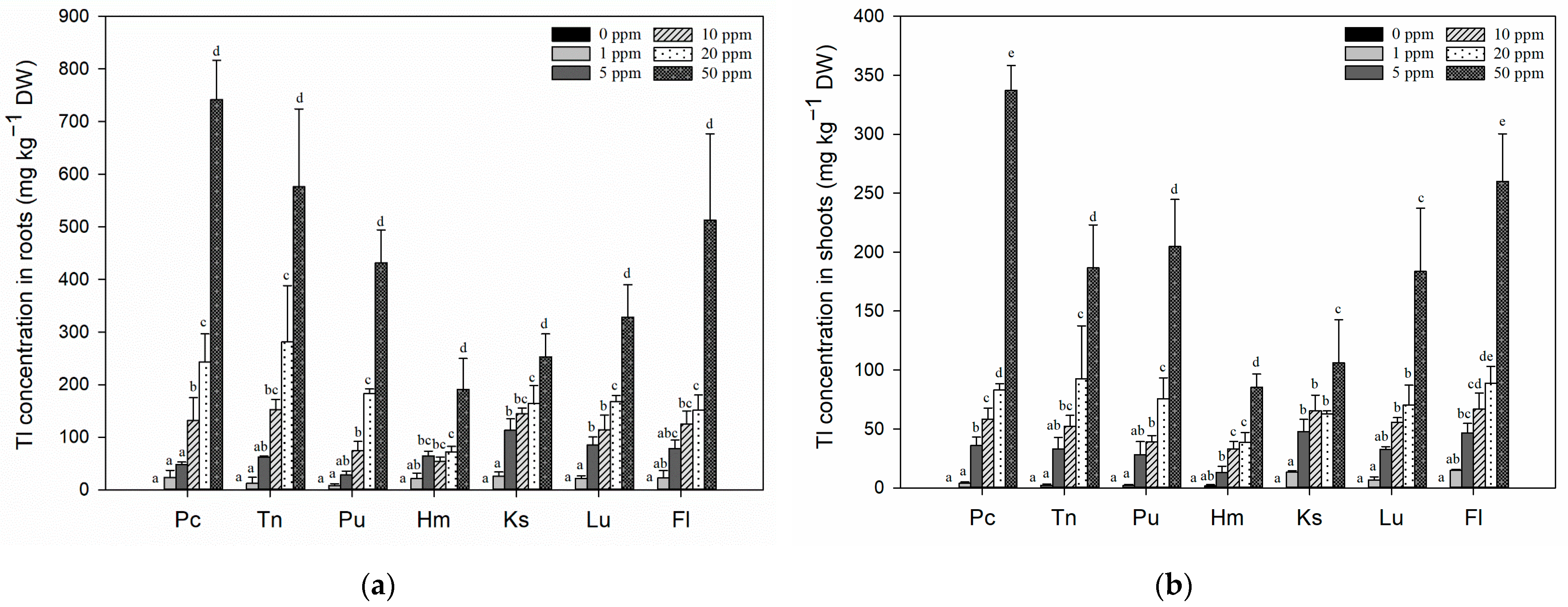
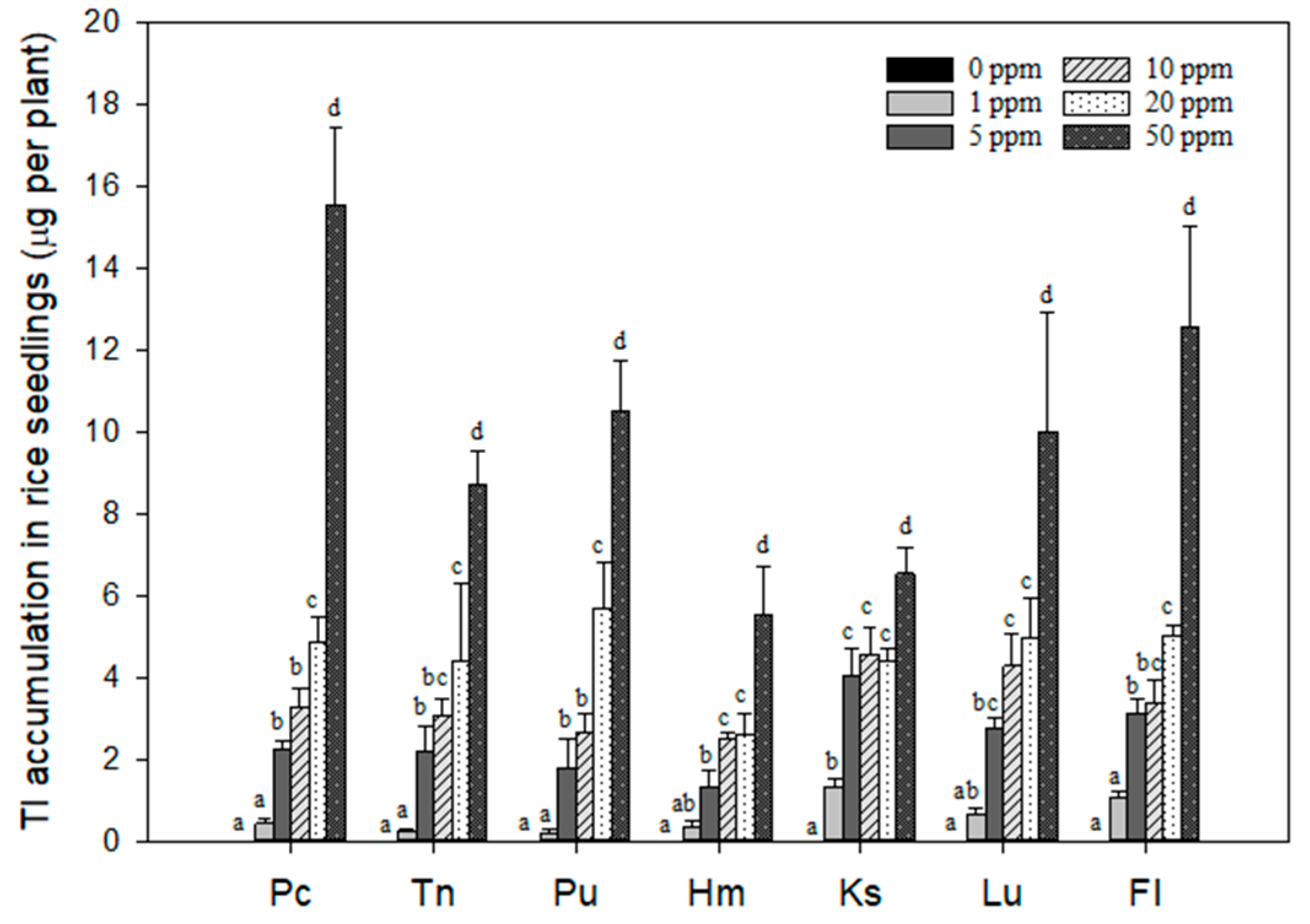
3.3. Effects of Tl Treatments on the Tl Contents in Rice Seedlings
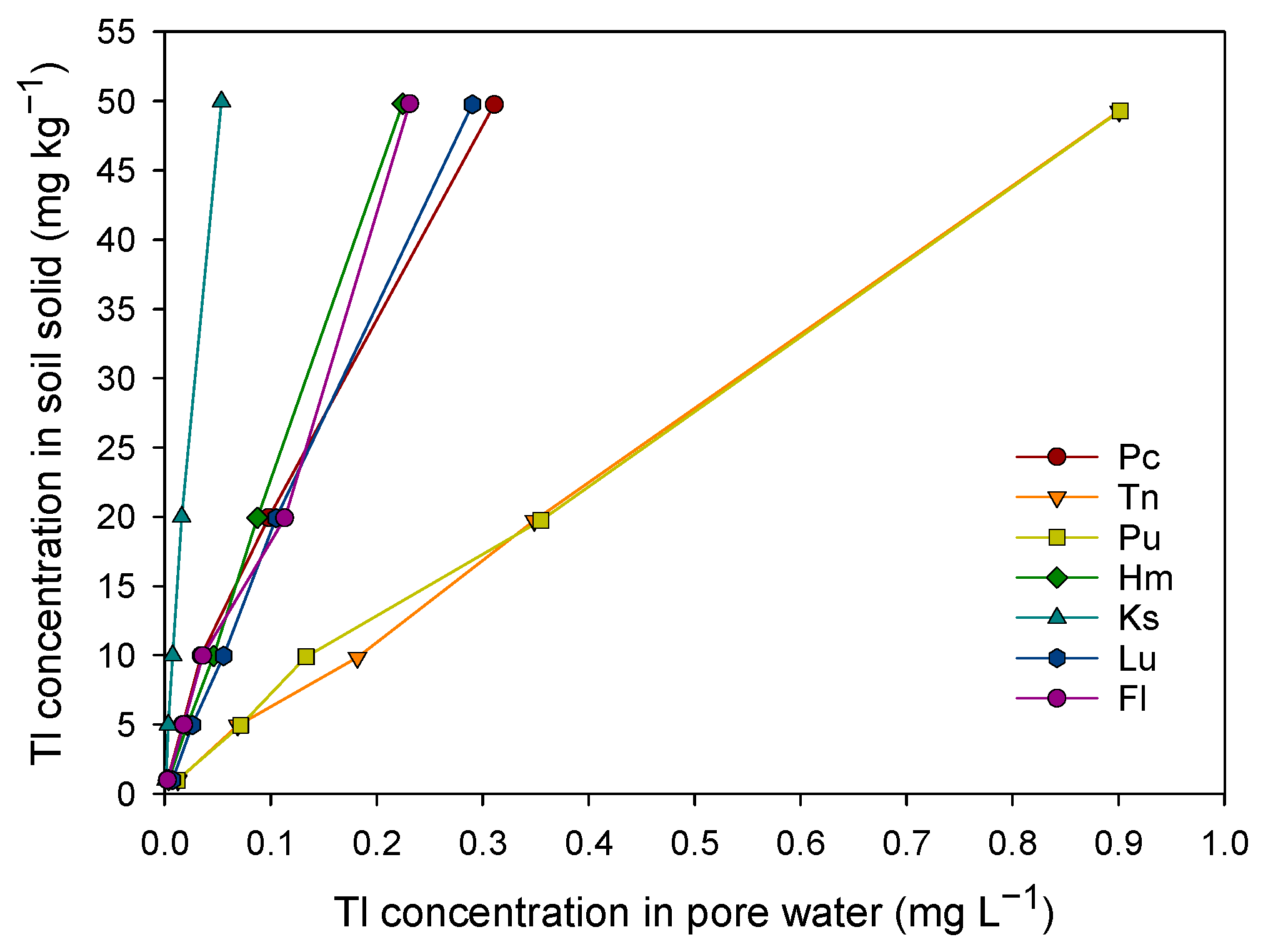
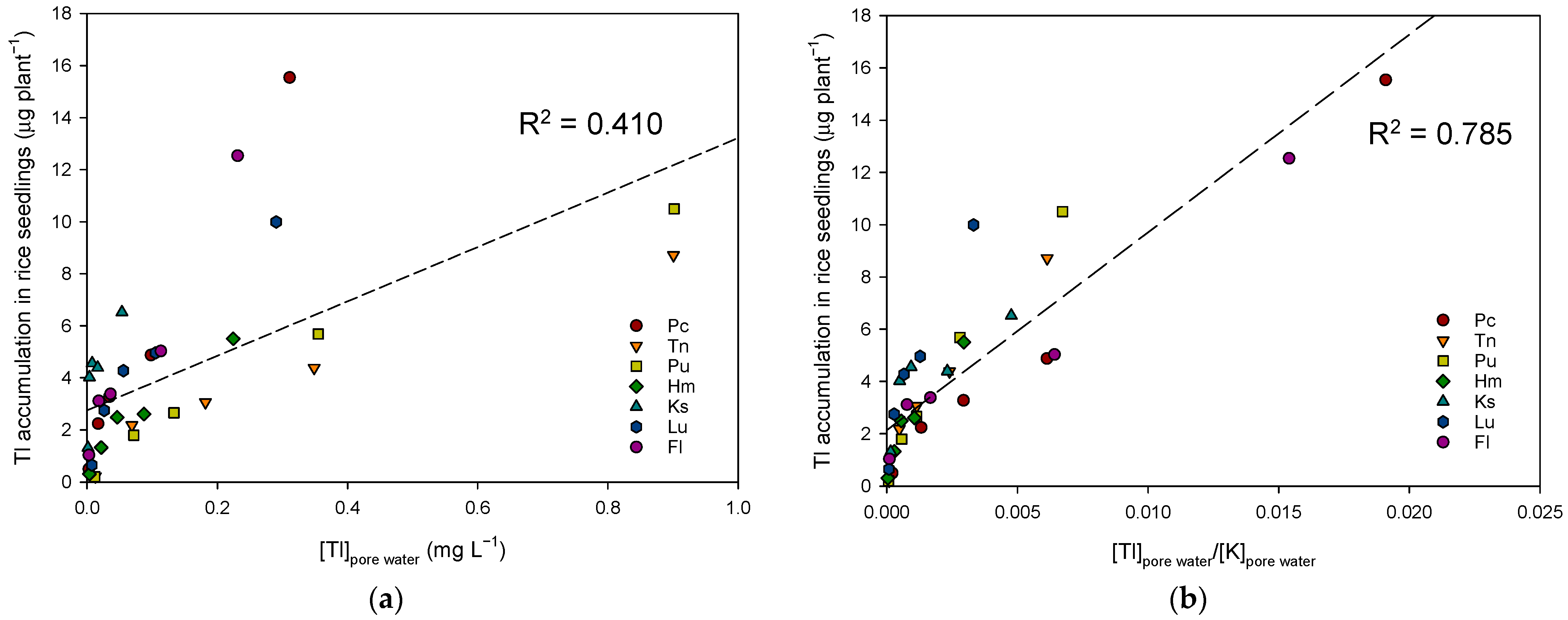
3.4. The Key Factors in Determining Soil Tl Availability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, A.L.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Viraraghavan, T.; Srinivasan, A. Thallium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 325–333. [Google Scholar]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Chen, Y.H.; Shen, C.C.; Jiang, X.Y.; Xie, X.F.; Chen, D.Y.; Lippold, H.; Wang, C.L. Thallium dispersal and contamination in surface sediments from South China and its source identification. Environ. Pollut. 2016, 213, 878–887. [Google Scholar] [CrossRef]
- Cobelo-Garcia, A.; Filella, M.; Croot, P.; Frazzoli, C.; Du Laing, G.; Ospina-Alvarez, N.; Rauch, S.; Salaun, P.; Schafer, J.; Zimmermann, S. COST action TD1407: Network on technology-critical elements (NOTICE)—From environmental processes to human health threats. Environ. Sci. Pollut. Res. Int. 2015, 22, 15188–15194. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A.; Jablonska-Czapla, M.; Grygoyc, K.; Rachwal, M. Studies on the Content of Selected Technology Critical Elements (Germanium, Tellurium and Thallium) in Electronic Waste. Materials 2021, 14, 3722. [Google Scholar] [CrossRef] [PubMed]
- Migaszewski, Z.M.; Gałuszka, A. Abundance and fate of thallium and its stable isotopes in the environment. Rev. Environ. Sci. Biotechnol. 2021, 20, 5–30. [Google Scholar] [CrossRef]
- LaCoste, C.; Robinson, B.; Brooks, R. Uptake of thallium by vegetables: Its significance for human health, phytoremediation, and phytomining. J. Plant Nutr. 2001, 24, 1205–1215. [Google Scholar] [CrossRef]
