Molecular Mechanism of Exogenous Magnesium in Regulating Cation Homeostasis in Roots of Peanut Seedlings under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material Culture
2.2. Experiment Design
2.3. Test Method
2.3.1. Determination of Physiological Phenotypic Indicators
2.3.2. Transcriptome (RNA-Seq) Sequencing
2.3.3. RT-qPCR Detection
2.4. Data Processing and Data Visualization Methods
2.4.1. Screening of Differentially Expressed Genes (DEGs)
2.4.2. GO Enrichment Analysis and KEGG Enrichment Analysis
3. Results
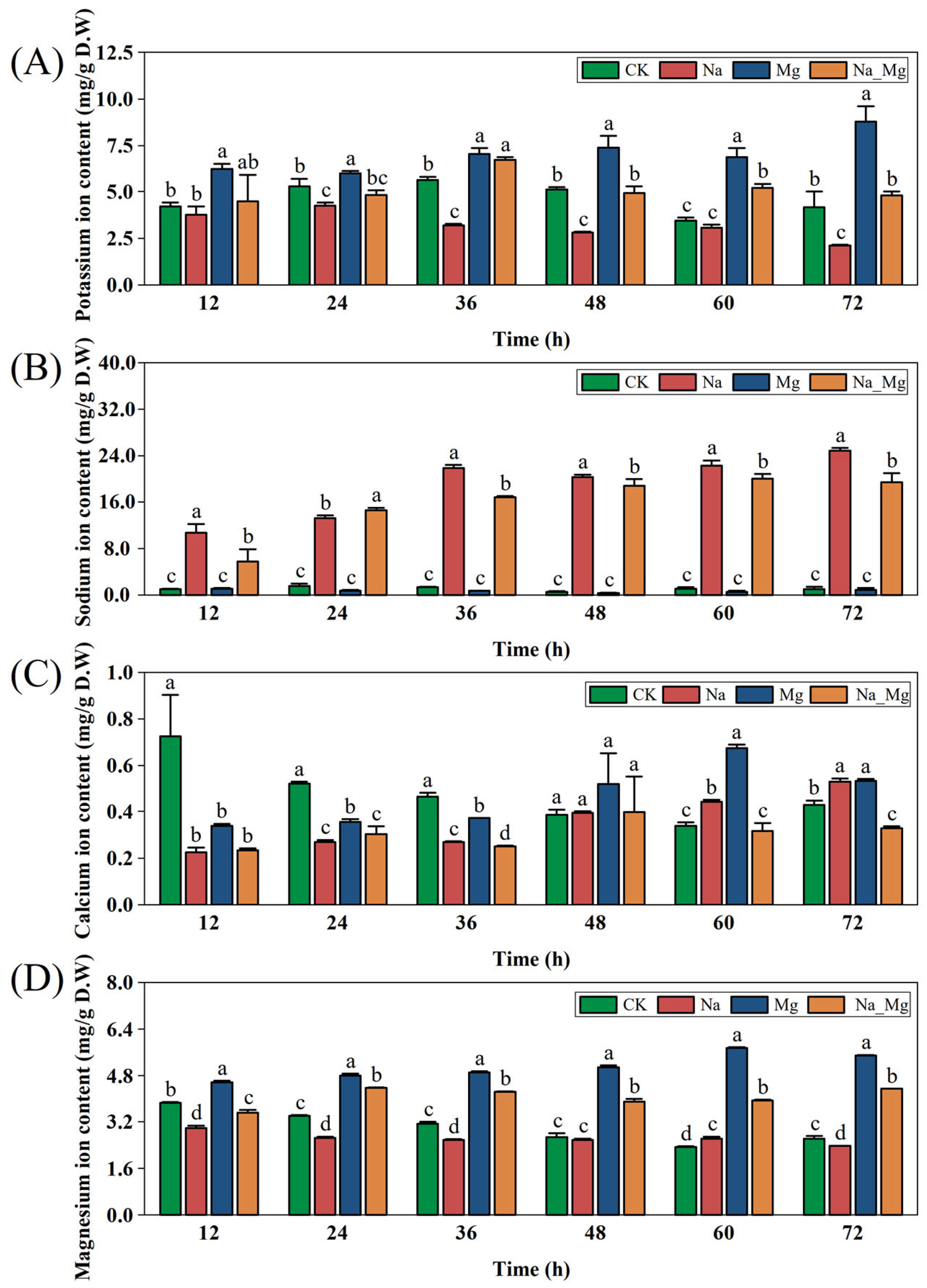
3.1. Exogenous Magnesium Alleviates Root Growth and Ion Homeostasis of Peanut Seedlings under Salt Stress
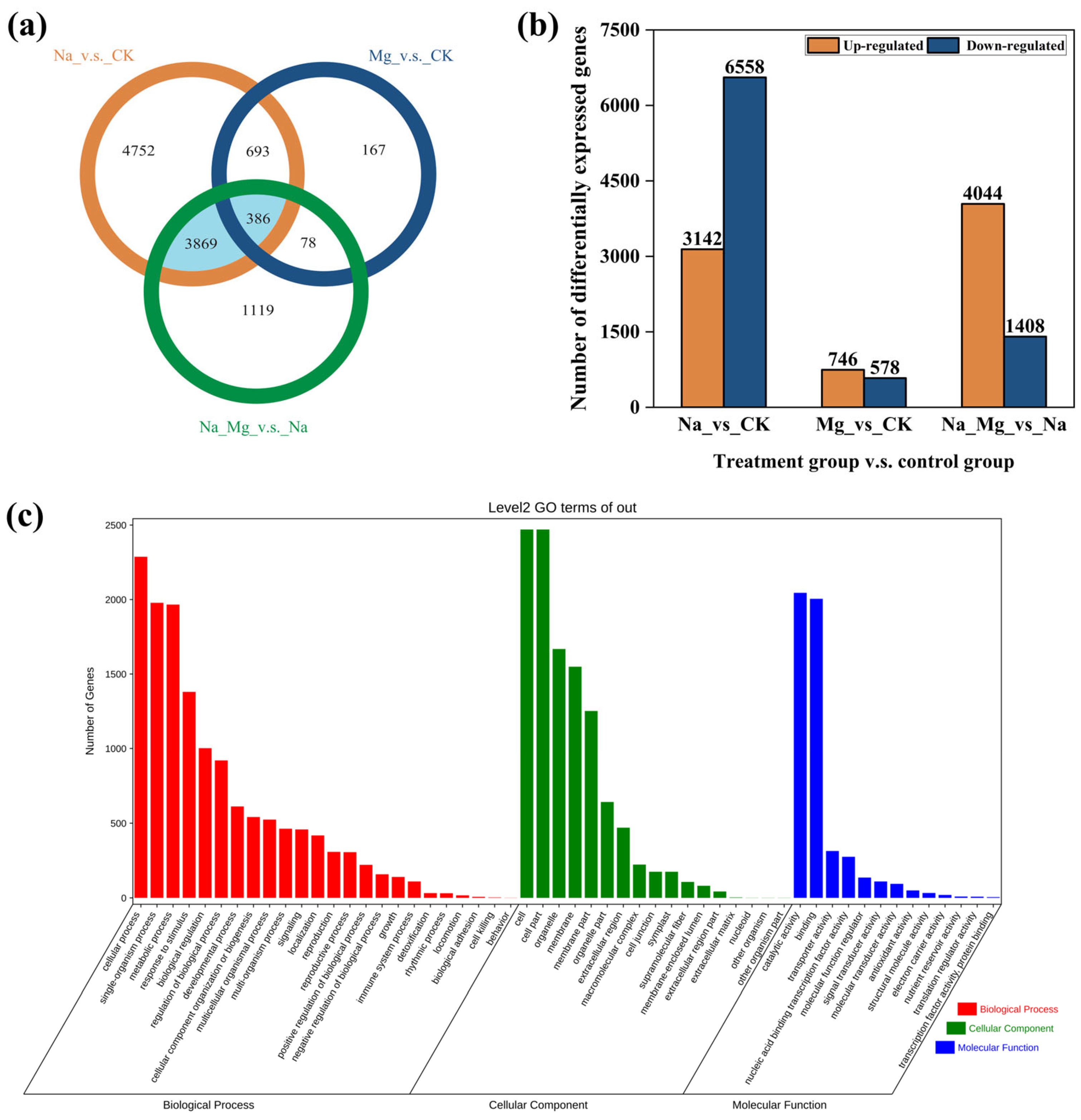
3.2. DEG Screening and KEGG Enrichment Analysis of DEGs
3.3. Mining Differentially Expressed Genes of Magnesium Ion in Response to Cation Homeostasis of Peanut Seedlings under Salt Stress
3.3.1. Regulation of Exogenous Mg2+ on Key Genes of Mg2+ Transport Channel and Non-Selective Ion Channel