- Pavlickova, J.; Zbiral, J.; Smatanova, M.; Habarta, P.; Houserova, P.; Kuban, V. Uptake of thallium from naturally-contaminated soils into vegetables. Food Addit. Contam. 2006, 23, 484–491. [Google Scholar] [CrossRef]
- Lehn, H.; Schoer, J. Thallium-transfer from soils to plants—Correlation between chemical form and plant uptake. Plant Soil 1987, 97, 253–265. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, X.; He, H.; She, J.; Liu, J.; Fang, F.; Zhang, W.; Liu, Y.; Wang, J.; Xiao, T.; et al. Transformation and fate of thallium and accompanying metal(loid)s in paddy soils and rice: A case study from a large-scale industrial area in China. J. Hazard. Mater. 2022, 423, 126997. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press: Boca Raton, FL, USA, 2015; p. 440. [Google Scholar]
- Jia, Y.L.; Xiao, T.F.; Zhou, G.Z.; Ning, Z.P. Thallium at the interface of soil and green cabbage (Brassica oleracea L. var. capitata L.): Soil-plant transfer and influencing factors. Sci. Total Environ. 2013, 450, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Maluszynski, M.J.; Maluszynska, I. Evaluation of the soil quality from areas with varying degrees of pollution. Desalin. Water Treat. 2016, 57, 1034–1037. [Google Scholar] [CrossRef]
- Xiao, T.; Guha, J.; Boyle, D.; Liu, C.-Q.; Chen, J. Environmental concerns related to high thallium levels in soils and thallium uptake by plants in southwest Guizhou, China. Sci. Total Environ. 2004, 318, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Madejon, P. Thallium. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: New York, NY, USA, 2013; pp. 543–549. [Google Scholar]
- Martin, L.A.; Wissocq, A.; Benedetti, M.F.; Latrille, C. Thallium (Tl) sorption onto illite and smectite: Implications for Tl mobility in the environment. Geochim. Cosmochim. Acta 2018, 230, 1–16. [Google Scholar] [CrossRef]
- Vanek, A.; Komarek, M.; Vokurkova, P.; Mihaljevic, M.; Sebek, O.; Panuskova, G.; Chrastny, V.; Drabek, O. Effect of illite and birnessite on thallium retention and bioavailability in contaminated soils. J. Hazard. Mater. 2011, 191, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Vanek, A.; Mihaljevic, M.; Galuskova, I.; Chrastny, V.; Komarek, M.; Penizek, V.; Zadorova, T.; Drabek, O. Phase-dependent phytoavailability of thallium—A synthetic soil experiment. J. Hazard. Mater. 2013, 250, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lippold, H.; Wang, J.; Lippmann-Pipke, J.; Chen, Y. Sorption of thallium(I) onto geological materials: Influence of pH and humic matter. Chemosphere 2011, 82, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Vanek, A.; Grosslova, Z.; Mihaljevic, M.; Ettler, V.; Chrastny, V.; Komarek, M.; Tejnecky, V.; Drabek, O.; Penizek, V.; Galuskova, I.; et al. Thallium contamination of soils/vegetation as affected by sphalerite weathering: A model rhizospheric experiment. J. Hazard. Mater. 2015, 283, 148–156. [Google Scholar] [CrossRef]
- Voegelin, A.; Pfenninger, N.; Petrikis, J.; Majzlan, J.; Plotze, M.; Senn, A.C.; Mangold, S.; Steininger, R.; Gottlicher, J. Thallium speciation and extractability in a thallium- and arsenic-rich soil developed from mineralized carbonate rock. Environ. Sci. Technol. 2015, 49, 5390–5398. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chuang, T.J.; Yang, P.T.; Guo, L.Y.; Wang, S.L. Adsorption and desorption of thallium(I) in soils: The predominant contribution by clay minerals. Appl. Clay Sci. 2021, 205, 106063. [Google Scholar] [CrossRef]