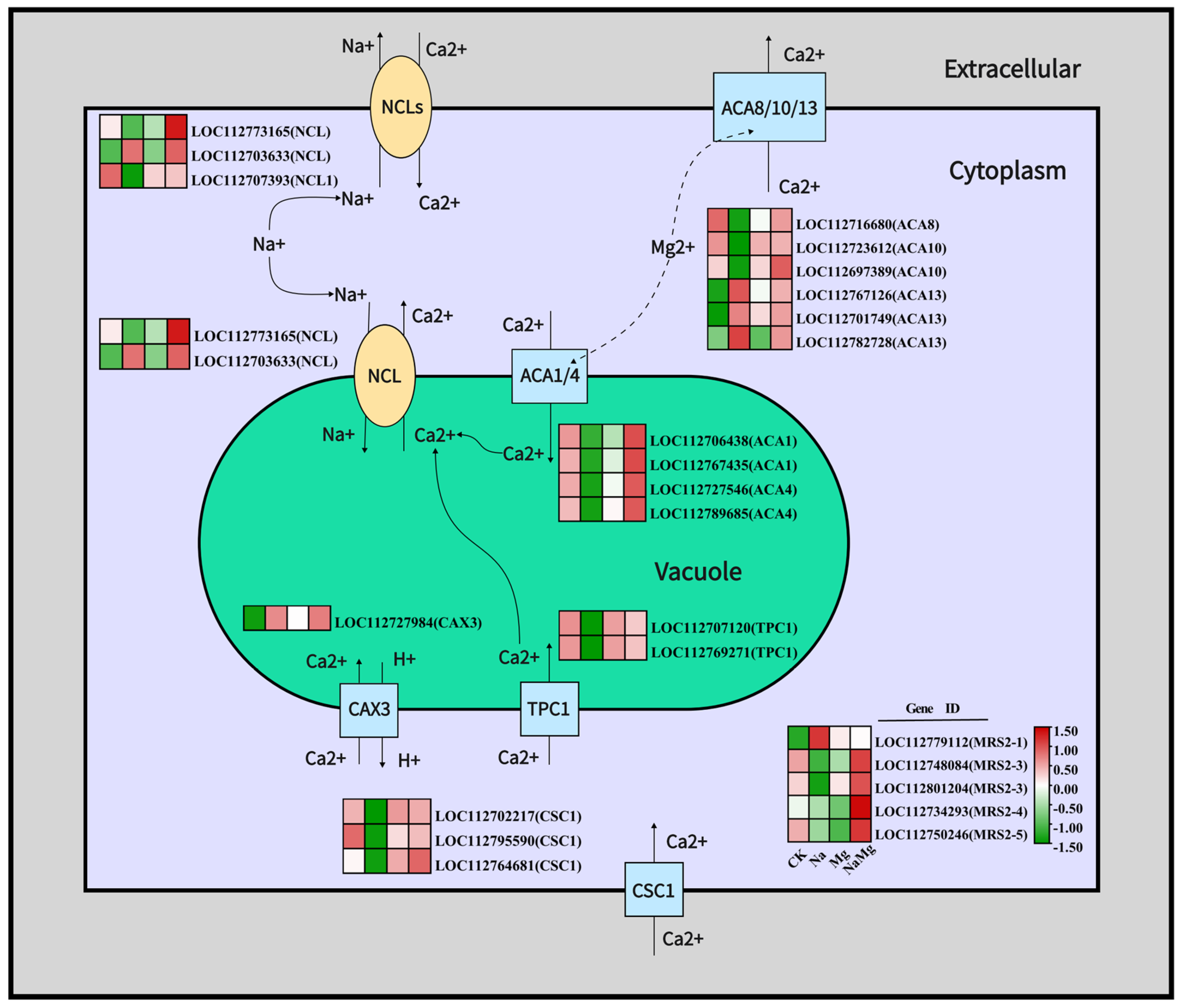
3.3.2. Regulation of Exogenous Mg2+ on Key Genes of Ca2+ Transport Channel and Na+/Ca2+ Ion Exchange Channel
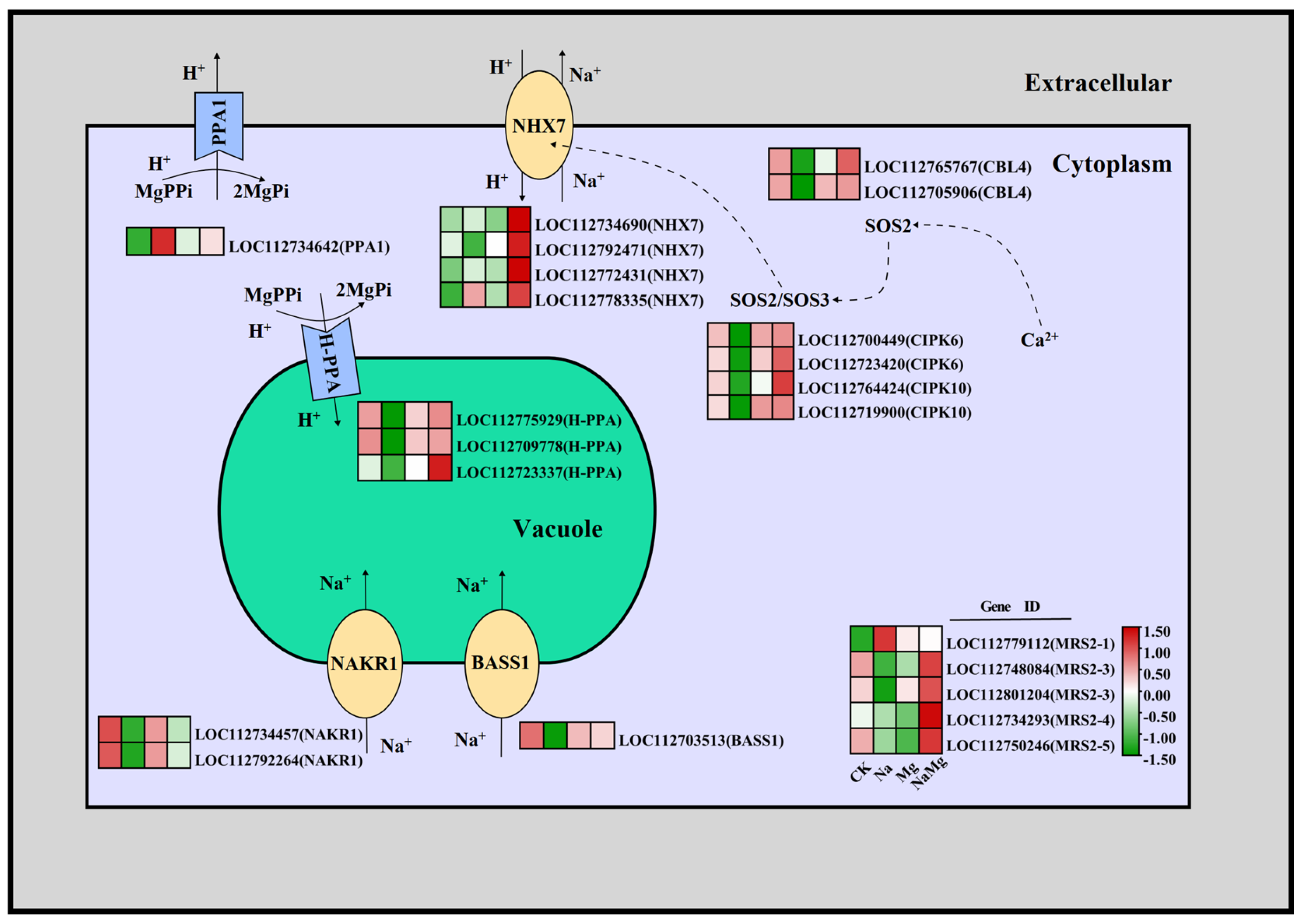
3.3.3. Regulation of Exogenous Mg2+ on Key Genes of H+ Transport Channel, Na+ Transport Channel, and Na+/H+ Ion Exchange Channel
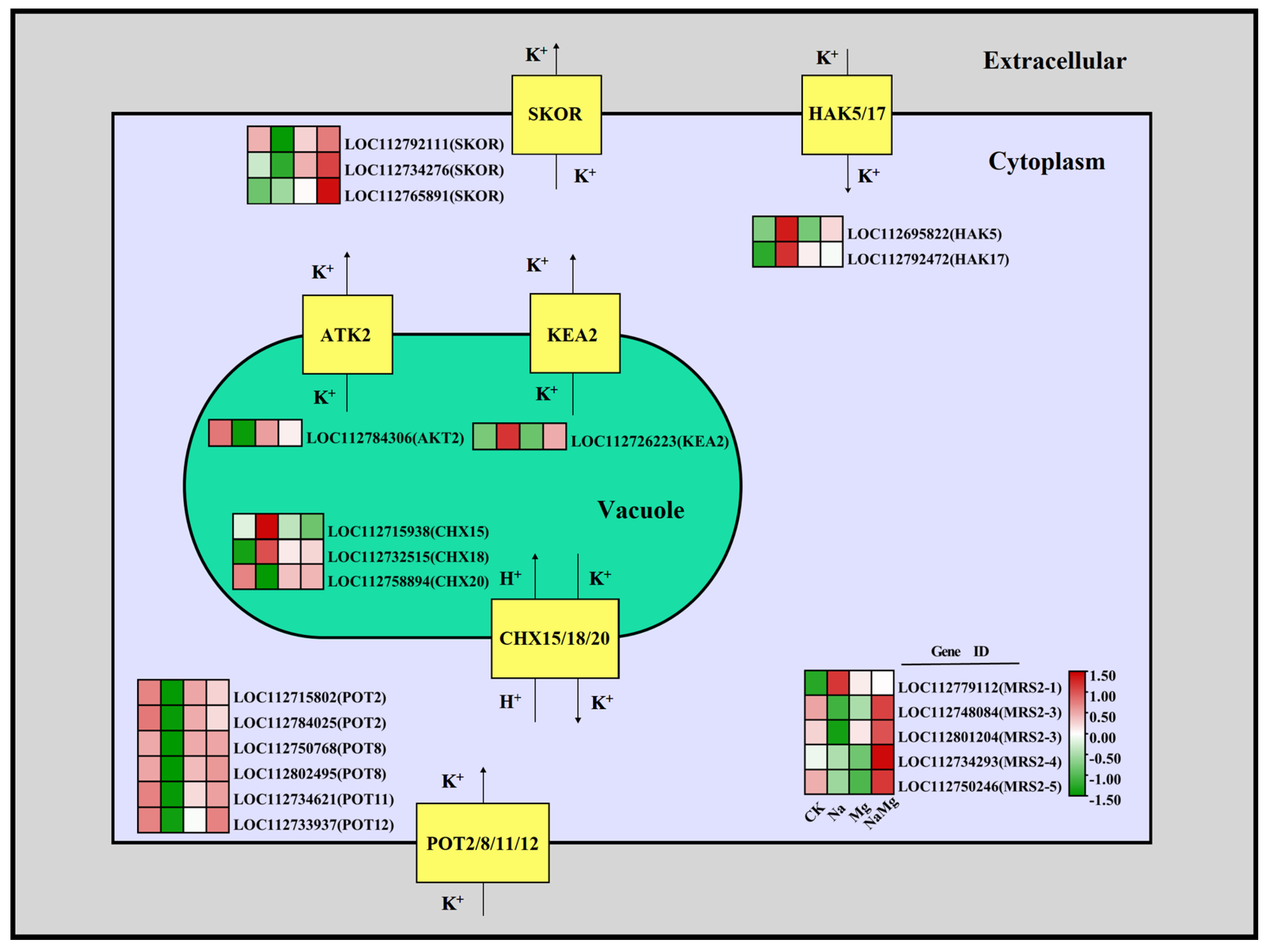
3.3.4. Regulation of Exogenous Mg2+ on Key Genes of K+ Transport Channel
3.4. RT−qPCR Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, A. Soil salinization management for sustainable development: A review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Nie, C.Y.; Li, Z.; Kang, J.; Wang, X.L.; Cui, Y.N. Physiological and transcriptional analyses provide insight into maintaining ion homeostasis of sweet sorghum under salt stress. Int. J. Mol. Sci. 2023, 24, 11045. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.; Li, J.; Zhang, J.; Zhang, X.; Hu, L.; Ding, D.; Bakpa, E.P.; Xie, J. Trehalose alleviated salt stress in tomato by regulating ROS metabolism, photosynthesis, osmolyte synthesis, and trehalose metabolic pathways. Front. Plant Sci. 2022, 13, 772948. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fu, R.; Li, M.; Song, Y.; Li, J.; Chen, C.; Gu, Y.; Liang, X.; Nie, W.; Ma, L.; et al. Transcriptome profiling reveals multiple regulatory pathways of tamarix chinensis in response to salt stress. Plant Cell Rep. 2023, 42, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Hanafy, R.S.; Elkady, F.M.A.M.; Mogazy, A.M.; Abdelhamid, M.T. Exogenous calcium reinforces photosynthetic pigment content and osmolyte, enzymatic, and non-enzymatic antioxidants abundance and alleviates salt stress in bread wheat. Plants 2023, 12, 1532. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Kobayashi, N.; Tanoi, K. Critical issues in the study of magnesium transport systems and magnesium deficiency symptoms in plants. Int. J. Mol. Sci. 2015, 16, 23076–23093. [Google Scholar] [CrossRef]
- Verbruggen, C.E.M.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Kleczkowski, L.A.; Igamberdiev, A.U. Magnesium signaling in plants. Int. J. Mol. Sci. 2021, 22, 1159. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Changes in soil properties and salt tolerance of safflower in response to biochar-based metal oxide nanocomposites of magnesium and manganese. Ecotoxicol. Environ. Saf. 2021, 211, 111904. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Gyushi, M.A.H.; Hemida, K.A.; El-Saadony, M.T.; Abd El-Mageed, S.A.; Abdalla, H.; AbuQamar, S.F.; El-Tarabily, K.A.; Abdelkhalik, A. Coapplication of effective microorganisms and nanomagnesium boosts the agronomic, physio-biochemical, osmolytes, and antioxidants defenses against salt stress in Ipomoea batatas. Front. Plant Sci. 2022, 13, 883274. [Google Scholar] [CrossRef]
- Rivelli, A.R.; De Maria, S.; Pizza, S.; Gherbin, P. Growth and physiological response of hydroponically-grown sunflower as affected by salinity and magnesium levels. J. Plant Nutr. 2010, 33, 1307–1323. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice1[W][OA]. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef]