- Wick, S.; Baeyens, B.; Fernandes, M.M.; Gottlicher, J.; Fischer, M.; Pfenninger, N.; Plotze, M.; Voegelin, A. Thallium sorption and speciation in soils: Role of micaceous clay minerals and manganese oxides. Geochim. Cosmochim. Acta 2020, 288, 83–100. [Google Scholar] [CrossRef]
- Bidoglio, G.; Gibson, P.N.; Ogorman, M.; Roberts, K.J. X-ray absorption spectroscopy investigation of surface redox transformations of thallium and chromium on colloidal mineral oxides. Geochim. Cosmochim. Acta 1993, 57, 2389–2394. [Google Scholar] [CrossRef]
- Cruz-Hernandez, Y.; Villalobos, M.; Marcus, M.A.; Pi-Puig, T.; Zanella, R.; Martinez-Villegas, N. Tl(I) sorption behavior on birnessite and its implications for mineral structural changes. Geochim. Cosmochim. Acta 2019, 248, 356–369. [Google Scholar] [CrossRef]
- Antic-Mladenovic, S.; Frohne, T.; Kresovic, M.; Stark, H.J.; Savic, D.; Licina, V.; Rinklebe, J. Redox-controlled release dynamics of thallium in periodically flooded arable soil. Chemosphere 2017, 178, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.R.; McBride, M.B.; Baveye, P.; Steenhuis, T.S. Environmental factors determining the trace-level sorption of silver and thallium to soils. Sci. Total Environ. 2005, 345, 191–205. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Jackson, M.L.; Lim, C.H.; Zelazny, L.W. Oxides, hydroxides, and aluminosilicates. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 101–150. [Google Scholar]
- Summer, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3. Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Solymosi, K.; Bertrand, M. Soil metals, chloroplasts, and secure crop production: A review. Agron. Sustain. Dev. 2012, 32, 245–272. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation-a critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L. The aqueous geochemistry of thallium: Speciation and solubility of thallium in low temperature systems. Environ. Chem. 2009, 6, 441–451. [Google Scholar] [CrossRef]
- Wick, S.; Baeyens, B.; Marques Fernandes, M.; Voegelin, A. Thallium adsorption onto illite. Environ. Sci. Technol. 2018, 52, 571–580. [Google Scholar] [CrossRef]
- Huang, X.; Li, N.; Wu, Q.; Long, J.; Luo, D.; Zhang, P.; Yao, Y.; Huang, X.; Li, D.; Lu, Y.; et al. Risk assessment and vertical distribution of thallium in paddy soils and uptake in rice plants irrigated with acid mine drainage. Environ. Sci. Pollut. Res. Int 2016, 23, 24912–24921. [Google Scholar] [CrossRef] [PubMed]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef]
- Jungk, A.; Claassen, N. Ion Diffusion in the Soil–Root System. Adv. Agron. 1997, 61, 53–110. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nolan, A.; Schaumlöffel, D.; Lombi, E.; Ouerdane, L.; Łobiński, R.; McLaughlin, M. Determination of Tl (I) and Tl (III) by IC-ICP-MS and application to Tl speciation analysis in the Tl hyperaccumulator plant Iberis intermedia. J. Anal. Atom. Spectrom. 2004, 19, 757–761. [Google Scholar] [CrossRef]
- Renkema, H.; Koopmans, A.; Hale, B.; Berkelaar, E. Thallium and potassium uptake kinetics and competition differ between durum wheat and canola. Environ. Sci. Pollut. Res. 2015, 22, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.; Smith, S. Some aspects of the kinetics of cadmium and thallium uptake by fronds of Lemna minor L. New Phytol. 1991, 117, 91–102. [Google Scholar] [CrossRef]