- Tian, G.; Liu, C.; Xu, X.; Xing, Y.; Liu, J.; Lyu, M.; Feng, Z.; Zhang, X.; Qin, H.; Jiang, H.; et al. Effects of magnesium on nitrate uptake and sorbitol synthesis and translocation in apple seedlings. Plant Physiol. Biochem. 2023, 196, 139–151. [Google Scholar] [CrossRef]
- Luo, L.; Wan, Q.; Zhang, K.; Zhang, X.; Guo, R.; Wang, C.; Zheng, C.; Liu, F.; Ding, Z.; Wan, Y. AhABI4s negatively regulate salt-stress response in peanut. Front. Plant Sci. 2021, 12, 741641. [Google Scholar] [CrossRef]
- Khan, M.A.; Gemenet, D.C.; Villordon, A. Root system architecture and abiotic stress tolerance: Current knowledge in root and tuber crops. Front. Plant Sci. 2016, 7, 1584. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Ding, H.; Wen, S.; Zhang, G.; Qin, F.; Dai, L. Comprehensive effects of salt stress and peanut cultivars on the rhizosphere bacterial community diversity of peanut. Arch. Microbiol. 2021, 204, 15. [Google Scholar] [CrossRef]
- Wen, S.; Ding, H.; Xu, Y.; Zhang, G.C.; Zhang, Z.M.; Dai, L.X. Photosynthetic and stress-resistant physiological response characteristics of different salt-tolerant peanut varieties to NaCl stress. Acta Bot. Northwest 2021, 41, 1535–1544. (In Chinese) [Google Scholar] [CrossRef]
- Miao, Y.; Hong-zhi, L.; Ying, Y.; Ai-min, S.; Li, L.; Hui, H.; Qiang, W.; Hong-wei, Y.; Xiao-he, W. Optimising germinated conditions to enhance yield of resveratrol content in peanut sprout using response surface methodology. Int. J. Food Sci. Technol. 2016, 51, 1754–1761. [Google Scholar] [CrossRef]
- Liang, Y. Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 1999, 209, 217–224. [Google Scholar] [CrossRef]
- Malmstadt, H.V.; Hadjiioannou, T.P. Plant Analyses, Rapid and Accurate Automatic Titration Method for Determination of Calcium and Magnesium in Plant Material with EDTA Titrant. Available online: https://pubs.acs.org/doi/abs/10.1021/jf60100a005 (accessed on 4 January 2024).