- Poscic, F.; Marchiol, L.; Schat, H. Hyperaccumulation of thallium is population-specific and uncorrelated with caesium accumulation in the thallium hyperaccumulator, Biscutella laevigata. Plant Soil 2013, 365, 81–91. [Google Scholar] [CrossRef]
- Soukup, D.A.; Buck, B.J.; Lynn, W.C. Preparing soils for mineralogical analyses. In Methods of Soil Analysis Part 5—Mineralogical Analysis; Ulery, A.L., Drees, L.R., Eds.; Soil Science Society of America: Madison, WI, USA, 2008; pp. 13–31. [Google Scholar]
- Harris, W.; White, G.N. X-ray diffraction techniques for soil mineral identification. In Methods of Soil Analysis Part 5—Mineralogical Methods; Ulery, A.L., Drees, L.R., Eds.; Soil Science Society of America: Madison, WI, USA, 2008; pp. 81–115. [Google Scholar]
- Johns, W.D.; Grim, R.E.; Bradley, W.F. Quantitative estimations of clay minerals by diffraction methods. J. Sediment. Petrol. 1954, 24, 242–251. [Google Scholar]
| Soil | pH | Soil Texture 1 | OM 2 (g kg−1) | CEC 3 (cmolc kg−1) | Exchangeable Cations (mmol kg−1) | Free Oxides (g kg−1) | Amorphous Oxides (g kg−1) | Tl (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caex | Mgex | Kex | Naex | Fed | Ald | Feo | Alo | ||||||
| Pc | 4.6 | SiCL | 13.5 | 7.6 | 4.2 | 0.8 | 2.9 | 0.3 | 1.22 | 3.02 | 0.93 | 1.22 | 0.57 |
| Tn | 5.0 | SiL | 11.4 | 6.6 | 5.4 | 1.8 | 3.0 | 0.4 | 0.35 | 0.37 | 1.31 | 0.35 | 0.77 |
| Pu | 5.7 | CL | 21.6 | 9.4 | 2.4 | 3.7 | 4.0 | 3.4 | 0.83 | 1.57 | 4.77 | 0.83 | 0.49 |
| Hm | 6.1 | CL | 27.8 | 14.6 | 30.8 | 10.3 | 3.2 | 2.8 | 0.33 | 3.34 | 4.21 | 0.33 | 0.74 |
| Ks | 6.9 | CL | 22.6 | 29.8 | 35.4 | 75.2 | 1.5 | 1.6 | 1.24 | 3.09 | 4.17 | 1.24 | 0.88 |
| Lu | 7.0 | SiCL | 11.9 | 10.5 | 31.0 | 7.2 | 2.6 | 1.9 | 0.37 | 0.39 | 3.96 | 0.37 | 0.23 |
| Fl | 8.0 | SiL | 9.8 | 5.5 | 36.7 | 2.6 | 0.9 | 0.7 | 0.51 | 0.56 | 1.63 | 0.51 | 0.70 |