- Ziemann, M.; Kaspi, A.; El-Osta, A. Digital expression explorer 2: A repository of uniformly processed RNA sequencing data. GigaScience 2019, 8, giz022. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2022, 51, D418–D427. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.-Y.; Yun, D.-J. A new insight of salt stress signaling in plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Horie, T.; Karahara, I.; Katsuhara, M. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice 2012, 5, 11. [Google Scholar] [CrossRef]
- Ren, W.; Chen, L.; Wang, Q.; Ren, Y. Transcriptome and metabolome analysis of upland cotton (Gossypium hirsutum) seed pretreatment with MgSO4 in response to salinity stress. Life 2022, 12, 921. [Google Scholar] [CrossRef]
- Xuan, T.D.; Huong, C.T.; Quan, N.V.; Anh, L.H.; Khanh, T.D.; Rayee, R. Improvement of salinity tolerance in rice seedlings by exogenous magnesium sulfate application. Soil Syst. 2022, 6, 69. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; He, H.; Yang, P.; Sun, X.; Zhang, Z. Genome-wide identification, characterization and experimental expression analysis of CNGC gene family in Gossypium. Int. J. Mol. Sci. 2023, 24, 4617. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-Y.; He, D.-D.; Bai, S.; Zeng, W.-Z.; Wang, Z.; Wang, M.; Wu, L.-Q.; Chen, Z.-C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-F.; Qian, X.-X.; Yang, Z.-N. Slowing development facilitates arabidopsis mgt mutants to accumulate enough magnesium for pollen formation and fertility restoration. Front. Plant Sci. 2021, 11, 621338. [Google Scholar] [CrossRef]
- Li, J.; Yokosho, K.; Liu, S.; Cao, H.R.; Yamaji, N.; Zhu, X.G.; Liao, H.; Ma, J.F.; Chen, Z.C. Diel magnesium fluctuations in chloroplasts contribute to photosynthesis in rice. Nat. Plants 2020, 6, 848–859. [Google Scholar] [CrossRef]
- Bin, M.; Yi, G.; Zhang, X. Discovery and characterization of magnesium transporter (MGT) gene family in Citrus sinensis and their role in magnesium deficiency stress. Plant Growth Regul. 2023, 100, 733–746. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Li, H.; Shuai, Y.; Chen, W.; Ma, D.; Lü, Z. Uncovering the role of wheat magnesium transporter family genes in abiotic responses. Front. Plant Sci. 2023, 14, 1078299. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, S.F.; Babaeian Jelodar, N.; Bagheri, N.; Nematzadeh, G.; Hashemipetroudi, S.H. New insights into comprehensive analysis of magnesium transporter (MGT) gene family in rice (Oryza Sativa L.). 3 Biotech 2023, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Theerawitaya, C.; Cha-um, S.; Kirdmanee, C.; Takabe, T. Expression and functional analysis of putative vacuolar Ca2+-transporters (CAXs and ACAs) in roots of salt tolerant and sensitive rice cultivars. Protoplasma 2014, 251, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, G.; Gonzales, N.; Guo, Y.; Hu, H.; Park, S.; Zhao, J. Ca2+-regulated and diurnal rhythm-regulated Na+/Ca2+ exchanger AtNCL affects flowering time and auxin signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 377–392. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, R.; Tripathi, A.K.