| Soil Tl Level (mg kg−1) | Pc Soil | Tn Soil | Pu Soil | Hm Soil | Ks Soil | Lu Soil | Fl Soil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCFR 1 | BCFS 2 | BCFR | BCFS | BCFR | BCFS | BCFR | BCFS | BCFR | BCFS | BCFR | BCFS | BCFR | BCFS | |
| 1 | 23.5 | 3.8 | 12.2 | 2.2 | 7.9 | 1.9 | 21.3 | 1.7 | 25.6 | 13.3 | 21.7 | 6.7 | 22.4 | 14.7 |
| 5 | 9.6 | 7.2 | 12.4 | 6.6 | 5.6 | 5.6 | 12.9 | 1.9 | 22.7 | 9.6 | 17.0 | 6.5 | 15.7 | 9.3 |
| 10 | 13.1 | 5.8 | 15.2 | 5.2 | 7.4 | 3.9 | 5.4 | 3.3 | 14.5 | 6.6 | 11.4 | 5.6 | 12.5 | 6.7 |
| 20 | 12.1 | 4.2 | 14.0 | 4.6 | 9.1 | 3.8 | 3.6 | 2.6 | 8.2 | 3.1 | 8.4 | 3.5 | 7.5 | 4.4 |
| 50 | 14.8 | 6.8 | 11.5 | 3.7 | 8.6 | 4.1 | 3.8 | 1.5 | 5.1 | 2.1 | 6.4 | 3.7 | 6.9 | 4.2 |
| Soil Properties | Correlations of Tl Accumulation in Rice Seedlings with Soil Properties | Intercorrelations among Soil Properties | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tl1 | Tl5 | Tl10 | Tl20 | Tl50 | pH | OM | CEC | Feo | Alo | Fed | Ald | Caex | Mgex | Kex | Naex | Kd | |
| pH | 0.706 | 0.558 | 0.445 | 0.056 | −0.192 | 1 | |||||||||||
| OM | −0.105 | −0.279 | −0.270 | −0.553 | −0.669 | −0.079 | 1 | ||||||||||
| CEC | 0.583 | 0.541 | 0.545 | −0.308 | −0.626 | 0.231 | 0.616 | 1 | |||||||||
| Feo | 0.022 | −0.046 | 0.084 | −0.124 | −0.636 | 0.296 | 0.728 | 0.549 | 1 | ||||||||
| Alo | 0.410 | 0.437 | 0.340 | 0.335 | 0.323 | −0.217 | 0.166 | 0.474 | −0.032 | 1 | |||||||
| Fed | 0.190 | 0.224 | 0.201 | 0.369 | 0.416 | −0.380 | 0.175 | 0.327 | −0.015 | 0.963 | 1 | ||||||
| Ald | 0.110 | −0.090 | −0.098 | −0.504 | −0.217 | −0.265 | 0.750 | 0.584 | 0.241 | 0.582 | 0.585 | 1 | |||||
| Caex | 0.731 | 0.526 | 0.502 | −0.363 | −0.415 | 0.874 | 0.122 | 0.463 | 0.289 | −0.199 | −0.373 | 0.048 | 1 | ||||