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Genome-wide investigation and expression analysis of sodium/calcium exchanger gene family in rice and Arabidopsis. Rice 2015, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic stress signaling and responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yu, X.; Jiang, Y.; Wang, Y.; Yang, Y.; Chen, S.; Chen, Q.; Guo, Y. Salt overly sensitive 1 is inhibited by clade D protein phosphatase 2C D6 and D7 in Arabidopsis thaliana. Plant Cell 2023, 35, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709.e5. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kuang, L.; Li, X.; Wu, L.; Wu, D.; Zhang, G. Metabolomic and transcriptomic analyses reveal the reasons why hordeum marinum has higher salt tolerance than Hordeum vulgare. Environ. Exp. Bot. 2018, 156, 48–61. [Google Scholar] [CrossRef]
- Meyer, K.; Stecca, K.L.; Ewell-Hicks, K.; Allen, S.M.; Everard, J.D. Oil and protein accumulation in developing seeds is influenced by the expression of a cytosolic pyrophosphatase in Arabidopsis[C][W][OA]. Plant Physiol. 2012, 159, 1221–1234. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Abdoli, S. Improving ATPase and PPase activities, nutrient uptake and growth of salt stressed ajowan plants by salicylic acid and iron-oxide nanoparticles. Plant Cell Rep. 2021, 40, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Himabindu, Y.; Chakradhar, T.; Reddy, M.C.; Kanygin, A.; Redding, K.E.; Chandrasekhar, T. Salt-tolerant genes from halophytes are potential key players of salt tolerance in glycophytes. Environ. Exp. Bot. 2016, 124, 39–63. [Google Scholar] [CrossRef]
- Li, Z.; Geng, W.; Tan, M.; Ling, Y.; Zhang, Y.; Zhang, L.; Peng, Y. Differential responses to salt stress in four white clover genotypes associated with root growth, endogenous polyamines metabolism, and sodium/potassium accumulation and transport. Front. Plant Sci. 2022, 13, 896436. [Google Scholar] [CrossRef] [PubMed]
- Long-Tang, H.; Li-Na, Z.; Li-Wei, G.; Anne-Aliénor, V.; Hervé, S.; Yi-Dong, Z. Constitutive expression of CmSKOR, an outward K+ channel gene from melon, in Arabidopsis thaliana involved in saline tolerance. Plant Sci. 2018, 274, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Tang, Q.; Cai, J.; Xu, B.; Xu, G.; Yu, L. Rice OsHAK16 functions in potassium uptake and translocation in shoot, maintaining potassium homeostasis and salt tolerance. Planta 2019, 250, 549–561. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Q.; Luo, L.; Yang, T.; Zhang, S.; Hu, Y.; Yu, L.; Xu, G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015, 38, 2747–2765. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Dong, X.; Gao, Y.; Hao, F.; Zhang, H.; Lin, G. Molecular Mechanism of Exogenous Magnesium in Regulating Cation Homeostasis in Roots of Peanut Seedlings under Salt Stress. Agronomy 2024, 14, 724. https://doi.org/10.3390/agronomy14040724
Wang R, Dong X, Gao Y, Hao F, Zhang H, Lin G. Molecular Mechanism of Exogenous Magnesium in Regulating Cation Homeostasis in Roots of Peanut Seedlings under Salt Stress. Agronomy. 2024; 14(4):724. https://doi.org/10.3390/agronomy14040724
Chicago/Turabian StyleWang, Rongjin, Xuan Dong, Yan Gao, Fei Hao, Hui Zhang, and Guolin Lin. 2024. "Molecular Mechanism of Exogenous Magnesium in Regulating Cation Homeostasis in Roots of Peanut Seedlings under Salt Stress" Agronomy 14, no. 4: 724. https://doi.org/10.3390/agronomy14040724