| Mgex | 0.714 | 0.715 | 0.652 | −0.157 | −0.515 | 0.295 | 0.432 | 0.967 | 0.402 | 0.535 | 0.348 | 0.458 | 0.467 | 1 | |||
| Kex | −0.900 | −0.815 | −0.610 | −0.030 | −0.062 | −0.730 | 0.360 | −0.265 | 0.286 | −0.119 | 0.109 | 0.124 | −0.735 | −0.430 | 1 | ||
| Naex | −0.273 | −0.376 | −0.282 | −0.116 | −0.500 | 0.135 | 0.744 | 0.264 | 0.924 | −0.136 | −0.051 | 0.229 | 0.067 | 0.088 | 0.512 | 1 | |
| Kd | 0.807 | 0.764 | 0.684 | −0.178 | −0.423 | 0.364 | 0.370 | 0.938 | 0.306 | 0.560 | 0.362 | 0.489 | 0.553 | 0.982 | −0.553 | −0.011 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-S.; Yang, P.-T.; Lu, Y.-A.; Liu, W.-L.; Chuang, T.-J.; Wang, S.-L. Uptake of Thallium(I) by Rice Seedlings Grown in Different Soils: Key Soil Properties Determining Soil Thallium Availability. Agronomy 2024, 14, 718. https://doi.org/10.3390/agronomy14040718
Huang L-S, Yang P-T, Lu Y-A, Liu W-L, Chuang T-J, Wang S-L. Uptake of Thallium(I) by Rice Seedlings Grown in Different Soils: Key Soil Properties Determining Soil Thallium Availability. Agronomy. 2024; 14(4):718. https://doi.org/10.3390/agronomy14040718
Chicago/Turabian StyleHuang, Liang-Sin, Puu-Tai Yang, Yu-An Lu, Wei-Lin Liu, Tsung-Ju Chuang, and Shan-Li Wang. 2024. "Uptake of Thallium(I) by Rice Seedlings Grown in Different Soils: Key Soil Properties Determining Soil Thallium Availability" Agronomy 14, no. 4: 718. https://doi.org/10.3390/agronomy14040718
APA StyleHuang, L.-S., Yang, P.-T., Lu, Y.-A., Liu, W.-L., Chuang, T.-J., & Wang, S.-L. (2024). Uptake of Thallium(I) by Rice Seedlings Grown in Different Soils: Key Soil Properties Determining Soil Thallium Availability. Agronomy, 14(4), 718. https://doi.org/10.3390/agronomy14040718






